Genetics and Epigenetics of Chemoinduced Oral Mucositis in Paediatric Patients with Haematological Malignancies—A Review
Abstract
1. Introduction
2. Methodology
3. Genetics and Oral Mucositis
3.1. Polymorphisms in the Folic Acid Metabolism Pathway
3.2. Polymorphisms in the Transport Proteins Pathway
3.3. Polymorphisms in the Epigenetic Machinery Pathway
3.4. Polymorphisms in the Others Pathways
4. Epigenetics and Oral Mucositis
4.1. DNA Methylation in the Epigenetic Machinery Pathway
4.2. DNA Methylation in the Inflammation, Oxidative Stress and Vitamin D Metabolism Pathways
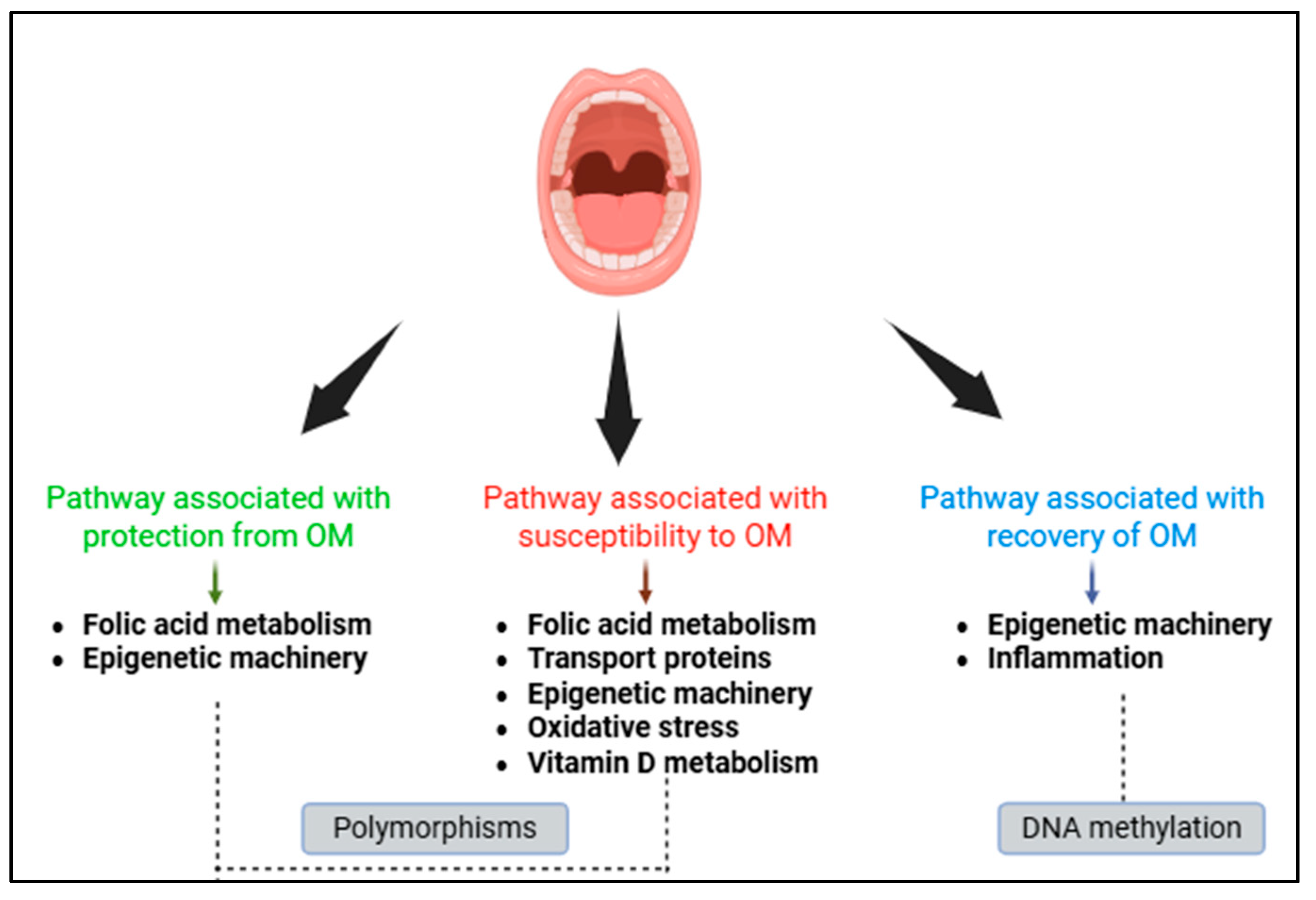
5. Biological Pathways Associated with Oral Mucositis
5.1. Pathways Associated with Protection from Oral Mucositis
5.2. Pathways Associated with Susceptibility to Oral Mucositis
5.3. Pathways Associated with Recovery of the Oral Mucosa
6. Final Considerations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Curra, M.; Gabriel, A.F.; Ferreira, M.B.C.; Martins, M.A.T.; Brunetto, A.T.; Gregianin, L.J.; Martins, M.D. Incidence and risk factors for oral mucositis in pediatric patients receiving chemotherapy. Support. Care Cancer 2021, 29, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Bachour, P.C.; Sonis, S.T. Predicting mucositis risk associated with cytotoxic cancer treatment regimens: Rationale, complexity, and challenges. Curr. Opin. Support. Palliat. Care 2018, 12, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Cheng, K.K.; Chang, A.M.; Yuen, M.P. Prevention of oral mucositis in paediatric patients treated with chemotherapy; a randomized crossover trial comparing two protocols of oral care. Eur. J. Cancer 2004, 40, 1208–1216. [Google Scholar] [CrossRef]
- World Health Organization. Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979; pp. 15–22. [Google Scholar]
- National Cancer Institute; National Institute of Health. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 5 November 2024).
- Korf, B.R. Basic genetics. Prim. Care 2004, 31, 461–478. [Google Scholar] [CrossRef]
- Jenkins, W.J. The Distribution of the Human Blood Groups and other Polymorphisms. J. Clin. Pathol. 1977, 30, 392–393. [Google Scholar] [CrossRef]
- Lappalainen, T.; MacArthur, D.G. From variant to function in human disease genetics. Science 2021, 373, 1464–1468. [Google Scholar] [CrossRef]
- Chakravorty, S.; Hegde, M. Inferring the effect of genomic variation in the new era of genomics. Hum. Mutat. 2018, 39, 756–773. [Google Scholar] [CrossRef]
- Peixoto, P.; Cartron, P.F.; Serandour, A.A.; Hervouet, E. From 1957 to Nowadays: A Brief History of Epigenetics. Int. J. Mol. Sci. 2010, 21, 7571. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzila, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.A.; El-Bostany, E.A.; Adly, A.A.; Abou El Asrar, M.; El-Ghouroury, E.A.; Abdulghaffar, E.E. Methylene tetrahydrofolate reductase gene polymorphism in Egyptian children with acute lymphoblastic leukemia. Blood Coagul. Fibrinolysis 2010, 21, 28–34. [Google Scholar] [CrossRef]
- Liu, S.G.; Li, Z.G.; Cui, L.; Gao, C.; Li, W.J.; Zhao, X.X. Effects of methylenetetrahydrofolate reductase gene polymorphisms on toxicities during consolidation therapy in pediatric acute lymphoblastic leukemia in a Chinese population. Leuk. Lymphoma 2011, 52, 1030–1040. [Google Scholar] [CrossRef]
- Faganel Kotnik, B.; Grabnar, I.; Bohanec Grabar, P.; Dolžan, V.; Jazbec, J. Association of genetic polymorphism in the folate met-abolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur. J. Clin. Pharmacol. 2011, 67, 993–1006. [Google Scholar] [CrossRef]
- Bektas-Kayhan, K.; Küçükhüseyin, Ö.; Karagöz, G.; Ünür, M.; Öztürk, O.; Ünüvar, A.; Devecioglu, O.; Yilmaz-Aydogan, H. Is the MDR1 C3435T polymorphism responsible for oral mucositis in children with acute lymphoblastic leukemia? Asian Pac. J. Cancer Prev. 2012, 13, 5251–5255. [Google Scholar] [CrossRef]
- Liu, S.G.; Gao, C.; Zhang, R.D.; Jiao, Y.; Cui, L.; Li, W.J.; Chen, Z.P.; Wu, M.Y.; Zheng, H.Y.; Zhao, X.X.; et al. FPGS rs1544105 polymorphism is associated with treatment outcome in pediatric B-cell precursor acute lymphoblastic leukemia. Cancer Cell Int. 2013, 13, 107. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Y.; Sheng, Q.; Lu, X.; Wang, F.; Lin, Z.; Tian, H.; Xu, A.; Zhang, J. Association of ABCC2 -24C>T polymorphism with high-dose methotrexate plasma concentrations and toxicities in childhood acute lymphoblastic leukemia. PLoS ONE 2014, 9, e82681. [Google Scholar] [CrossRef]
- López-López, E.; Gutiérrez-Camino, Á.; Piñán, M.Á.; Sánchez-Toledo, J.; Uriz, J.J.; Ballesteros, J.; García-Miguel, P.; Navajas, A.; García-Orad, Á. Pharmacogenetics of microRNAs and microRNAs biogenesis machinery in pediatric acute lymphoblastic leu-kemia. PLoS ONE 2014, 9, e91261. [Google Scholar] [CrossRef] [PubMed]
- Zaruma-Torres, F.; Lares-Asseff, I.; Reyes-Espinoza, A.; Loera-Castañeda, V.; Chairez-Hernández, I.; Sosa-Macías, M.; Galaviz-Hernández, C.; Almanza-Reyes, H. Association of ABCB1, ABCC5 and xanthine oxidase genetic polymorphisms with methotrexate adverse reactions in Mexican pediatric patients with ALL. Drug Metab. Pers. Ther. 2015, 3, 195–201. [Google Scholar] [CrossRef]
- Ramírez-Pacheco, A.; Moreno-Guerrero, S.; Alamillo, I.; Medina-Sanson, A.; Lopez, B.; Moreno-Galván, M. Mexican Childhood Acute Lymphoblastic Leukemia: A Pilot Study of the MDR1 and MTHFR Gene Polymorphisms and Their Associations with Clinical Outcomes. Genet. Test. Mol. Biomark. 2016, 10, 597–602. [Google Scholar] [CrossRef]
- Liu, S.G.; Gao, C.; Zhang, R.D.; Zhao, X.X.; Cui, L.; Li, W.J.; Chen, Z.P.; Yue, Z.X.; Zhang, Y.Y.; Wu, M.Y.; et al. Polymorphisms in methotrexate transporters and their relationship to plasma methotrexate levels, toxicity of high-dose methotrexate, and outcome of pediatric acute lymphoblastic leukemia. Oncotarget 2017, 23, 37761–37772. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Camino, A.; Oosterom, N.; den Hoed, M.A.H.; Lopez-Lopez, E.; Martin-Guerrero, I.; Pluijm, S.M.F.; Pieters, R.; De Jonge, R.; Tissing, W.J.; Heil, S.G.; et al. The miR-1206 microRNA variant is associated with methotrexate-induced oral mucositis in pediatric acute lymphoblastic leukemia. Pharmacogenet. Genom. 2017, 27, 303–306. [Google Scholar] [CrossRef]
- Oosterom, N.; Berrevoets, M.; den Hoed, M.A.H.; Zolk, O.; Hoerning, S.; Pluijm, S.M.F.; Pieters, R.; de Jonge, R.; Tissing, W.J.; van den Heuvel-Eibrink, M.M.; et al. The role of genetic polymorphisms in the thymidylate synthase (TYMS) gene in methotrexate-induced oral mucositis in children with acute lymphoblastic leukemia. Pharmacogenet. Genom. 2018, 10, 223–229. [Google Scholar] [CrossRef]
- Gutierrez-Camino, Á.; Umerez, M.; Lopez-Lopez, E.; Santos-Zorrozua, B.; Martin-Guerrero, I.; de Andoin, N.G.; Ana, S.; Navajas, A.; Astigarraga, I.; Garcia-Orad, A.; et al. Involvement of miRNA polymorphism in mucositis development in childhood acute lymphoblastic leukemia treatment. Pharmacogenomics 2018, 19, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Kotur, N.; Lazic, J.; Ristivojevic, B.; Stankovic, B.; Gasic, V.; Dokmanovic, L.; Krstovski, N.; Milosevic, G.; Janic, D.; Zukic, B.; et al. Pharmacogenomic Markers of Methotrexate Response in the Consolidation Phase of Pediatric Acute Lymphoblastic Leukemia Treatment. Genes 2020, 1, 468. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, M.H.; Zhuang, Q.; Lin, B.J.; Chen, Y.Y.; Yang, L.; Liu, M.B.; Que, W.C.; Qiu, H.Q. Genetic factors involved in delayed methotrexate elimination in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2021, 68, e28858. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, L.; Wang, L.; Chen, J.; Chen, F.; Ma, Y.; Xu, Z.; Sun, Y.; Luo, L.; Shi, C.; et al. Association of MTHFR and ABCB1 polymorphisms with MTX-induced mucositis in Chinese paediatric patients with acute lymphoblastic leukaemia, lymphoma or osteosarcoma-A retrospective cohort study. J. Clin. Pharm. Ther. 2021, 46, 1557–1563. [Google Scholar] [CrossRef]
- Huang, S.; Jin, L.; Yang, J.; Duan, L.Y.; Zhang, M.; Zhou, J.C.; Zhang, H.Y. Study on Relationships of Tumor Status and Gene Polymorphism With Blood Concentration of MTX and Toxicities in 63 Pediatric Mature B Cell Lymphoma in Chinese Population. Technol. Cancer Res. Treat. 2021, 20, 1533033821995288. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhu, X.; Li, W.; Chen, H.; Zhou, D.; Zhen, Z.; Sun, F.; Huang, J.; Zhu, J.; Wang, J.; et al. Influence of Methylenetetrahy-drofolate Reductase C677T and A1298C Polymorphism on High-Dose Methotrexate-Related Toxicities in Pediatric Non-Hodgkin Lymphoma Patients. Front. Oncol. 2021, 11, 598226. [Google Scholar]
- Viana Filho, J.M.C.; Coêlho, M.C.; Ribeiro, I.L.A.; Persuhn, D.C.; Valença, A.M.G.; Oliveira, N.F.P. ABCG2 polymorphism, age and leukocyte count may contribute to oral mucositis in oncopediatric patients. Braz. Dent. J. 2021, 32, 14–26. [Google Scholar] [CrossRef]
- Coêlho, M.C.; Viana Filho, J.M.C.; Souza, B.F.; Valença, A.M.G.; Persuhn, D.C.; Oliveira, N.F.P. Genetic polymorphisms of genes involved in oxidative stress and inflammatory management in oncopediatric patients with chemo-induced oral mucositis. J. Appl. Oral Sci. 2022, 30, e20210490. [Google Scholar] [CrossRef]
- Viana Filho, J.M.C.; de Souza, B.F.; Coêlho, M.C.; Valença, A.M.G.; Persuhn, D.C.; de Oliveira, N.F.P. Polymorphism but not methylation status in the vitamin D receptor gene contributes to oral mucositis in children. Oral Dis. 2023, 29, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- de Souza, B.F.; Viana Filho, J.M.C.; de Queiroz Neto, J.N.; Coêlho, M.C.; Valença, A.M.G.; Persuhn, D.C.; de Oliveira, N.F.P. DNA Methyltransferase Genes Are Associated with Oral Mucositis and Creatinine Levels in Oncopediatric Patients. Genes 2023, 14, 1136. [Google Scholar] [CrossRef]
- Zarou, M.M.; Vazquez, A.; Vignir Helgason, G. Folate metabolism: A re-emerging therapeutic target in haematological cancers. Leukemia 2021, 35, 1539–1551. [Google Scholar] [CrossRef]
- Cao, M.; Guo, M.; Wu, D.Q.; Meng, L. Pharmacogenomics of Methotrexate: Current Status and Future Outlook. Curr. Drug Metabolism 2018, 19, 1182–1187. [Google Scholar] [CrossRef]
- Taylor, Z.L.; Vang, J.; Lopez-Lopez, E.; Oosterom, N.; Mikkelsen, T.; Ramsey, L.B. Systematic Review of Pharmacogenetic Factors That Influence High-Dose Methotrexate Pharmacokinetics in Pediatric Malignancies. Cancers 2021, 13, 2837. [Google Scholar] [CrossRef]
- Chinn, L.W.; Kroetz, D.L. ABCB1 pharmacogenetics: Progress, pitfalls, and promise. Clin. Pharmacol. Ther. 2007, 81, 265–269. [Google Scholar] [CrossRef]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Oosterom, N.; Griffioen, P.H.; den Hoed, M.A.H.; Pieters, R.; de Jonge, R.; Tissing WJEet, a.l. Global methylation in relation to methotrexate-induced oral mucositis in children with acute lymphoblastic leukemia. PLoS ONE 2018, 13, e0199574. [Google Scholar] [CrossRef]
- Viana Filho, J.M.C.; Castro Coêlho, M.; Queiroz Neto, J.N.; Souza, B.F.; Valença, A.M.G.; Oliveira, N.F.P. TNF-? promoter hypomethylation is frequent in oncopediatric patients who recovered from mucositis. Braz. Oral Res. 2024, 38, e042. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; de Souza, B.F.; Filho, J.M.C.V.; Damascena, L.C.L.; Valença, A.M.G.; Persuhn, D.C.; de Oliveira, N.F.P. Epigenetic mechanisms and oral mucositis in children with acute lymphoblastic leukaemia. Eur. J. Oral Sci. 2024, 132, e13009. [Google Scholar] [CrossRef]
- Iglesias-Bartolome, R.; Uchiyama, A.; Molinolo, A.A.; Abusleme, L.; Brooks, S.R.; Callejas-Valera, J.L.; Edwards, D.; Doci, C.; Asselin-Labat, M.L.; Onaitis, M.W.; et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 2018, 10, eaap8798. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, Q.; Tang, R.; Li, W.; Wang, J.; Yang, F.; Zhao, J.; Zhu, J.; Pang, W.; Li, N.; et al. DNA methyltransferase 1 deficiency improves macrophage motility and wound healing by ameliorating cholesterol accumulation. NPJ Regen. Med. 2023, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Miramini, S.; Patel, M.; Richardson, M.; Ebeling, P.; Zhang, L. Role of TNF-alpha in early-stage fracture healing under normal and diabetic conditions. Comput. Methods Programs Biomed. 2022, 213, 106536. [Google Scholar] [CrossRef]
- Giletti, A.; Esperon, P. Genetic markers in methotrexate treatments. Pharmacogenom. J. 2018, 18, 689–703. [Google Scholar] [CrossRef]
- Kang, D.W.; Choi, K.-Y.; Min, D.S. Functional regulation of phospholipase D expression in cancer and inflammation. J. Biol. Chem. 2014, 289, 22575–22582. [Google Scholar] [CrossRef]
- Gomez-Cambronero, J.; Fite, K.; Miller, T.E. How miRs and mRNA deadenylases could post-transcriptionally regulate expression of tumor-promoting protein PLD. Adv. Biol. Regul. 2018, 68, 107–119. [Google Scholar] [CrossRef]
- Domi, E.; Hoxha, M.; Hoxha, B.; Zappacosta, B. The Interaction between Arachidonic Acid Metabolism and Homocysteine. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kodydková, J.; Vávrová, L.; Kocík, M.; Žák, A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 2014, 60, 153–167. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza- Meza, M.R.; Vizmanos-Lamotte, B.; ParraRojas, I.; Cruz-Mosso, U. Association of vitamin D metabolism gene polymorphisms with autoimmunity: Evidence in population genetic studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef]
- Ti, D.; Li, M.; Fu, X.; Han, W. Causes and consequences of epigenetic regulation in wound healing. Wound Repair Regen. 2014, 22, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhan, M.; Li, Q.; Li, D.; Xu, Q. DNA methyltransferase DNMT1 inhibits lipopolysaccharide induced inflammatory response in human dental pulp cells involving the methylation changes of IL 6 and TRAF6. Mol. Med. Rep. 2020, 21, 959–968. [Google Scholar] [CrossRef]
- Freitas, M.O.; Fonseca, A.P.; Aguiar, M.T.; Dias, C.C.; Avelar, R.L.; Sousa, F.B.; Alves, A.P.N.N.; de Barros Silva, P.G. Tumor necrosis factor alpha (TNF-α) blockage reduces acute inflammation and delayed wound healing in oral ulcer of rats. Inflammopharmacology 2022, 30, 1781–1798. [Google Scholar] [CrossRef]
- Suraweera, A.; O’Byrne, K.J.; Richard, D.J. Epigenetic drugs in cancer therapy. Cancer Metastasis Rev. 2025, 44, 37. [Google Scholar] [CrossRef]
| Scale | Gradation | Characteristics |
|---|---|---|
| Grade 1 | Normality of voice, swallowing ability, texture of lips, tongue, jugal mucosa, palate, lip mucosa, gums and saliva consistency | |
| OAG (modified) | Grade 2 | Hoarse voice, pain on swallowing, dry/cracked lips, apparently shiny tongue with or without papillae and redness, buccal mucosa, palate and labial mucosa reddish/white but without ulceration, swollen gums with or without redness and thick saliva consistency/viscous |
| Grade 3 | Difficulty speaking/pain, inability to swallow, swollen/bleeding lips, blistered/cracked tongue, buccal mucosa, palate and labial mucosa with ulcers with or without bleeding, bleeding gums with or without stimulation and lack of saliva | |
| Grade 0 | Without changes | |
| Grade 1 | Erythema, irritations, pain | |
| WHO | Grade 2 | Erythema, ulcers (solid diet) |
| Grade 3 | Ulcers (liquid diet) | |
| Grade 4 | Inability to feed | |
| Grade 0 | Without changes | |
| Grade 1 | No symptoms or mild symptoms, intervention not indicated | |
| NCI- CTCAE | Grade 2 | Moderate pain or ulcers that do not interfere with swallowing, diet modification indicated |
| Grade 3 | Severe pain interfering with swallowing | |
| Grade 4 | Life-threatening consequences, urgent intervention indicated | |
| Grade 5 | Death |
| Country Year [Reference] | Underlying Disease/ Diagnosis of OM | Sample | Chemotherapy Regimen (Includes MTX?) | Gene-Polymorphism | Technique/ Tissue | Outcome and Conclusion |
|---|---|---|---|---|---|---|
| Egypt 2010 [17] | Leukaemia/ No scale informed | 40 | Yes, with HD | MTHFR rs1801131, rs1801133 | PCR-RFLP/Blood | MTHFR rs1801133 TT genotype was associated with susceptibility to OM |
| China 2011 [18] | Leukaemia/ CI-CTCAE | 181 | Yes | MTHFR rs1801131, rs1801133 | PCR-RFLP/Blood | No association with OM |
| Slovenia 2011 [19] | Leukaemia or lymphoma/ WHO | 64 | Yes, with HD | SLC19A1 rs1051266, MTHFR rs1801133, rs1801131, MS rs1805087, MTRR rs1801394, TYMS rs34743033, ABCB1 rs2032582, rs1045642 | PCR-RFLP, AS-PCR or TaqMan/Blood | MTHFR rs1801133 TT genotype was associated with susceptibility to OM; TYMS rs34743033 3R3R genotype was a protective factor for OM |
| Turquia 2012 [20] | Leukaemia/ WHO | 115 | Chemotherapy regimen not mentioned | ABCB1 rs1045642 | PCR-RFLP/Blood | ABCB1 rs1045642 CT genotype was associated with susceptibility to OM |
| China 2013 [21] | Leukaemia/ NCI-CTCAE | 164 | Yes | FPGS rs1544105 | PCR-RFLP/Blood | No association with OM |
| China 2014 [22] | Leukaemia/ NCI-CTCAE | 112 | Yes, with HD | ABCC2 rs717620, rs3740065, ABCC4 rs9516519, rs868853, rs2274407 ABCG2 rs2231137 | Microarray/ Blood | ABCC2 rs717620 T allele was associated with susceptibility to OM |
| Spain 2014 [23] | Leukaemia/ WHO adapted | 152 | Yes, with HD | 118 SNPs in 21 genes involved in the biogenesis and processing of miRNA | PCR-TaqMan/Blood | No association with OM |
| Mexico 2015 [24] | Leukaemia/ WHO and NCI-CTCAE | 35 | Yes | XO rs1701368, rs17323235 ABCB1 rs1128503 ABCC5 rs9838667, rs3792585 | PCR-TaqMan/ tissue not mentioned | No association with OM |
| Mexico 2016 [25] | Leukaemia/ No scale informed | 109 | Yes, with HD | ABCB1 rs1045642 MTHFR rs1801133 | PCR-RFLP/Blood | MTHFR rs1801133 CC genotype was associated with susceptibility to OM |
| China 2017 [26] | Leukaemia/ NCI-CTCAE | 322 | Yes, with HD | 12 SNPs in 4 genes: SLCO1B1, SLC19A1, ABCB1, ABCG2 | PCR- Mass spectrometry/bone marrow | No association with OM |
| Dutch 2017 [27] | Leukaemia/ NCI-CTCAE | 117 | Yes | CNOT4 rs3812265, miR-1206 rs2114358, miR-2053 rs10505168 | AS-PCR and Taqman/Blood | miR-1206 rs2114358 GG genotype was associated with susceptibility to OM |
| Dutch 2018 [28] | Leukaemia/ NCI-CTCAE | 117 | Yes, with HD | TYMS rs34743033, rs2853542, rs151264360 | PCR-RFLP/Blood | No association with OM |
| Spain 2018 [29] | Leukaemia/ WHO adapted | 179 | Yes | 213 SNPs in 206 genes of miRNAs | Microarray/ Blood or bone marrow | miR-3683 rs6977967 GG genotype was associated with susceptibility to OM; miR-4268 rs4674470 AG/GG genotypes were protective factors for OM |
| Serbia 2020 [30] | Leukaemia/ NCI-CTCAE | 148 | Yes, with HD | 11 SNPs in 5 genes: TYMS, MTHFR, DHFR, SLC19A1, SLCO1B | ASO-PCR and sequencing/ tissue not mentioned | No association with OM |
| China 2021 [31] | Leukaemia/ NCI-CTCAE | 647 | Yes, with HD | 28 SNPs in 14 genes: ABCG2, ABCB1, ABCC1, ABCC2, ABCC4, SLCO1B, SLCO1A2, SLC19A1, MTHFR, ATIC, DHFR, GGH, FPGS, CCND1 | PCR and sequencing/Blood | SLCO1B1 rs2306283 AG/GG genotypes were associated with susceptibility to OM |
| China 2021 [32] | Leukaemia or lymphoma/ NCI-CTCAE | 80 | Yes, with HD | 23 SNPs in 15 genes: SLC28A2, SLCO1B1, ABCB1, ABCC2, ABCC4, ABCG2, FPGS, GGH, MTHFR, MTHFD1, MTR, MTRR, GSTP1, ATIC, CCND1 | PCR- Mass spectrometry/Blood | ABCB1 rs1128503 T, rs1045642 T, MTHFR rs1801133 T alleles were associated with susceptibility to OM |
| China 2021 [33] | Lymphoma/ NCI-CTCAE | 63 | Yes, with HD | MTHFR rs1801133, SLCO1B1 rs11045879, rs4149056 | PCR-HRM and TaqMan/ tissue not mentioned | No association with OM |
| China 2021 [34] | Lymphoma/ NCI-CTCAE | 93 | Yes, with HD | MTHFR rs1801133, rs1801131 | PCR and sequencing/ tissue not mentioned | MTHFR rs1801133 CT/TT genotypes were associated with susceptibility to OM |
| Brazil 2021 [35] | Leukaemia/ OAG | 64 | Yes | MTHFR rs1801133, DNMT3B rs2424913, ABCC2 rs717620, ABCG2 rs2231137, rs2231142 | PCR-RFLP/ Oral mucosa | ABCG2 rs2231142 CA genotype were associated with susceptibility to OM |
| Brazil 2022 [36] | Leukaemia or lymphoma/ OAG | 95 | Yes | SOD2 rs4880, CAT rs7943316, TNF-α rs1800629, IL6 rs1800795 | PCR-RFLP/ Oral mucosa | CAT rs7943316 AA genotype was associated with susceptibility to OM |
| Brazil 2023 [37] | Leukaemia or lymphoma/ OAG | 102 | Yes | VDR rs1544410, rs2228570, rs731236 | PCR-RFLP/ Oral mucosa | VDR rs1544410 G allele and rs2228570 CT genotype were associated with susceptibility to OM |
| Brazil 2023 [38] | Leukaemia or lymphoma/ OAG | 102 | Yes | DNMT1 rs2228611, DNMT3A rs7590760, DNMT3B rs6087990 | PCR-RFLP/ Oral mucosa | No association with OM |
| Country Year [Reference] | Underlying Disease/ Diagnosis of OM | Sample | Chemotherapy Regimen (Includes MTX?) | Methylation | Technique/Tissue | Outcome and Conclusion |
|---|---|---|---|---|---|---|
| The Netherlands 2018 [44] | Leukaemia/ NCI-CTCAE | 82 | Yes, with HD | Global (LINE-1) | PCR- Mass spectrometry/ Blood | No association with OM |
| Brazil 2023 [37] | Leukaemia or lymphoma/ OAG | 81 | Yes | Site-specific in VDR gene | MSP/ Oral mucosa | No association with OM |
| Brazil 2023 [38] | Leukaemia or lymphoma/ OAG | 85 | Yes | Site-specific in DNMT1, DNMT3A, DNMT3B genes | MSP/ Oral mucosa | DNMT1 methylation was associated with mucosal recovery |
| Brazil 2024 [45] | Leukaemia or lymphoma/ OAG | 85 | Yes | Site-specific in CAT, SOD3, IL-6, TNF-α genes | MSP/ Oral mucosa | TNF-α hypomethylation was associated with mucosal recovery |
| Brazil 2024 [46] | Leukaemia/ OAG | 76 | Yes | Global, site-specific in miR-9-1, miR-9-3 genes | ELISA, MSP/ Oral mucosa | Lower global methylation levels were associated with mucosal recovery |
| Pathway | Targets | Country |
|---|---|---|
| Folic acid metabolism | MTHFR 2, MTRR, TYMS 1, MTHFD1, MTR, MS, FPGS, GGH, DHFR | Egypt, China, Slovenia, Mexico, Dutch, Serbia, Brazil |
| Transport proteins | ABCB1 2, ABCC1, ABCC2 2, ABCC4, ABCC5, ABCG2 2, SLC19A1, SLC28A2, SLCO1A2, SLCO1B 2 | Slovenia, Turquia, China, Mexico, Serbia, Brazil |
| Epigenetic machinery | miR 1,2, global methylation 3, DNMT1 3, DNMT3A, DNMT3B | Spain, Dutch, Netherlands, Brazil |
| Other pathways (purine metabolism, cell cycle, transcriptional regulator, detoxification, inflammation, oxidative stress, vitamin D metabolism) | XO, ATIC, CCND1, CNOT4, GSTP1, IL6, TNF-α 3, VDR 2, SOD2, SOD3, CAT 2 | Mexico, Dutch, China, Brazil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, J.R.; Viana Filho, J.M.C.; de Oliveira, N.F.P. Genetics and Epigenetics of Chemoinduced Oral Mucositis in Paediatric Patients with Haematological Malignancies—A Review. Epigenomes 2025, 9, 16. https://doi.org/10.3390/epigenomes9020016
Guimarães JR, Viana Filho JMC, de Oliveira NFP. Genetics and Epigenetics of Chemoinduced Oral Mucositis in Paediatric Patients with Haematological Malignancies—A Review. Epigenomes. 2025; 9(2):16. https://doi.org/10.3390/epigenomes9020016
Chicago/Turabian StyleGuimarães, Juliana Ramalho, José Maria Chagas Viana Filho, and Naila Francis Paulo de Oliveira. 2025. "Genetics and Epigenetics of Chemoinduced Oral Mucositis in Paediatric Patients with Haematological Malignancies—A Review" Epigenomes 9, no. 2: 16. https://doi.org/10.3390/epigenomes9020016
APA StyleGuimarães, J. R., Viana Filho, J. M. C., & de Oliveira, N. F. P. (2025). Genetics and Epigenetics of Chemoinduced Oral Mucositis in Paediatric Patients with Haematological Malignancies—A Review. Epigenomes, 9(2), 16. https://doi.org/10.3390/epigenomes9020016







