Effect of Different Light Spectrum in Helicoverpa armigera Larvae during HearNPV Induced Tree-Top Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Larvae and Virus
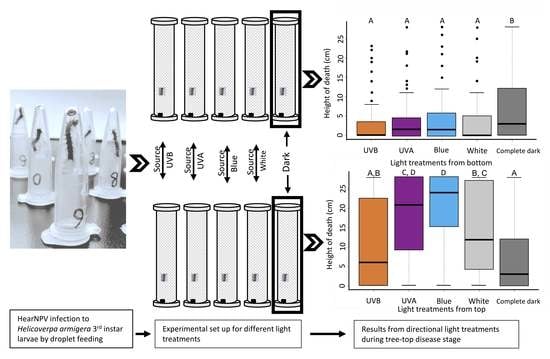
2.2. Behavioral Assay 1: Light from Above
2.3. Behavioral Assay 2: Light from Below
2.4. Statistical Analysis
3. Results
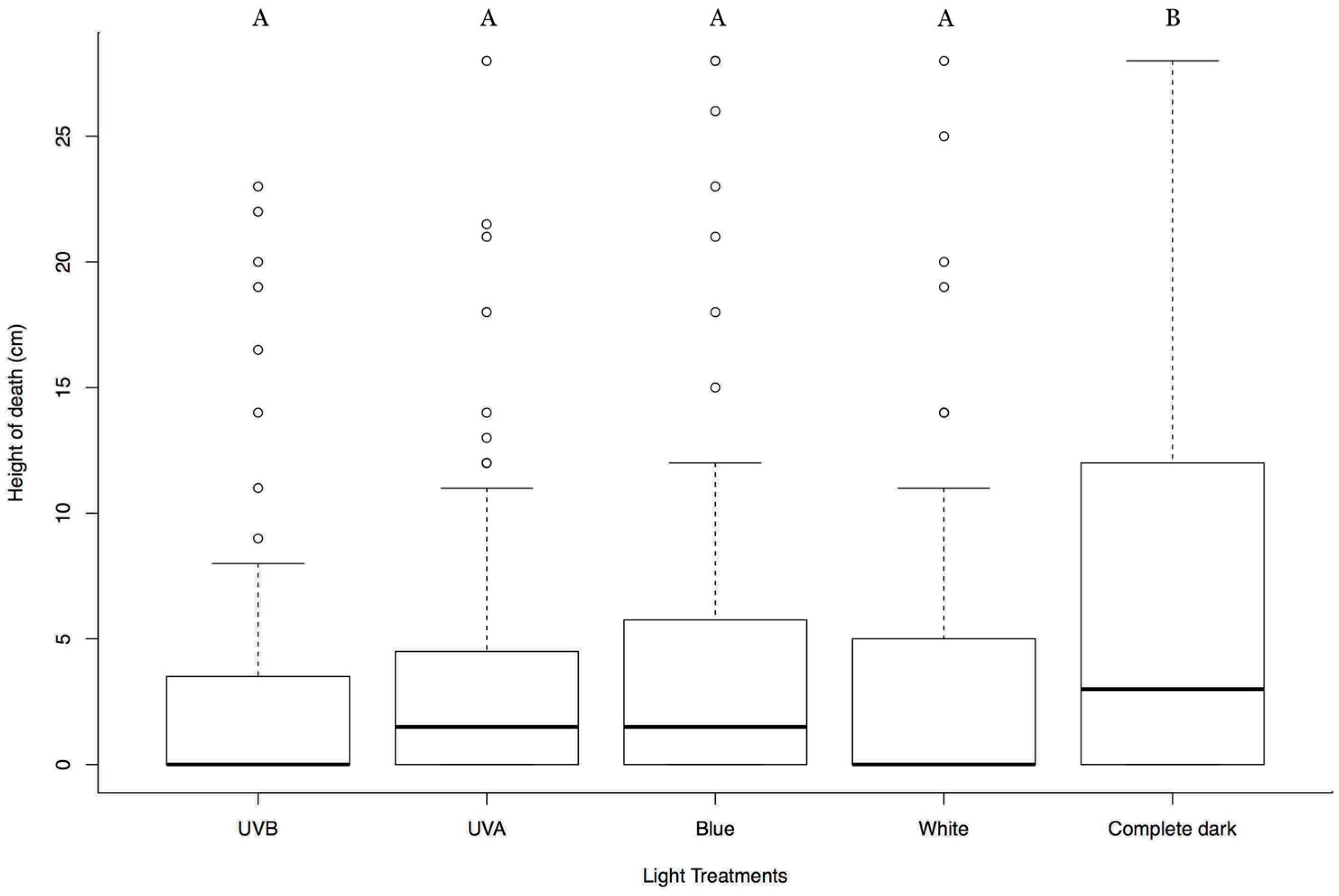
3.1. Different Lights from Above Affecting Tree-Top Disease
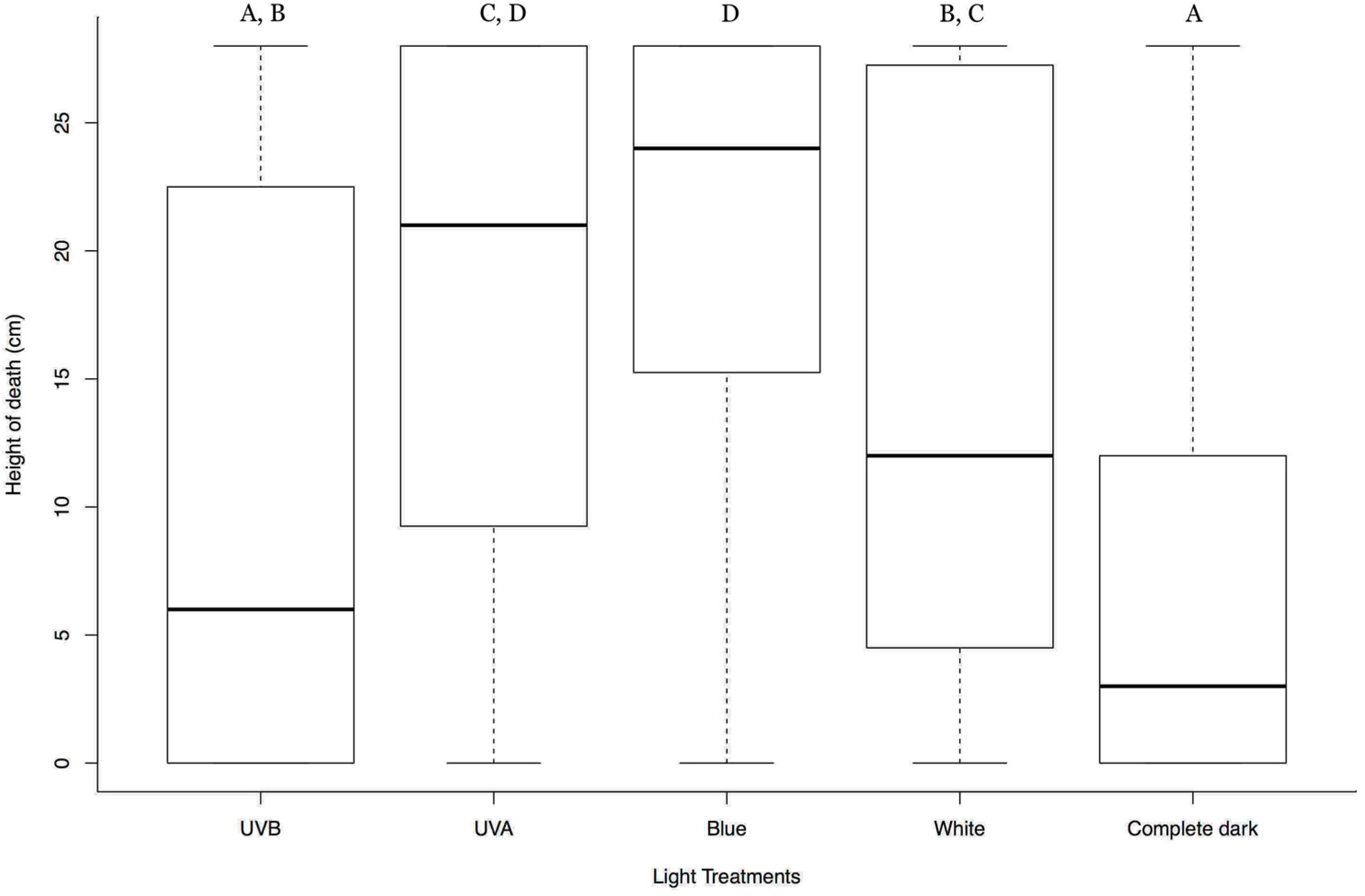
3.2. Effect of Lights from Below on Tree-Top Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulin, R. Adaptive changes in the behaviour of parasitized animals: A critical review. Int. J. Parasitol. 1995, 25, 1371–1383. [Google Scholar] [CrossRef]
- Heil, M. Host Manipulation by Parasites: Cases, Patterns, and Remaining Doubts. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef]
- Moore, J. Parasites and the Behavior of Animals; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Poulin, R. Parasite Manipulation of Host Behavior: An Update and Frequently Asked Questions. In Advances in the Study of Behavior; Academic Press: Cambridge, MA, USA, 2010; Volume 41, pp. 151–186. [Google Scholar]
- Lefèvre, T.; Lebarbenchon, C.; Gauthier-Clerc, M.; Missé, D.; Poulin, R.; Thomas, F. The ecological significance of manipulative parasites. Trends Ecol. Evol. 2009, 24, 41–48. [Google Scholar] [CrossRef]
- Lobo, I.; Shaw, K. Phenotypic range of gene expression: Environmental influence. Nat. Educ. 2008, 1, 12. [Google Scholar]
- Cézilly, F.; Grégoire, A.; Bertin, A. Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology 2000, 120, 625–630. [Google Scholar] [CrossRef]
- Brodeur, J.; McNeil, J.N. Overwintering microhabitat selection by an endoparasitoid (Hymenoptera: Aphidiidae): Induced phototactic and thigmokinetic responses in dying hosts. J. Insect Behav. 1990, 3, 751–763. [Google Scholar] [CrossRef]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef]
- Zimmer, C. Parasite Rex: Inside the Bizarre World of Nature’s Most Dangerous Creatures; Simon and Schuster: New York City, NY, USA, 2000. [Google Scholar]
- Williams, T.; Bergoin, M.; van Oers, M.M. Diversity of large DNA viruses of invertebrates. J. Invertebr. Pathol. 2017, 147, 4–22. [Google Scholar] [CrossRef]
- Van Houte, S.; Ros, V.I.D.; van Oers, M.M. Hyperactivity and tree-top disease induced by the baculovirus AcMNPV in Spodoptera exigua larvae are governed by independent mechanisms. Naturwissenschaften 2014, 101, 347–350. [Google Scholar] [CrossRef]
- Kamita, S.G.; Nagasaka, K.; Chua, J.W.; Shimada, T.; Mita, K.; Kobayashi, M.; Maeda, S.; Hammock, B.D. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a lepidopteran host. Proc. Natl. Acad. Sci. USA 2005, 102, 2584–2589. [Google Scholar] [CrossRef]
- Bhattarai, U.R.; Katuwal Bhattarai, M.; Li, F.; Wang, D. Insights into the temporal gene expression pattern in Lymantria dispar larvae during the baculovirus induced hyperactive stage. Virol. Sin. 2018. [Google Scholar] [CrossRef]
- Goulson, D. Wipfelkrankheit: Modification of host behaviour during baculoviral infection. Oecologia 1997, 109, 219–228. [Google Scholar] [CrossRef]
- Hoover, K.; Grove, M.; Gardner, M.; Hughes, D.P.; McNeil, J.; Slavicek, J. A gene for an extended phenotype. Science 2011, 333, 1401. [Google Scholar] [CrossRef]
- Ros, V.I.D.; van Houte, S.; Hemerik, L.; van Oers, M.M. Baculovirus-induced tree-top disease: How extended is the role of egt as a gene for the extended phenotype? Mol. Ecol. 2015, 24, 249–258. [Google Scholar] [CrossRef]
- Han, Y.; van Houte, S.; Drees, G.F.; van Oers, M.M.; Ros, V.I.D. Parasitic Manipulation of Host Behaviour: Baculovirus SeMNPV EGT Facilitates Tree-Top Disease in Spodoptera exigua Larvae by Extending the Time to Death. Insects 2015, 6, 716–731. [Google Scholar] [CrossRef]
- Van Houte, S.; van Oers, M.M.; Han, Y.; Vlak, J.M.; Ros, V.I.D. Baculovirus infection triggers a positive phototactic response in caterpillars to induce ‘tree-top’ disease. Biol. Lett. 2014, 10. [Google Scholar] [CrossRef]
- Han, Y.; van Houte, S.; van Oers, M.M.; Ros, V.I.D. Timely trigger of caterpillar zombie behaviour: Temporal requirements for light in baculovirus-induced tree-top disease. Parasitology 2017. [Google Scholar] [CrossRef]
- Hariyama, T.; Saini, R.K. Odor Bait Changes the Attractiveness of Color for the Tsetse Fly. Tropics 2001, 10, 581–589. [Google Scholar] [CrossRef]
- Shimoda, M.; Honda, K.-I. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Vaishampayan, S.M.; Kogan, M.; Waldbauer, G.P.; Woolley, J.T. Spectral specific responses in the visual behavior of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae). Entomol. Exp. Appl. 1975, 18, 344–356. [Google Scholar] [CrossRef]
- Webb, R.E.; Smith, F.F.; Affeldt, H.; Thimijan, R.W.; Dudley, R.F.; Webb, H.F. Trapping greenhouse whitefly with coloured surfaces: Variables affecting efficacy. Crop Prot. 1985, 4, 381–393. [Google Scholar] [CrossRef]
- Ogino, T.; Uehara, T.; Muraji, M.; Yamaguchi, T.; Ichihashi, T.; Suzuki, T.; Kainoh, Y.; Shimoda, M. Violet LED light enhances the recruitment of a thrip predator in open fields. Sci. Rep. 2016, 6, 32302. [Google Scholar] [CrossRef] [PubMed]
- Hardie, J. Spectral specificity for targeted flight in the black bean aphid, Aphis fabae. J. Insect Physiol. 1989, 35, 619–626. [Google Scholar] [CrossRef]
- Sakai, Y.; Osakabe, M. Spectrum-specific Damage and Solar Ultraviolet Radiation Avoidance in the Two-spotted Spider Mite. Photochem. Photobiol. 2010, 86, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Sedaratian-Jahromi, A.; Ghane-Jahromi, M. Possible effects of Ultraviolet ray (UV-C) on biological traits of Callosobruchus maculatus (Col.: Chrysomelidae). J. Stored Prod. Res. 2016, 69, 91–98. [Google Scholar] [CrossRef]
- Nakajima, M.; Yoshida, H. Studies on Ultraviolet Sensitivity in Silkworm, with Special Reference to Variations in Its Killing Effect during the Larval Instar Stage. Jpn. J. Appl. Entomol. Zool. 1971, 15, 17–22. [Google Scholar] [CrossRef]
- Lah, E.F.C.; Musa, R.N.A.R.; Ming, H.T. Effect of germicidal UV-C light (254 nm) on eggs and adult of house dustmites, Dermatophagoides pteronyssinus and Dermatophagoides farinae (Astigmata: Pyroglyhidae). Asian Pac. J. Trop. Biomed. 2012, 2, 679–683. [Google Scholar] [CrossRef]
- Hori, M.; Shibuya, K.; Sato, M.; Saito, Y. Lethal effects of short-wavelength visible light on insects. Sci. Rep. 2014, 4, 7383. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, B.; Meng, J.; Xu, J.; Liu, T.X.; Wang, D. Recombinant Helicoverpa armigera nucleopolyhedrovirus with arthropod-specific neurotoxin gene RjAa17f from Rhopalurus junceus enhances the virulence against the host larvae. Insect Sci. 2017, 24, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Meng, J.; Xu, J.; Liu, T.-X.; Wang, D. A Novel Neurotoxin Gene ar1b Recombination Enhances the Efficiency of Helicoverpa armigera Nucleopolyhedrovirus as a Pesticide by Inhibiting the Host Larvae Ability to Feed and Grow. PLoS ONE 2015, 10, e0135279. [Google Scholar] [CrossRef]
- El-Salamouny, S.; Ranwala, D.; Shapiro, M.; Shepard, B.M.; Farrar, R.R., Jr. Tea, Coffee, and Cocoa as ultraviolet radiation protectants for the beet armyworm nucleopolyhedrovirus. J. Econ. Entomol. 2009, 102, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.; El Salamouny, S.; Merle Shepard, B. Green tea extracts as ultraviolet protectants for the beet armyworm, Spodoptera exigua nucleopolyhedrovirus. Biocontrol Sci. Technol. 2008, 18, 591–603. [Google Scholar] [CrossRef]
- McIntosh, A.H.; Grasela, J.J.; Lua, L.; Braunagel, S.C. Demonstration of the protective effects of fluorescent proteins in baculoviruses exposed to ultraviolet light inactivation. J. Insect Sci. 2004, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grasela, J.J.; McIntosh, A.H.; Ignoffo, C.M.; Goodman, C.L. Insect cells and their potential as stabilization barriers for DNA of multiple and single nucleopolyhedroviruses against ultraviolet-B simulated sunlight inactivation. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 173–177. [Google Scholar]
- Van Houte, S.; van Oers, M.M.; Han, Y.; Vlak, J.M.; Ros, V.I.D. Baculovirus infection triggers a positive phototactic response in caterpillars: A response to Dobson et al., (2015). Biol. Lett. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Benesh, D.P.; Duclos, L.M.; Nickol, B.B. The behavioral response of amphipods harboring Corynosoma constrictum (Acanthocephala) to various components of light. J. Parasitol. 2005, 91, 731–736. [Google Scholar] [CrossRef]
- Johansen, N.S.; Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Shipp, L. In the light of new greenhouse technologies: Direct effects of artificial lighting on arthropods and integrated pest management in greenhouse crops. Ann. Appl. Biol. 2011, 159, 1–27. [Google Scholar] [CrossRef]
- Sun, Y.X.; Tian, A.; Zhang, X.B.; Zhao, Z.G.; Zhang, Z.W.; Ma, R.Y. Phototaxis of Grapholitha molesta (Lepidoptera: Olethreutidae) to different light sources. J. Econ. Entomol. 2014, 107, 1792–1799. [Google Scholar] [CrossRef]
- Singh, A.K.; Saxena, K.N. Attraction of larvae of the armyworm Spodoptera litura (Lepidoptera: Noctuidae) to coloured surfaces. Eur. J. Entomol. 2004, 101, 697–699. [Google Scholar] [CrossRef]
- Tokushima, Y.; Uehara, T.; Yamaguchi, T.; Arikawa, K.; Kainoh, Y.; Shimoda, M. Broadband photoreceptor are involved in violet light preference in the parasitoid fly Exorista japonica. PLoS ONE 2016, 11, e0160441. [Google Scholar] [CrossRef]
- Castrejon, F.; Rojas, J.C. Behavioral responses of larvae and adults of Estigmene acrea (Lepidoptera: Arctiidae) to light of different wavelengths. Fla. Entomol. 2011, 93, 505–509. [Google Scholar] [CrossRef]
- Otsuna, H.; Shinomiya, K.; Ito, K. Parallel neural pathways in higher visual centers of the Drosophila brain that mediate wavelength-specific behavior. Front. Neural Circuits 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Gorostiza, E.A.; Colomb, J.; Brembs, B. A decision underlies phototaxis in an insect. Open Biol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Desplan, C.; Heisenberg, M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 5634–5639. [Google Scholar] [CrossRef] [PubMed]
- Daiker, V.; Häder, D.-P.; Richter, P.R.; Lebert, M. The involvement of a protein kinase in phototaxis and gravitaxis of Euglena gracilis. Planta 2011, 233, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Iseki, M. Photomovement in Euglena. In Euglena: Biochemistry, Cell and Molecular Biology; Schwartzbach, S.D., Shigeoka, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 207–235. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Nakamura, H.; Ren, S.; Hori, K.; Masuda, S. Genetics of the Blue Light-Dependent Signal Cascade That Controls Phototaxis in the Cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2017, 58, 458–465. [Google Scholar] [PubMed]
- Muntz, W.R.A. The Development of Phototaxis in the Frog (Rana Temporaria). J. Exp. Biol. 1963, 40, 371. [Google Scholar] [PubMed]
- Kitabatake, S.; Shimizu, I.; Kato, M. Wavelength-dependent properties of phototaxis in larvae of Bombyx mori. Photochem. Photobiol. 1983, 37, 321–327. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, M.K.; Bhattarai, U.R.; Feng, J.-n.; Wang, D. Effect of Different Light Spectrum in Helicoverpa armigera Larvae during HearNPV Induced Tree-Top Disease. Insects 2018, 9, 183. https://doi.org/10.3390/insects9040183
Bhattarai MK, Bhattarai UR, Feng J-n, Wang D. Effect of Different Light Spectrum in Helicoverpa armigera Larvae during HearNPV Induced Tree-Top Disease. Insects. 2018; 9(4):183. https://doi.org/10.3390/insects9040183
Chicago/Turabian StyleBhattarai, Mandira Katuwal, Upendra Raj Bhattarai, Ji-nian Feng, and Dun Wang. 2018. "Effect of Different Light Spectrum in Helicoverpa armigera Larvae during HearNPV Induced Tree-Top Disease" Insects 9, no. 4: 183. https://doi.org/10.3390/insects9040183
APA StyleBhattarai, M. K., Bhattarai, U. R., Feng, J.-n., & Wang, D. (2018). Effect of Different Light Spectrum in Helicoverpa armigera Larvae during HearNPV Induced Tree-Top Disease. Insects, 9(4), 183. https://doi.org/10.3390/insects9040183