Tripartite Interaction of Epigenetic Regulation, Brain Aging, and Neuroinflammation: Mechanistic Insights and Therapeutic Implications
Abstract
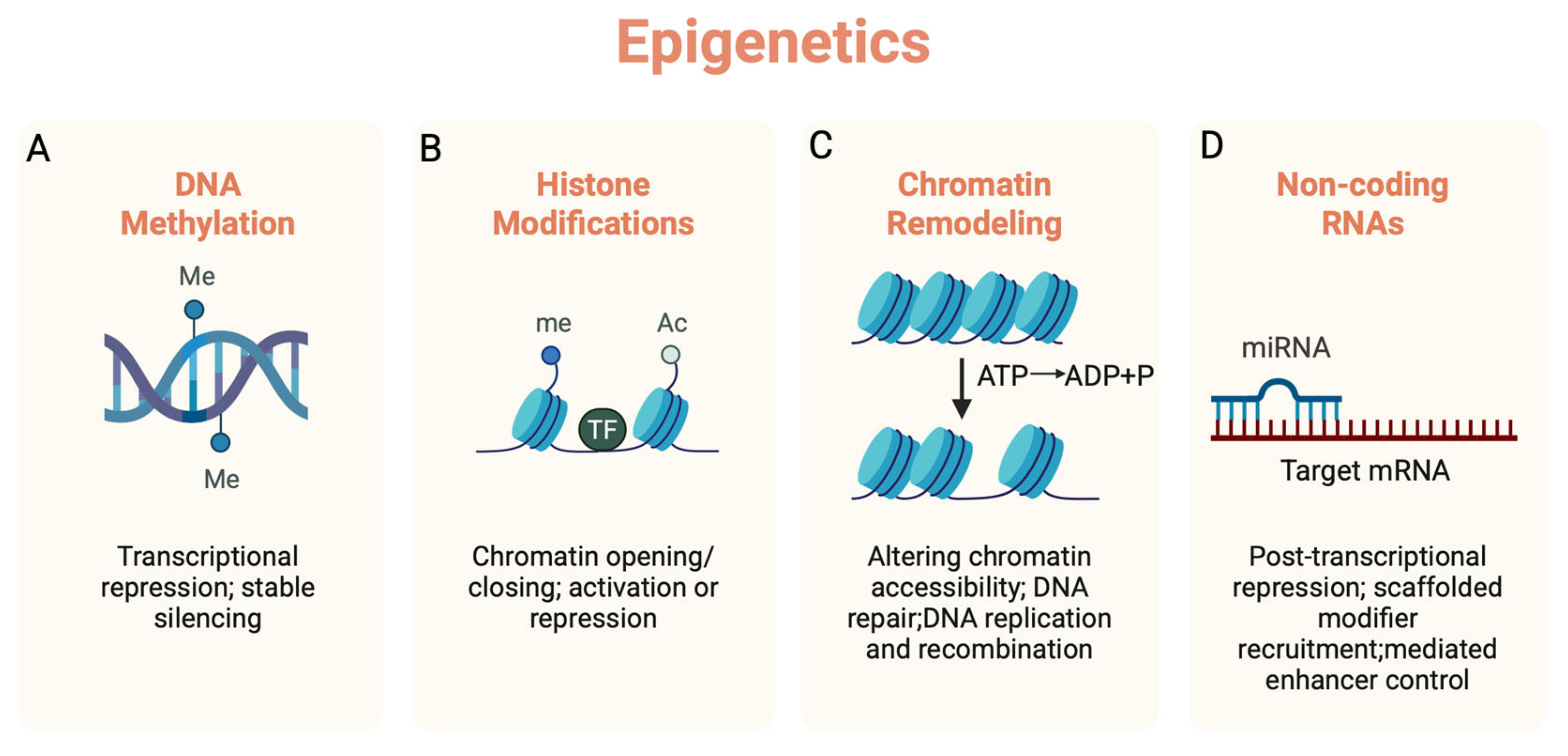
1. Introduction
2. The Role of Epigenetics in Brain Aging
2.1. Neural Stem Cell Aging
2.2. Neuronal Aging
2.3. Astrocytic Aging
2.4. Oligodendrocytic Aging
2.5. Microglial Aging
3. The Role of Epigenetics in Neuroinflammation
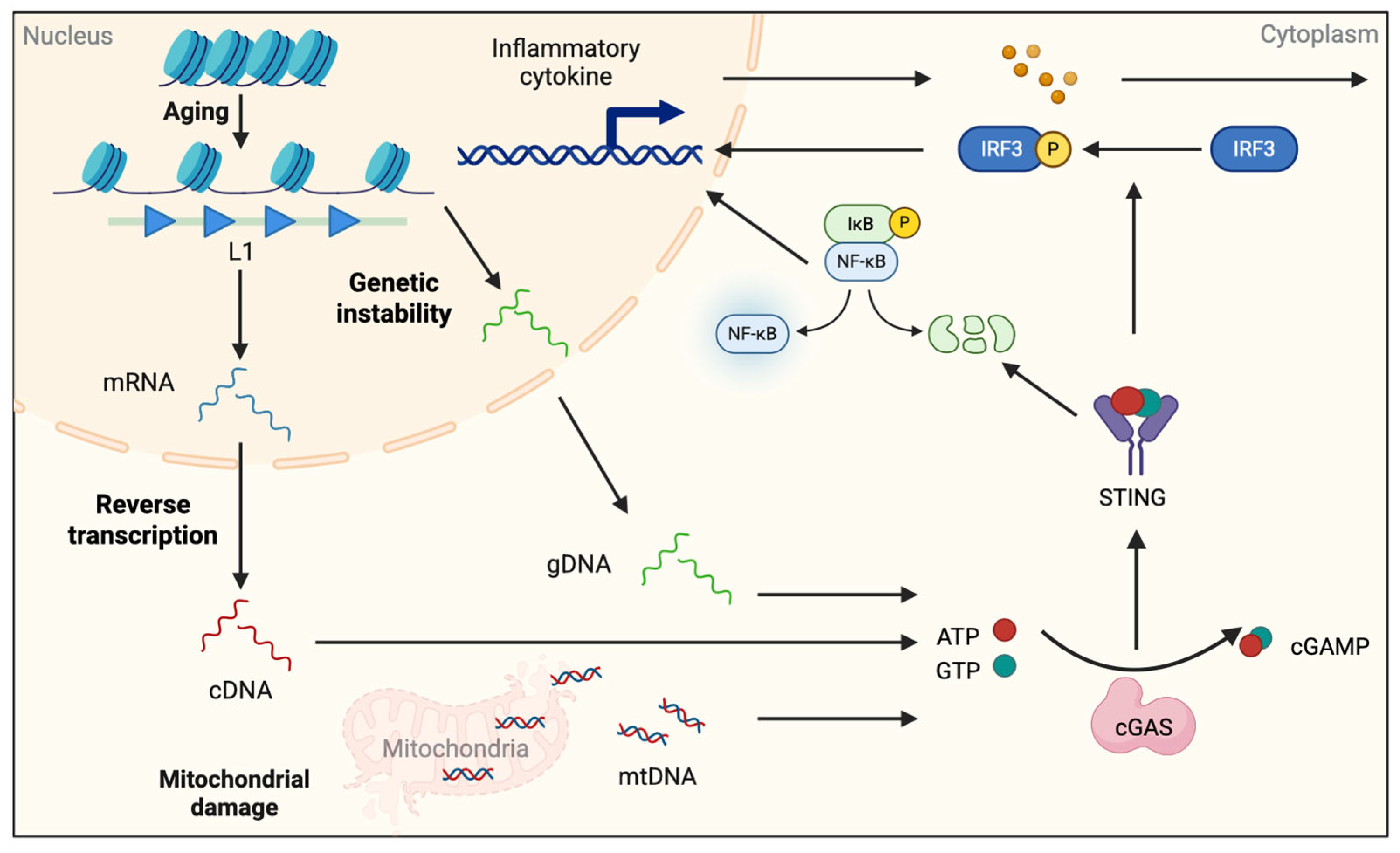
3.1. Oxidative Stress
3.2. Endogenous DNA Ligands
3.3. Chromatin Remodeling Factors and ncRNAs
3.4. Environmental Factors Affecting the Interaction of Epigenetic Alteration and Inflammation
3.4.1. Circadian Clock
3.4.2. PM2.5
3.4.3. VPA
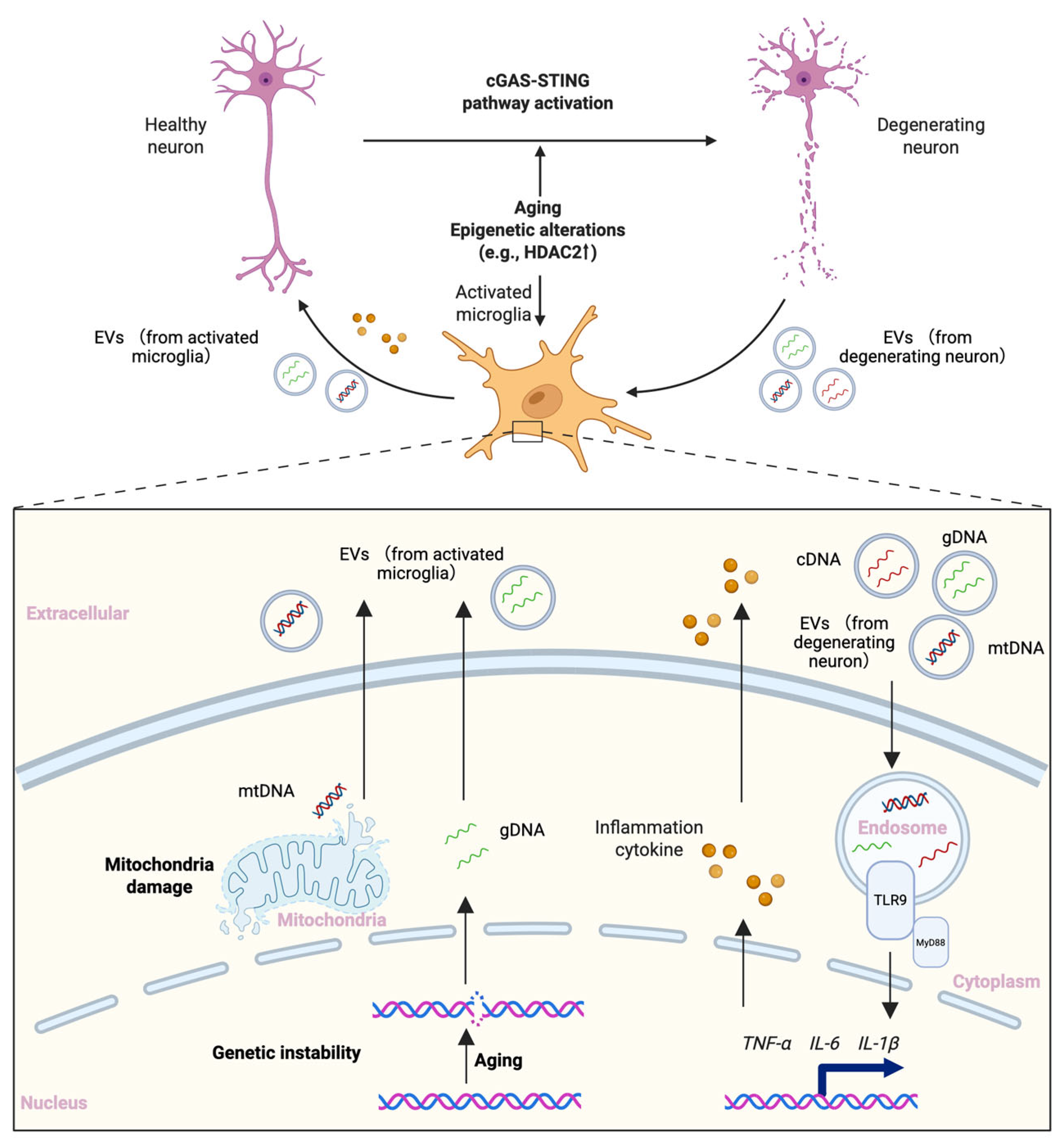
4. The Tripartite Model: A Dynamic Feed-Forward Loop of Brain Aging, Neuroinflammation, and Epigenetics
4.1. Model Overview: Interdependency and Amplification
4.2. Case Example of the Network Disorder: Neurodegenerative Diseases
4.2.1. Alzheimer’s Disease (AD)
4.2.2. Parkinson’s Disease (PD)
4.2.3. Amyotrophic Lateral Sclerosis (ALS)
5. Epigenetics-Related Therapeutic Interventions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weismann, A. Essays Upon Heredity; Clarendon Press: Oxford, UK, 1891. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Barter, J.D.; Foster, T.C. Aging in the Brain: New Roles of Epigenetics in Cognitive Decline. Neuroscience 2018, 24, 516–525. [Google Scholar] [CrossRef]
- Jurcau, M.C.; Jurcau, A.; Cristian, A.; Hogea, V.O.; Diaconu, R.G.; Nunkoo, V.S. Inflammaging and Brain Aging. Int. J. Mol. Sci. 2024, 25, 10535. [Google Scholar] [CrossRef]
- Giallongo, S.; Longhitano, L.; Denaro, S.; D’aprile, S.; Torrisi, F.; La Spina, E.; Giallongo, C.; Mannino, G.; Furno, D.L.; Zappalà, A.; et al. The Role of Epigenetics in Neuroinflammatory-Driven Diseases. Int. J. Mol. Sci. 2022, 23, 15218. [Google Scholar] [CrossRef]
- Bin Imtiaz, M.K.; Jaeger, B.N.; Bottes, S.; Machado, R.A.; Vidmar, M.; Moore, D.L.; Jessberger, S. Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity. Cell Stem Cell 2021, 28, 967–977.e8. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Houtman, J.; Eguiguren, J.S.; Ghassemzadeh, S.; Rund, N.; Novaresi, N.M.; Hu, L.; Parylak, S.L.; Denli, A.M.; Randolph-Moore, L.; et al. Lamin B1 decline underlies age-related loss of adult hippocampal neurogenesis. EMBO J. 2021, 40, 967–977. [Google Scholar] [CrossRef]
- Zhao, T.; Hong, Y.; Yan, B.; Huang, S.; Ming, G.-L.; Song, H. Epigenetic maintenance of adult neural stem cell quiescence in the mouse hippocampus via Setd1a. Nat. Commun. 2024, 15, 5674. [Google Scholar] [CrossRef]
- Matsubara, S.; Matsuda-Ito, K.; Sekiryu, H.; Doi, H.; Nakagawa, T.; Murao, N.; Oda, H.; Nakashima, K.; Matsuda, T. Epigenetic regulation of neural stem cell aging in the mouse hippocampus by Setd8 downregulation. EMBO J. 2025, 44, 3645–3668. [Google Scholar] [CrossRef] [PubMed]
- Gontier, G.; Iyer, M.; Shea, J.M.; Bieri, G.; Wheatley, E.G.; Ramalho-Santos, M.; Villeda, S.A. Tet2 Rescues Age-Related Regenerative Decline and Enhances Cognitive Function in the Adult Mouse Brain. Cell Rep. 2018, 22, 1974–1981. [Google Scholar] [CrossRef]
- Janke, C.; Montagnac, G. Causes and Consequences of Microtubule Acetylation. Curr. Biol. 2017, 27, R1287–R1292. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.-S.; Haggarty, S.J.; Giacometti, E.; Dannenberg, J.-H.; Joseph, N.; Gao, J.; Nieland, T.J.F.; Zhou, Y.; Wang, X.; Mazitschek, R.; et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009, 459, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhong, S.; Wan, H.; Guo, X.; Yao, X.; Liu, Q.; Chen, L.; Wang, J.-Z.; Xiao, S. Upregulated astrocyte HDAC7 induces Alzheimer-like tau pathologies via deacetylating transcription factor-EB and inhibiting lysosome biogenesis. Mol. Neurodegener. 2025, 20, 5. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.-C.; Xiao, B.-J.; Guo, X.-D.; Zheng, Q.-X.; Wu, B. Age-related Changes in the Global DNA Methylation Profile of Oligodendrocyte Progenitor Cells Derived from Rat Spinal Cords. Curr. Med. Sci. 2019, 39, 67–74. [Google Scholar] [CrossRef]
- Cho, S.-H.; Chen, J.A.; Sayed, F.; Ward, M.E.; Gao, F.; Nguyen, T.A.; Krabbe, G.; Sohn, P.D.; Lo, I.; Minami, S.; et al. SIRT1 Deficiency in Microglia Contributes to Cognitive Decline in Aging and Neurodegeneration via Epigenetic Regulation of IL-1β. J. Neurosci. 2015, 35, 807–818. [Google Scholar] [CrossRef]
- Carrillo-Jimenez, A.; Deniz, Ö.; Niklison-Chirou, M.V.; Ruiz, R.; Bezerra-Salomão, K.; Stratoulias, V.; Amouroux, R.; Yip, P.K.; Vilalta, A.; Cheray, M.; et al. TET2 Regulates the Neuroinflammatory Response in Microglia. Cell Rep. 2019, 29, 697–713.e8. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, I.; Paterlini, M.; Zamboni, M.; Ziegenhain, C.; Giatrellis, S.; Saghaleyni, R.; Björklund, Å.; Alkass, K.; Tata, M.; Druid, H.; et al. Identification of proliferating neural progenitors in the adult human hippocampus. Science 2025, 389, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Van Steensel, B.; Belmont, A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 2017, 169, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Richman, J.; Puthanveettil, S.V. Dissecting mechanisms of brain aging by studying the intrinsic excitability of neurons. Front. Aging Neurosci. 2015, 6, 337. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.T.; Hannon, E.; Levine, M.E.; Crimmins, E.M.; Lunnon, K.; Mill, J.; Geschwind, D.H.; Horvath, S. Genetic architecture of epigenetic and neuronal ageing rates in human brain regions. Nat. Commun. 2017, 8, 15353. [Google Scholar] [CrossRef]
- Singh, P.; Thakur, M.K. Histone Deacetylase 2 Inhibition Attenuates Downregulation of Hippocampal Plasticity Gene Expression during Aging. Mol. Neurobiol. 2018, 55, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Pao, P.-C.; Patnaik, D.; Watson, L.A.; Gao, F.; Pan, L.; Wang, J.; Adaikkan, C.; Penney, J.; Cam, H.P.; Huang, W.-C.; et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 2020, 11, 2484. [Google Scholar] [CrossRef]
- Labarta-Bajo, L.; Allen, N.J. Astrocytes in aging. Neuron 2025, 113, 109–126. [Google Scholar] [CrossRef]
- Namihira, M.; Kohyama, J.; Semi, K.; Sanosaka, T.; Deneen, B.; Taga, T.; Nakashima, K. Committed Neuronal Precursors Confer Astrocytic Potential on Residual Neural Precursor Cells. Dev. Cell 2009, 16, 245–255. [Google Scholar] [CrossRef]
- Sanosaka, T.; Imamura, T.; Hamazaki, N.; Chai, M.; Igarashi, K.; Ideta-Otsuka, M.; Miura, F.; Ito, T.; Fujii, N.; Ikeo, K.; et al. DNA Methylome Analysis Identifies Transcription Factor-Based Epigenomic Signatures of Multilineage Competence in Neural Stem/Progenitor Cells. Cell Rep. 2017, 20, 2992–3003. [Google Scholar] [CrossRef]
- Takizawa, T.; Nakashima, K.; Namihira, M.; Ochiai, W.; Uemura, A.; Yanagisawa, M.; Fujita, N.; Nakao, M.; Taga, T. DNA Methylation Is a Critical Cell-Intrinsic Determinant of Astrocyte Differentiation in the Fetal Brain. Dev. Cell 2001, 1, 749–758. [Google Scholar] [CrossRef]
- Chisholm, N.C.; Henderson, M.L.; Selvamani, A.; Park, M.J.; Dindot, S.; Miranda, R.C.; Sohrabji, F. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 2015, 10, 142–152. [Google Scholar] [CrossRef]
- Windener, F.; Grewing, L.; Thomas, C.; Dorion, M.-F.; Otteken, M.; Kular, L.; Jagodic, M.; Antel, J.; Albrecht, S.; Kuhlmann, T. Physiological aging and inflammation-induced cellular senescence may contribute to oligodendroglial dysfunction in MS. Acta Neuropathol. 2024, 147, 82. [Google Scholar] [CrossRef]
- Shen, S.; Liu, A.; Li, J.; Wolubah, C.; Casaccia-Bonnefil, P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiol. Aging 2008, 29, 452–463. [Google Scholar] [CrossRef]
- Liu, X.; Xin, D.E.; Zhong, X.; Zhao, C.; Li, Z.; Zhang, L.; Dourson, A.J.; Lee, L.; Mishra, S.; Bayat, A.E.; et al. Small-molecule-induced epigenetic rejuvenation promotes SREBP condensation and overcomes barriers to CNS myelin regeneration. Cell 2024, 187, 2465–2484.e22. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, Y.; Zhang, Y.; Wu, J.; Xu, Z.; Huang, Y.; Cai, L.; Gao, S.; Liu, T.; et al. Publisher Correction: Transcriptional and epigenetic decoding of the microglial aging process. Nat. Aging 2023, 4, 276. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J.; Han, T.; Kojima, N. Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress. Brain Sci. 2025, 15, 534. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Cheng, D.; Tsukamoto, M.R.; A Koike, M.; Wes, P.D.; Vasilevko, V.; Cribbs, D.H.; LaFerla, F.M. Blocking IL-1 Signaling Rescues Cognition, Attenuates Tau Pathology, and Restores Neuronal β-Catenin Pathway Function in an Alzheimer’s Disease Model. J. Immunol. 2011, 187, 6539–6549. [Google Scholar] [CrossRef]
- Wendeln, A.-C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef]
- Gosselin, D.; Skola, D.; Coufal, N.G.; Holtman, I.R.; Schlachetzki, J.C.M.; Sajti, E.; Jaeger, B.N.; O’Connor, C.; Fitzpatrick, C.; Pasillas, M.P.; et al. An environment-dependent transcriptional network specifies human microglia identity. Science 2017, 356, eaal3222. [Google Scholar] [CrossRef] [PubMed]
- Gjoneska, E.; Pfenning, A.R.; Mathys, H.; Quon, G.; Kundaje, A.; Tsai, L.-H.; Kellis, M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 2015, 518, 365–369. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Wan, M.; Liu, Y.; Li, D.; Snyder, R.J.; Elkin, L.B.; Day, C.R.; Rodriguez, J.; Grunseich, C.; Mahley, R.W.; Watts, J.A.; et al. The enhancer RNA, AANCR, regulates APOE expression in astrocytes and microglia. Nucleic Acids Res. 2024, 52, 10235–10254. [Google Scholar] [CrossRef]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacology 2017, 42, 318–333. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Invest. 2017, 217, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.M.; Zimmerman, J.D.; Lawson, M.A.; Bustamante, A.C.; Uddin, M.; Johnson, R.W. Inhibition of DNA Methylation With Zebularine Alters Lipopolysaccharide-Induced Sickness Behavior and Neuroinflammation in Mice. Front. Neurosci. 2018, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Seddon, A.R.; MacArthur, C.P.; Hampton, M.B.; Stevens, A.J. Inflammation and DNA methylation in Alzheimer’s disease: Mechanisms of epigenetic remodelling by immune cell oxidants in the ageing brain. Redox Rep. 2024, 29, 2428152. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, S.; Yan, F.; Cheng, J.; Huang, L.; Chen, H.; Liu, Q.; Ji, X.; Yuan, Z. HDAC2 Selectively Regulates FOXO3a-Mediated Gene Transcription during Oxidative Stress-Induced Neuronal Cell Death. J. Neurosci. 2015, 35, 1250–1259. [Google Scholar] [CrossRef]
- Peleg, S.; Sananbenesi, F.; Zovoilis, A.; Burkhardt, S.; Bahari-Javan, S.; Agis-Balboa, R.C.; Cota, P.; Wittnam, J.L.; Gogol-Doering, A.; Opitz, L.; et al. Altered Histone Acetylation Is Associated with Age-Dependent Memory Impairment in Mice. Science 2010, 328, 753–756. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; I Aladjem, M.; Kohn, K.W. SIRT1/PARP1 crosstalk: Connecting DNA damage and metabolism. Genome Integr. 2013, 4, 6. [Google Scholar] [CrossRef]
- Kauppinen, T.M.; Gan, L.; Swanson, R.A. Poly(ADP-ribose) polymerase-1-induced NAD+ depletion promotes nuclear factor-κB transcriptional activity by preventing p65 de-acetylation. Biochim. Biophys. Acta 2013, 1833, 1985–1991. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Guedes, J.R.; Pereira de Almeida, L.; Pedroso de Lima, M.C. miR-155 modulates microglia-mediated immune re-sponse by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2012, 135, 73–88. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, L. Inflamma-MicroRNAs in Alzheimer’s Disease: From Disease Pathogenesis to Therapeutic Potentials. Front. Cell Neurosci. 2021, 15, 785433. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Ramesh, S.; Beasley, M.; Lynn, G.; Wallace, C.; Labeau, S.; Pathak, S.; Nadar, R.; Moore, T.; Dhanasekaran, M. Role of cGAS-Sting Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 8151. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Shinkai, Y. SETDB1-Mediated Silencing of Retroelements. Viruses 2020, 12, 596. [Google Scholar] [CrossRef]
- Padeken, J.; Methot, S.P.; Gasser, S.M. Establishment of H3K9-methylated heterochromatin and its functions in tissue differentia-tion and maintenance. Nat. Rev. Mol. Cell Biol. 2022, 23, 623–640. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.-C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Matsuda, T.; Murao, N.; Katano, Y.; Juliandi, B.; Kohyama, J.; Akira, S.; Kawai, T.; Nakashima, K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat. Commun. 2015, 6, 6514. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H.; Bohr, V.A. Signaling by cGAS–STING in Neurodegeneration, Neuroinflammation, and Aging. Trends Neurosci. 2021, 44, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Udeochu, J.C.; Amin, S.; Huang, Y.; Fan, L.; Torres, E.R.S.; Carling, G.K.; Liu, B.; McGurran, H.; Coronas-Samano, G.; Kauwe, G.; et al. Tau activation of microglial cGAS-IFN reduces MEF2C-mediated cognitive resilience. Nat. Neurosci. 2023, 26, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, B.K.; Erwin, J.A.; Song, J.-J.; Lee, J.T. Polycomb Proteins Targeted by a Short Repeat RNA to the Mouse X Chromosome. Science 2008, 322, 750–756. [Google Scholar] [CrossRef]
- Kaneko, S.; Bonasio, R.; Saldaña-Meyer, R.; Yoshida, T.; Son, J.; Nishino, K.; Umezawa, A.; Reinberg, D. Interactions between JARID2 and Noncoding RNAs Regulate PRC2 Recruitment to Chromatin. Mol. Cell 2014, 53, 290–300. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Kagey, M.H.; Newman, J.J.; Bilodeau, S.; Zhan, Y.; Orlando, D.A.; Van Berkum, N.L.; Ebmeier, C.C.; Goossens, J.; Rahl, P.B.; Levine, S.S.; et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 2010, 467, 430–435. [Google Scholar] [CrossRef]
- Schaukowitch, K.; Joo, J.-Y.; Liu, X.; Watts, J.K.; Martinez, C.; Kim, T.-K. Enhancer RNA Facilitates NELF Release from Immediate Early Genes. Mol. Cell 2014, 56, 29–42. [Google Scholar] [CrossRef]
- Tsai, P.-F.; Dell’oRso, S.; Rodriguez, J.; Vivanco, K.O.; Ko, K.-D.; Jiang, K.; Juan, A.H.; Sarshad, A.A.; Vian, L.; Tran, M.; et al. A Muscle-Specific Enhancer RNA Mediates Cohesin Recruitment and Regulates Transcription In trans. Mol. Cell 2018, 71, 129–141.e8. [Google Scholar] [CrossRef]
- Varambally, S.; Cao, Q.; Mani, R.S.; Shankar, S.; Wang, X.; Ateeq, B.; Laxman, B.; Cao, X.; Jing, X.; Ramnarayanan, K.; et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 2008, 322, 1695–1699. [Google Scholar] [CrossRef]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. microRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson’s Disease. J. Neurosci. 2016, 36, 2383–2390. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU.1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Saba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Rao, D.S.; Boldin, M.P.; Taganov, K.D.; O’COnnell, R.M.; Baltimore, D. NF-κB dysregulation in microRNA-146a–deficient mice drives the development of myeloid malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 9184–9189. [Google Scholar] [CrossRef]
- Kim, T.-K.; Hemberg, M.; Gray, J.M.; Costa, A.M.; Bear, D.M.; Wu, J.; Harmin, D.A.; Laptewicz, M.; Barbara-Haley, K.; Kuersten, S.; et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010, 465, 182–187. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, Z.; Yang, M.; Yu, W.; Luo, R.; Zhou, J.; He, J.; Chen, Q.; Song, Z.; Cheng, S. Non-Coding RNA in Microglia Activation and Neuroinflammation in Alzheimer’s Disease. J. Inflamm. Res. 2023, 16, 4165–4211. [Google Scholar] [CrossRef]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer Disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef]
- Griffin, P.; Dimitry, J.M.; Sheehan, P.W.; Lananna, B.V.; Guo, C.J.; Robinette, M.L.; Hayes, M.E.; Cedeño, M.R.; Nadarajah, C.; Ezerskiy, L.A.; et al. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 5102–5107. [Google Scholar] [CrossRef]
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N. Differential Control of Bmal1 Circadian Transcription by REV-ERB and ROR Nuclear Receptors. J. Biol. Rhythm. 2005, 20, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, B.; Damle, M.; Guan, D.; Li, Z.; Kim, Y.H.; Gannon, M.; Lazar, M.A. HNF6 and Rev-erbα integrate hepatic lipid metabolism by overlapping and distinct transcriptional mechanisms. Genes Dev. 2016, 30, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.Y.; Cho, H.; Lesch, H.P.; Gosselin, D.; Heinz, S.; Tanaka-Oishi, Y.; Benner, C.; Kaikkonen, M.U.; Kim, A.S.; Kosaka, M.; et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 2013, 498, 511–515. [Google Scholar] [CrossRef]
- Kim, Y.H.; Marhon, S.A.; Zhang, Y.; Emmett, M.J.; Damle, M.; Kyoung-Jae, W.; Lazar, M.A. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science 2018, 359, 1274–1277. [Google Scholar] [CrossRef]
- Hor, C.N.; Yeung, J.; Jan, M.; Emmenegger, Y.; Hubbard, J.; Xenarios, I.; Naef, F.; Franken, P. Sleep–wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 25773–25783. [Google Scholar] [CrossRef]
- Rider, C.F.; Carlsten, C. Air pollution and DNA methylation: Effects of exposure in humans. Clin. Epigenetics 2019, 11, 131. [Google Scholar] [CrossRef]
- Wu, Y.; Qie, R.; Cheng, M.; Zeng, Y.; Huang, S.; Guo, C.; Zhou, Q.; Li, Q.; Tian, G.; Han, M.; et al. Air pollution and DNA methylation in adults: A systematic review and meta-analysis of observational studies. Environ. Pollut. 2021, 284, 117152. [Google Scholar] [CrossRef]
- Prunicki, M.; Cauwenberghs, N.; Lee, J.; Zhou, X.; Movassagh, H.; Noth, E.; Lurmann, F.; Hammond, S.K.; Balmes, J.R.; Desai, M.; et al. Air pollution exposure is linked with methylation of immunoregulatory genes, altered immune cell profiles, and increased blood pressure in children. Sci. Rep. 2021, 11, 7043. [Google Scholar] [CrossRef]
- Ku, T.; Li, B.; Gao, R.; Zhang, Y.; Yan, W.; Ji, X.; Li, G.; Sang, N. NF-κB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol. 2017, 14, 34. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Ebelt, S.; Gearing, M.; Kobor, M.S.; Konwar, C.; Maclsaac, J.L.; Dever, K.; Wingo, A.P.; Levey, A.A.; et al. Differential DNA methylation in the brain as potential mediator of the association between traffic-related PM2.5 and neuro-pathology markers of Alzheimer’s disease. Alzheimers Dement. 2024, 20, 2538–2551. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [CrossRef]
- Abematsu, M.; Tsujimura, K.; Yamano, M.; Saito, M.; Kohno, K.; Kohyama, J.; Namihira, M.; Komiya, S.; Nakashima, K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J. Clin. Investig. 2010, 120, 3255–3266. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, J.L.; Morell, M.; Scheys, J.O.; Kulpa, D.A.; Morell, S.; Carter, C.C.; Hammer, G.D.; Collins, K.L.; O’sHea, K.S.; Menendez, P.; et al. Epigenetic silencing of engineered L1 retrotransposition events in human embryonic carcinoma cells. Nature 2010, 466, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Zapata-Bodalo, I.; Klein, A.; Plassat, J.-L.; Knauer-Meyer, T.; Keyes, W.M. Aberrant induction of p19Arf-mediated cellular senescence contributes to neurodevelopmental defects. PLOS Biol. 2022, 20, e3001664. [Google Scholar] [CrossRef]
- Coninx, E.; Chew, Y.C.; Yang, X.; Guo, W.; Coolkens, A.; Baatout, S.; Moons, L.; Verslegers, M.; Quintens, R. Hippocampal and cortical tissue-specific epigenetic clocks indicate an increased epigenetic age in a mouse model for Alzheimer’s disease. Aging 2020, 12, 20817–20834. [Google Scholar] [CrossRef]
- Watson, C.T.; Roussos, P.; Garg, P.; Ho, D.J.; Azam, N.; Katsel, P.L.; Haroutunian, V.; Sharp, A.J. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290. [Google Scholar] [CrossRef]
- Mancuso, R.; Fattorelli, N.; Martinez-Muriana, A.; Davis, E.; Wolfs, L.; Van Den Daele, J.; Geric, I.; Premereur, J.; Polanco, P.; Bijnens, B.; et al. Xenografted human microglia display diverse transcriptomic states in response to Alzheimer’s disease-related amyloid-b pathology. Nat. Neurosci. 2024, 27, 886–900. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Marzi, S.J.; Leung, S.K.; Ribarska, T.; Hannon, E.; Smith, A.R.; Pishva, E.; Poschmann, J.; Moore, K.; Troakes, C.; Al-Sarraj, S.; et al. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat. Neurosci. 2018, 21, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Nativio, R.; Donahue, G.; Berson, A.; Lan, Y.; Amlie-Wolf, A.; Tuzer, F.; Toledo, J.B.; Gosai, S.J.; Gregory, B.D.; Torres, C.; et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 497–505. [Google Scholar] [CrossRef]
- Arvanitaki, E.S.; Goulielmaki, E.; Gkirtzimanaki, K.; Niotis, G.; Tsakani, E.; Nenedaki, E.; Rouska, I.; Kefalogianni, M.; Xydias, D.; Kalafatakis, I.; et al. Microglia-derived extracellular vesicles trigger age-related neurodegeneration upon DNA damage. Proc. Natl. Acad. Sci. USA 2024, 121, e2317402121. [Google Scholar] [CrossRef]
- Heikenwalder, M.; Polymenidou, M.; Junt, T.; Sigurdson, C.; Wagner, H.; Akira, S.; Zinkernagel, R.; Aguzzi, A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 2004, 10, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-W.; Chang, N.P.; Krishnagiri, M.; Patel, A.P.; Lindman, M.; Angel, J.P.; Kung, P.-L.; Atkins, C.; Daniels, B.P. Fibrillar α-synuclein induces neurotoxic astrocyte activation via RIP kinase signaling and NF-κB. Cell Death Dis. 2021, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Song, N.; Chen, L.; Xie, J. Alpha-Synuclein Handling by Microglia: Activating, Combating, and Worsening. Neurosci. Bull. 2021, 37, 751–753. [Google Scholar] [CrossRef]
- Rostami, J.; Mothes, T.; Kolahdouzan, M.; Eriksson, O.; Moslem, M.; Bergström, J.; Ingelsson, M.; O’Callaghan, P.; Healy, L.M.; Falk, A.; et al. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein aggregates. J. Neu-roinflamm. 2021, 18, 124. [Google Scholar] [CrossRef]
- Matsumoto, L.; Takuma, H.; Tamaoka, A.; Kurisaki, H.; Date, H.; Tsuji, S.; Iwata, A. CpG Demethylation Enhances Alpha-Synuclein Expression and Affects the Pathogenesis of Parkinson’s Disease. PLoS ONE 2010, 5, e15522. [Google Scholar] [CrossRef]
- De Boni, L.; Riedel, L.; Schmitt, I.; Kraus, T.F.; Kaut, O.; Piston, D.; Akbarian, S.; Wüllner, U. DNA methylation levels of α-synuclein intron 1 in the aging brain. Neurobiol. Aging 2015, 36, 3334.e7–3334.e11. [Google Scholar] [CrossRef]
- Gu, J.; Barrera, J.; Yun, Y.; Murphy, S.K.; Beach, T.G.; Woltjer, R.L.; Serrano, G.E.; Kantor, B.; Chiba-Falek, O. Cell-Type Specific Changes in DNA Methylation of SNCA Intron 1 in Synucleinopathy Brains. Front. Neurosci. 2021, 15, 652226. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Morales, E.; Meier, K.; Sandoval-Carrillo, A.; Salas-Pacheco, J.; Vázquez-Cárdenas, P.; Arias-Carrión, O. Implications of DNA methylation in parkinson’s disease. Front. Mol. Neurosci. 2017, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Loygorri, J.I.; Villarejo-Zori, B.; Viedma-Poyatos, Á.; Zapata-Muñoz, J.; Benítez-Fernández, R.; Frutos-Lisón, M.D.; Tomás-Barberán, F.A.; Espín, J.C.; Area-Gómez, E.; Gomez-Duran, A.; et al. Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat. Commun. 2024, 15, 830. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Patel, J.; Panicker, N.; Karuppagounder, S.S.; Biswas, D.; Belingon, B.; Chen, R.; Brahmachari, S.; Pletnikova, O.; Troncoso, J.C.; et al. STING mediates neurodegeneration and neuroinflammation in nigrostriatal α-synucleinopathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2118819119. [Google Scholar] [CrossRef]
- Standaert, D.G.; Childers, G.M. Alpha-synuclein–mediated DNA damage, STING activation, and neuroinflammation in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2204058119. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Sinha, S.C.; Amin, S.; Gan, L. Mechanism and therapeutic potential of targeting cGAS-STING signaling in neuro-logical disorders. Mol. Neurodegener. 2023, 18, 79. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Tian, T.; Yao, H.; Xia, X.-M.; Wang, C.; Cao, L.; Hu, G.; Du, R.-H.; Lu, M. The cGAS-STING-YY1 axis accelerates progression of neurodegeneration in a mouse model of Parkinson’s disease via LCN2-dependent astrocyte senescence. Cell Death Differ. 2023, 30, 2280–2292. [Google Scholar] [CrossRef]
- Szego, E.M.; Malz, L.; Bernhardt, N.; Rösen-Wolff, A.; Falkenburger, B.H.; Luksch, H. Constitutively active STING causes neuroinflammation and degeneration of dopaminergic neurons in mice. Elife 2022, 11, e81943. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Qiu, J.; Gao, S.; Yu, S.; Sun, D.; Lou, H. The STING inhibitor C-176 attenuates MPTP-induced neuroinflammation and neurodegeneration in mouse parkinsonian models. Int. Immunopharmacol. 2023, 124, 110827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, M.; Wu, H.; Zhu, J.; Jin, T. The cGAS-STING pathway drives neuroinflammation and neurodegeneration via cellular and molecular mechanisms in neurodegenerative diseases. Neurobiol. Dis. 2024, 202, 106710. [Google Scholar] [CrossRef]
- Fedotova, E.Y.; Iakovenko, E.V.; Abramycheva, N.Y.; Illarioshkin, S.N. SNCA Gene Methylation in Parkinson’s Disease and Multiple System Atrophy. Epigenomes 2023, 7, 5. [Google Scholar] [CrossRef]
- Yu, C.-H.; Davidson, S.; Harapas, C.R.; Hilton, J.B.; Mlodzianoski, M.J.; Laohamonthonkul, P.; Louis, C.; Low, R.R.J.; Moecking, J.; De Nardo, D.; et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell 2020, 183, 636–649.e18. [Google Scholar] [CrossRef]
- Marques, C.; Held, A.; Dorfman, K.; Sung, J.; Song, C.; Kavuturu, A.S.; Aguilar, C.; Russo, T.; Oakley, D.H.; Albers, M.W.; et al. Neuronal STING activation in amyotrophic lateral sclerosis and frontotemporal dementia. Acta Neuropathol. 2024, 147, 56. [Google Scholar] [CrossRef]
- Tan, H.Y.; Yong, Y.K.; Xue, Y.C.; Liu, H.; Furihata, T.; Shankar, E.M.; Ng, C.S. cGAS and DDX41-STING mediated intrinsic immunity spreads intercellularly to promote neuroinflammation in SOD1 ALS model. iScience 2022, 25, 104404. [Google Scholar] [CrossRef]
- Krug, L.; Chatterjee, N.; Borges-Monroy, R.; Hearn, S.; Liao, W.-W.; Morrill, K.; Prazak, L.; Rozhkov, N.; Theodorou, D.; Hammell, M.; et al. Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLOS Genet. 2017, 13, e1006635. [Google Scholar] [CrossRef]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; NYGC ALS Consortium; Fagegaltier, D.; Harris, B.T.; et al. Postmortem Cortex Samples Identify Distinct Molecular Sub-types of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e5. [Google Scholar] [CrossRef]
- Pereira, G.C.; Sanchez, L.; Schaughency, P.M.; Rubio-Roldán, A.; Choi, J.A.; Planet, E.; Batra, R.; Turelli, P.; Trono, D.; Ostrow, L.W.; et al. Properties of LINE-1 proteins and repeat element expression in the context of amyotrophic lateral sclerosis. Mob. DNA 2018, 9, 35. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Dubnau, J. Endogenous retroviruses and TDP-43 proteinopathy form a sustaining feedback driving intercellular spread of Drosophila neurodegeneration. Nat. Commun. 2023, 14, 966. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, L.; Wang, J.; Wu, Y.; Li, Y.; Du, L.; Li, L.; Fang, Z.; Zhang, X. The cytosolic DNA-sensing cGAS–STING pathway in neurodegeneration. CNS Neurosci. Ther. 2024, 30, e14671. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS–STING. Proc. Natl. Acad. Sci. USA 2021, 118, e2011226118. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Jeong, J.-H.; Park, J.-C.; Han, J.W.; Lee, Y.; Kim, J.-I.; Mook-Jung, I. Blockade of STING activation alleviates microglial dysfunction and a broad spectrum of Alzheimer’s disease pathologies. Exp. Mol. Med. 2024, 56, 1936–1951. [Google Scholar] [CrossRef]
- Xie, X.; Ma, G.; Li, X.; Zhao, J.; Zhao, Z.; Zeng, J. Activation of innate immune cGAS-STING pathway contributes to Alzheimer’s pathogenesis in 5×FAD mice. Nat. Aging 2023, 3, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, Z.P.; Perdok, A.; Hellemans, R.; North, K.; Vorsters, I.; Cappel, C.; Dehairs, J.; Swinnen, J.V.; Sannerud, R.; Bretou, M.; et al. Phospholipase D3 degrades mitochondrial DNA to regulate nucleotide signaling and APP metabolism. Nat. Commun. 2023, 14, 2847. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Zuniga, G.; Ramirez, P.; Fernandez, R.; Wang, C.-P.; Li, J.; Davila, L.; Pelton, K.; Gomez, S.; Sohn, C.; et al. A Phase IIa clinical trial to evaluate the effects of anti-retroviral therapy in Alzheimer’s disease (ART-AD). NPJ Dement. 2025, 1, 2. [Google Scholar] [CrossRef]
- Sun, Z.; Kwon, J.-S.; Ren, Y.; Chen, S.; Walker, C.K.; Lu, X.; Cates, K.; Karahan, H.; Sviben, S.; Fitzpatrick, J.A.J.; et al. Modeling late-onset Alzheimer’s disease neuropathology via direct neuronal reprogramming. Science 2024, 385, adl2992. [Google Scholar] [CrossRef]
- Vallés-Saiz, L.; Ávila, J.; Hernández, F. Lamivudine (3TC), a Nucleoside Reverse Transcriptase Inhibitor, Prevents the Neuropathological Alterations Present in Mutant Tau Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 11144. [Google Scholar] [CrossRef]
- Wahl, D.; Smith, M.E.; McEntee, C.M.; Cavalier, A.N.; Osburn, S.C.; Burke, S.D.; Grant, R.A.; Nerguizian, D.; Lark, D.S.; Link, C.D.; et al. The reverse transcriptase inhibitor 3TC protects against age-related cognitive dysfunction. Aging Cell 2023, 22, e13798. [Google Scholar] [CrossRef]
- Carling, G.K.; Fan, L.; Foxe, N.R.; Norman, K.; Wong, M.Y.; Zhu, D.; Corona, C.; Razzoli, A.; Yu, F.; Yarahmady, A.; et al. Alzheimer’s disease-linked risk alleles elevate microglial cGAS-associated senescence and neurodegeneration in a tauopathy model. Neuron 2024, 112, 3877–3896.e8. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Mittra, N.; Bhattacharjee, A.; Wang, H.; Song, S.; Zhao, S.; Liu, F.; Han, X. Microglial cGAS Deletion Preserves Intercellular Communication and Alleviates Amyloid-β-Induced Pathogenesis of Alzheimer’s Disease. Adv. Sci. 2025, 12, e2410910. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, Y.; Li, S.; Ma, C.; Huang, J.; Wen, S.; Yang, S.; Wang, B. Microglial cGAS drives neuroinflammation in the MPTP mouse models of Parkinson’s disease. CNS Neurosci. Ther. 2023, 29, 2018–2035. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.E.; O’rOurke, J.G.; Yáñez, A.; Markman, J.L.; Ho, R.; Wang, X.; Chen, S.; Lall, D.; Jin, M.; Muhammad, A.K.M.G.; et al. C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature 2020, 585, 96–101. [Google Scholar] [CrossRef]
- Gräff, J.; Rei, D.; Guan, J.-S.; Wang, W.-Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.F.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef]
- Simon, M.; Van Meter, M.; Ablaeva, J.; Ke, Z.; Gonzalez, R.S.; Taguchi, T.; De Cecco, M.; Leonova, K.I.; Kogan, V.; Helfand, S.L.; et al. LINE1 Derepression in Aged Wild-Type and SIRT6-Deficient Mice Drives Inflammation. Cell Metab. 2019, 29, 871–885.e5. [Google Scholar] [CrossRef]
- Eichmüller, O.L.; Knoblich, J.A. Human cerebral organoids—A new tool for clinical neurology research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G.-L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Coleman, K.; Jiang, S.; Kriz, A.J.; Marciano, J.H.; Luo, C.; Cai, C.; Manam, M.D.; Caglayan, E.; Lai, A.; et al. Spatial transcriptomics reveals human cortical layer and area specification. Nature 2025, 644, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ditzer, N.; Senoglu, E.; Kolodziejczyk, A.; Schütze, T.M.; Nikolaidi, A.; Küster, K.; Sameith, K.; Dietz, S.; Derihaci, R.P.; Birdir, C.; et al. Epigenome profiling identifies H3K27me3 regulation of extracellular matrix composition in human corticogenesis. Neuron 2025, 113, 2927–2944.e10. [Google Scholar] [CrossRef]
- Lee, C.N.; Fu, H.; Cardilla, A.; Zhou, W.; Deng, Y. Spatial joint profiling of DNA methylome and transcriptome in tissues. Nature 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]

| Cell Type | Epigenetic Alterations | Outcome | References |
|---|---|---|---|
| NSC | LaminB1 expression progressively declines | Silencing of neurogenic genes | [6,7] |
| Loss of Setd1a (H3K4me) | Promotes NSC activation | [8] | |
| Downregulation of Setd8 expression | Upregulation of quiescence-associated genes, inhibition of NSC proliferation | [9] | |
| Downregulation of Tet2 expression | Impairing adult neurogenesis | [10] | |
| Neuron | α-tubulin undergoes hyperacetylation | Loss of dendritic arbors and spines | [11] |
| Increased expression of Hdac2 | Synaptic loss and reduced plasticity | [12] | |
| Astrocyte | Increased expression of HDAC7 | Contributes to tau accumulation and degeneration of neurons | [13] |
| Oligodendrocyte/OPC | Reduced expression of Dnmt1 | Global DNA hypomethylation was observed, reduced remyelination efficiency and impaired OPC differentiation | [14] |
| Microglia | Downregulation of Sirt1 expression | Upregulation of Il-1β expression | [15] |
| Increased expression of TET2 | Microglial activation | [16] |
| Disease | Conclusions | Models | Strategy | Reference |
|---|---|---|---|---|
| Aging | Reduced inflammation associated with aging. Resulting in improved tissue function. | Aging mice | H-151, si-STING | [56] |
| AD | Microglia transition from a harmful phenotype to a protective phenotype. Decreased expression of IL-6, Il-1β, and Tnf-α. Increased expression of Arg1 and Fizz1. Reduced degree of aging. | APP/PS1 mice | H-151, si-STING | [131] |
| Reduced brain inflammation and microglial synaptic phagocytosis. Significantly improved Aβ burden, tau phosphorylation, and cognitive impairment. | AppNL-G-F/hTau-double KI mice | H-151 | [132] | |
| Increased Aβ clearance. Suppression of neurotoxic A1 astrocytes. Decreased expression of Ifn-β, Il-6, Tnf-α, and Il-1α. Enhanced phagocytic activity of microglia. Alleviation of cognitive impairment and Aβ pathological changes. | 5×FAD mice | H-151 | [133] | |
| Reduced of cleaved caspase 3. | PLD3 knockout SH-SY5Y cells | H-151 | [134] | |
| Decreased GFAP in cerebrospinal fluid [reduced neuroinflammation). Elevated Ab42/40 ratio in plasma (reduced plaque burden in the brain). | Human | Lamivudine (3TC) | [135] | |
| Reduced expression of single-stranded DNA. Reduced DNA damage. Reduced neuronal death. Increased expression of PI16 and ADAMDEC1. reduction of Aβ deposition p-tau and K63-linked ubiquitin-positive tau | 3D neural spheroids generated from late-onset AD patient fibroblasts via direct neural reprogramming technology | 3TC | [136] | |
| Reduced tau phosphorylation, inflammation, neuronal death, and hippocampal atrophy. Alleviated motor deficits (Rotarod test) and improved short-term memory (Y-maze test). Inhibited the insertion of L1. | P301S mice | 3TC | [137] | |
| Improved cognitive function. Reduced inflammation and anxiety. Reduced Iba1 and GFAP expression. | rTg4510 mice | 3TC | [138] | |
| Enhanced the neuronal Mef2c transcriptional network. Restored synaptic integrity, plasticity, and memory. | P301S transgenic mice | TDI-6570 | [65] | |
| Reduced of p21 positive cells. | Mouse microglia cells | TDI-6570 | [139] | |
| Restricted Aβ deposition. Alleviated neuroinflammation. Reduced neuronal damage. Improved cognitive behavioral. | 5xFAD; cGASfl/fl; Cx3cr1+/− mice | STING deficiency | [140] | |
| PD | Reduced interferon expression in the striatum. Treatment of motor dysfunction, pathological α-synuclein deposition, and dopaminergic neuron loss. | Stinggt mice | STING deficiency | [115] |
| Attenuated PD-associated behavioral phenotypes. Reduced loss of TH-positive neurons. Decreased the number of activated microglia. Lowered levels of factors associated with cGAS-dependent inflammation. | MPTP PD mice | RU.521 | [141] | |
| Reduced expression of Ifn-β, Tnf-α, Il-1β, and Il-6. Decreased expression of Nlpr3 and caspase-1. Alleviated neuroinflammation associated with MPTP neurotoxicity. Protected substantia nigra striatal dopaminergic neurons from degeneration. Lowered levels of reactive astrocytes. | MPTP PD mice | C-176 | [120] | |
| ALS | Reduced of IFN-I, ISGs, pro-inflammatory, and chemokines genes. Improved motor functions. | SOD1 ALS mice | C-176, H-151, RU.521 | [125] |
| Reduced the expression of Il-1β, Il-6, Tnf, Mx1, Infb 1. Decreased neuronal loss. | TDP-43 mutant mice | H-151 | [123] | |
| Preventing motor neuron death. | Human iPSC-derived motor neurons | |||
| Reduced levels of TNF-α, IL-6 and CXCL10. Suppressed inflammatory responses. | Human iPSC-derived neurons | H-151, RU.521 | [124] | |
| C9orf72 mice | ||||
| Reduced of ISGs expression | C9orf72 knockout mice | H-151 | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, S.; Nakashima, H.; Nakashima, K. Tripartite Interaction of Epigenetic Regulation, Brain Aging, and Neuroinflammation: Mechanistic Insights and Therapeutic Implications. Epigenomes 2025, 9, 38. https://doi.org/10.3390/epigenomes9040038
Mi S, Nakashima H, Nakashima K. Tripartite Interaction of Epigenetic Regulation, Brain Aging, and Neuroinflammation: Mechanistic Insights and Therapeutic Implications. Epigenomes. 2025; 9(4):38. https://doi.org/10.3390/epigenomes9040038
Chicago/Turabian StyleMi, Shenghui, Hideyuki Nakashima, and Kinichi Nakashima. 2025. "Tripartite Interaction of Epigenetic Regulation, Brain Aging, and Neuroinflammation: Mechanistic Insights and Therapeutic Implications" Epigenomes 9, no. 4: 38. https://doi.org/10.3390/epigenomes9040038
APA StyleMi, S., Nakashima, H., & Nakashima, K. (2025). Tripartite Interaction of Epigenetic Regulation, Brain Aging, and Neuroinflammation: Mechanistic Insights and Therapeutic Implications. Epigenomes, 9(4), 38. https://doi.org/10.3390/epigenomes9040038






