TNFR1 Absence Is Not Crucial for Different Types of Cell Reaction to TNF: A Study of the TNFR1-Knockout Cell Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Cell Lines
2.2. Plasmid Assembly for TNFRSF1A Gene Knockout
2.3. Transgenic Cell Line Production
2.4. Flow Cytometry: Evaluation of TNF Receptor Expression and Co-Expression
2.5. Flow Cytofluorimetry: Assessment of the Functional Activity of Cells
2.6. Evaluation of the Profile of Expressed Genes in Cells upon Activation of Specific TNF Receptors
2.7. Statistical Analysis
3. Results
3.1. TNFRSF1A Gene Deletion Characterization in Cells after Subcloning
3.2. Verification of Knockout Efficacy and Changes in TNFR2 Expression Levels in Knockout Lines
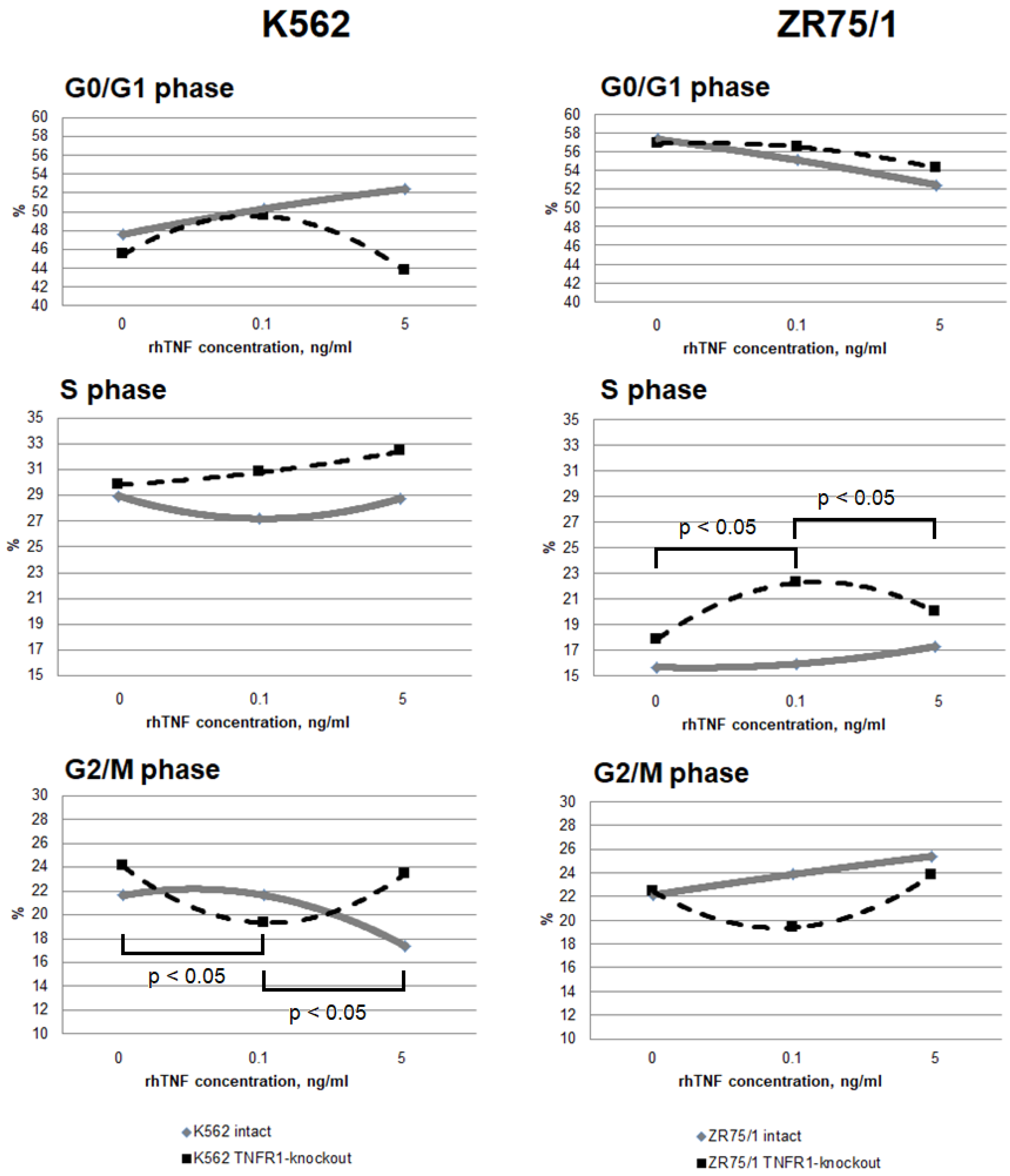
3.3. Study of the Dose-Dependent Effect of Cytokine on the Functional Response of Cells Depending on Receptor Co-Expression
3.4. Evaluation of the Profile of Expressed Genes in Cells upon Activation of Specific TNF Receptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salomon, B.L. Insights into the biology and therapeutic implications of TNF and regulatory T cells. Nat. Rev. Rheumatol. 2021, 17, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Mac-rophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF-TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Fischer, R.; Marsal, J.; Guttà, C.; Eisler, S.A.; Peters, N.; Bethea, J.R.; Pfizenmaier, K.; Kontermann, R.E. Novel strategies to mimic transmembrane tumor necrosis factor-dependent activation of tumor necrosis fac-tor receptor 2. Sci. Rep. 2017, 7, 6607. [Google Scholar] [CrossRef]
- Sennikov, S.V.; Alshevskaya, A.A.; Zhukova, J.; Belomestnova, I.; Karaulov, A.V.; Lopatnikova, J.A. Expres-sion Density of Receptors as a Potent Regulator of Cell Function and Property in Health and Pathol-ogy. Int. Arch. Allergy Immunol. 2019, 178, 182–191. [Google Scholar] [CrossRef]
- Sander, C.A.; Rush, E.A.; Shi, J.; Arantes, L.M.R.B.; Tesi, R.J.; Ross, M.A.; Calderon, M.J.; Watkins, S.C.; Kirk-wood, J.M.; Ferris, R.L.; et al. Co-expression of TNF receptors 1 and 2 on mela-nomas facilitates soluble TNF-induced resistance to MAPK pathway inhibitors. J. Transl. Med. 2022, 20, 331. [Google Scholar] [CrossRef]
- Leone, G.M.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Fagone, P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. J. Clin. Med. 2023, 12, 1630. [Google Scholar] [CrossRef]
- Medler, J.; Kucka, K.; Wajant, H. Tumor Necrosis Factor Receptor 2 (TNFR2): An Emerging Target in Cancer Therapy. Cancers 2022, 14, 2603. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective Targeting of TNF Receptors as a Novel Thera-peutic Approach. Front. Cell Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Fotin-Mleczek, M.; Henkler, F.; Samel, D.; Reichwein, M.; Hausser, A.; Parmryd, I.; Scheurich, P.; Schmid, J.A.; Wajant, H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J. Cell Sci. 2002, 115 Pt 13, 2757–2770. [Google Scholar] [CrossRef]
- Alshevskaya, A.; Koneva, O.; Belomestnova, I.; Lopatnikova, J.; Evsegneeva, I.; Zhukova, J.; Kireev, F.; Karaulov, A.; Sennikov, S. Ligand-Regulated Expression of TNF Receptors 1 and 2 Determines Re-ceptor-Mediated Functional Responses. Int. Arch. Allergy Immunol. 2021, 182, 1077–1088. [Google Scholar] [CrossRef]
- Dolskiy, A.A.; Bodnev, S.A.; Nazarenko, A.A.; Smirnova, A.M.; Pyankova, O.G.; Matveeva, A.K.; Grishchen-ko, I.V.; Tregubchak, T.V.; Pyankov, O.V.; Ryzhikov, A.B.; et al. Deletion of BST2 Cytoplasmic and Transmembrane N-Terminal Domains Results in SARS-CoV, SARS-CoV-2, and Influenza Virus Production Suppression in a Vero Cell Line. Front. Mol. Biosci. 2020, 7, 616798. [Google Scholar] [CrossRef]
- Pietkiewicz, S.; Schmidt, J.H.; Lavrik, I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods 2015, 423, 99–103. [Google Scholar] [CrossRef]
- Alshevskaya, A.A.; Belomestnova, I.; Lopatnikova, J.A.; Zhukova, J.; Evsegneeva, I.; Koneva, O.; Karaulov, A.V.; Sennikov, S.V. Co-Expression of Membrane-Bound Tumor Necrosis Factor-Alpha Receptor Types 1 and 2 by Tumor Cell Lines. Int. Arch. Allergy Immunol. 2020, 181, 249–256. [Google Scholar] [CrossRef]
- Van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2022, 15, 289–303. [Google Scholar] [CrossRef]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11, 585880. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Dostert, C.; Grusdat, M.; Letellier, E.; Brenner, D. The TNF Family of Ligands and Receptors: Commu-nication Modules in the Immune System and Beyond. Physiol. Rev. 2019, 99, 115–160. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Lazo, P.S. Signal transduction by tumor necrosis factor receptors. Cell. Signal. 2012, 24, 1297–1305. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.M.; Zhang, G.R.; Cheng, Y.; Chen, X.; Gu, W.; Guo, X.J. TNF-TNFR2 Signaling Inhibits Th2 and Th17 Polarization and Alleviates Allergic Airway Inflammation. Int. Arch. Allergy Immunol. 2019, 178, 281–290. [Google Scholar] [CrossRef]
- Kawano, S.; Mitoma, H.; Inokuchi, S.; Yamauchi, Y.; Yokoyama, K.; Nogami, J.; Semba, Y.; Ayano, M.; Kimoto, Y.; Akahoshi, M.; et al. TNFR2 Signaling En-hances Suppressive Abilities of Human Circulating T Follicular Regulatory Cells. J. Immunol. 2022, 208, 1057–1065. [Google Scholar] [CrossRef]
- Lo, C.H.; Huber, E.C.; Sachs, J.N. Conformational states of TNFR1 as a molecular switch for receptor function. Protein Sci. 2020, 29, 1401–1415. [Google Scholar] [CrossRef]
- Barbati, C.; Colasanti, T.; Vomero, M.; Ceccarelli, F.; Celia, A.I.; Perricone, C.; Spinelli, F.R.; Conti, F.; Valesini, G.; Alessandri, C. Up-regulation of autophagy by etanercept treatment results in TNF-induced apopto-sis reduction in EA.hy926 endothelial cell line. Clin. Exp. Rheumatol. 2021, 39, 606–611. [Google Scholar] [CrossRef]
- Gane, J.M.; Stockley, R.A.; Sapey, E. TNF-α Autocrine Feedback Loops in Human Monocytes: The Pro- and Anti-Inflammatory Roles of the TNF-α Receptors Support the Concept of Selective TNFR1 Blockade In Vivo. J. Immunol. Res. 2016, 2016, 1079851. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Artime, N.; Iglesias, J.; Prado, M.A.; Ugarte-Gil, L.; Casado, P.; Fernández-García, B.; Darnay, B.G.; Lazo, P.S. A TRAF2 binding independent region of TNFR2 is responsible for TRAF2 depletion and enhancement of cytotoxicity driven by TNFR1. Oncotarget 2014, 5, 224–236. [Google Scholar] [CrossRef]
- Droessler, L.; Cornelius, V.; Markov, A.G.; Amasheh, S. Tumor Necrosis Factor Alpha Effects on the Porcine Intestinal Epithelial Barrier Include Enhanced Expression of TNF Receptor 1. Int. J. Mol. Sci. 2021, 22, 8746. [Google Scholar] [CrossRef]
- You, K.; Gu, H.; Yuan, Z.; Xu, X. Tumor Necrosis Factor Alpha Signaling and Organogenesis. Front. Cell Dev. Biol. 2021, 9, 727075. [Google Scholar] [CrossRef]
- Cobson, E.C.J.; Jain, L.; Vickers, M.H.; Olins, A.L.; Olins, D.E.; Perry, J.K.; O’Sullivan, J.M. TNF-α Differen-tially Regulates Cell Cycle Genes in Promyelocytic and Granulocytic HL-60/S4 Cells. G3 Genes Genomes Genet. 2019, 9, 2775–2786. [Google Scholar] [CrossRef]
- Imbach, K.J.; Treadway, N.J.; Prahalad, V.; Kosters, A.; Arafat, D.; Duan, M.; Gergely, T.; Ponder, L.A.; Chandrakasan, S.; Ghosn, E.E.B.; et al. Profiling the peripheral immune response to ex vivo TNF stimulation in untreated juvenile idiopathic arthritis using single cell RNA sequencing. Pediatr. Rheumatol. Online J. 2023, 21, 17. [Google Scholar] [CrossRef]




| Name | Sequence 5′-3′ | Reference |
|---|---|---|
| TNFRSF1A_G3.1_S_BbsI | CACCGGTGGACTGGTCCCTCACCTAG | GenBank: AH003016.2 |
| TNFRSF1A_G3.1_AS_BbsI | AAACCTAGGTGAGGGACCAGTCCACC | GenBank: AH003016.2 |
| TNFRSF1A_G3.2_S_BsaI | TGCTGGTGGACAGTCATTGTACAAGT | GenBank: AH003016.2 |
| TNFRSF1A_G3.2_AS_BsaI | AAACACTTGTACAATGACTGTCCACC | GenBank: AH003016.2 |
| 14R | ATAAGGTCATGTACTGGGCACA | Addgene, #48138 |
| A-del_TNFRSF1A_3.2-F | CCACACACCACTCAAGACC | GenBank: AH003016.2 |
| A-del_TNFRSF1A_both_3.2+4.2-R | CCCTCCCTCTCTTGATGGTGTC | GenBank: AH003016.2 |
| Gene | Cytokine Signaling | Lymphocyte Activation | Host-Pathogen Interaction | Innate Immune System | Adaptive Immune System | Immunometabolism | NLR Signaling | Oxidative Stress | Chemokine Signaling | Complement System | Hemostasis | TLR Signaling | TNF Family Signaling | Apoptosis | Th17 Differentiation | Phagocytosis and Degradation | Cell Adhesion | Transcriptional Regulation | Autophagy | T Cell Receptor Signaling | Type II Interferon Signaling | Lymphocyte Trafficking | MHC Class I Antigen Presentation | MHC Class II Antigen Presentation | NF-kB Signaling | Type I Interferon Signaling | B Cell Receptor Signaling | Inflammasomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRKCD | + | + | − | + | − | − | + | + | + | − | + | − | − | + | − | − | − | − | + | − | + | − | − | − | − | − | − | − |
| TRAF6 | + | + | + | + | + | − | + | − | − | − | − | + | − | − | − | − | − | − | + | + | − | − | − | − | + | − | − | − |
| ITGAM | + | − | + | + | − | − | − | − | − | + | + | + | − | − | − | + | + | − | − | − | − | + | − | − | − | − | − | − |
| SOCS3 | + | − | + | − | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | − | + | − | − | + | − | − |
| APP | + | − | − | + | − | − | + | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| CD4 | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − |
| CXCL1 | + | − | + | + | − | − | + | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| NCF4 | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | + | − | − | − | − | − |
| CXCL10 | + | − | + | − | − | − | − | − | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ABL1 | − | + | + | + | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CD74 | − | + | + | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| CD80 | + | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − |
| IL1R1 | + | − | + | − | − | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − | − |
| IL18 | + | + | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| AHR | − | + | − | − | − | + | − | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − |
| ARG1 | − | − | + | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| FCGR2B | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | + | − |
| ICAM2 | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − |
| IL23A | + | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| BCL3 | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| CD55 | − | + | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CR1 | − | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PTPN22 | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| ADA | − | + | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ATG12 | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
| BID | − | + | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| C1QA | − | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| C1S | − | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CARD9 | − | − | + | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CIITA | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| LAG3 | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| TLR3 | − | − | + | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| C6 | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CCR10 | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CEACAM6 | − | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| FCGR2A/C | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| IFI35 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| IL1RAP | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ARG2 | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| BCL6 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CCL24 | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CCL26 | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CD70 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| EGR2 | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| GZMA | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IL18RAP | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IL1R2 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IL1RAP | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PRF1 | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| RAG1 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CD96 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CSF3R | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ICAM5 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IL16 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IL20 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| KLRC4 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| KLRF1 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| LTB4R | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| S1PR1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ABCB1 | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| AICDA | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| AIRE | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CD82 | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| HFE | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IL11RA | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| KLRG2 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MAP4K1 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| NFKBIZ | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| NT5E | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PIGR | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshevskaya, A.A.; Lopatnikova, J.A.; Zhukova, J.V.; Perik-Zavodskaia, O.Y.; Alrhmoun, S.; Obleukhova, I.A.; Matveeva, A.K.; Savenkova, D.A.; Imatdinov, I.R.; Yudkin, D.V.; et al. TNFR1 Absence Is Not Crucial for Different Types of Cell Reaction to TNF: A Study of the TNFR1-Knockout Cell Model. Epigenomes 2024, 8, 15. https://doi.org/10.3390/epigenomes8020015
Alshevskaya AA, Lopatnikova JA, Zhukova JV, Perik-Zavodskaia OY, Alrhmoun S, Obleukhova IA, Matveeva AK, Savenkova DA, Imatdinov IR, Yudkin DV, et al. TNFR1 Absence Is Not Crucial for Different Types of Cell Reaction to TNF: A Study of the TNFR1-Knockout Cell Model. Epigenomes. 2024; 8(2):15. https://doi.org/10.3390/epigenomes8020015
Chicago/Turabian StyleAlshevskaya, Alina A., Julia A. Lopatnikova, Julia V. Zhukova, Olga Y. Perik-Zavodskaia, Saleh Alrhmoun, Irina A. Obleukhova, Anna K. Matveeva, Darya A. Savenkova, Ilnaz R. Imatdinov, Dmitry V. Yudkin, and et al. 2024. "TNFR1 Absence Is Not Crucial for Different Types of Cell Reaction to TNF: A Study of the TNFR1-Knockout Cell Model" Epigenomes 8, no. 2: 15. https://doi.org/10.3390/epigenomes8020015
APA StyleAlshevskaya, A. A., Lopatnikova, J. A., Zhukova, J. V., Perik-Zavodskaia, O. Y., Alrhmoun, S., Obleukhova, I. A., Matveeva, A. K., Savenkova, D. A., Imatdinov, I. R., Yudkin, D. V., & Sennikov, S. V. (2024). TNFR1 Absence Is Not Crucial for Different Types of Cell Reaction to TNF: A Study of the TNFR1-Knockout Cell Model. Epigenomes, 8(2), 15. https://doi.org/10.3390/epigenomes8020015






