Seasonal Change in Distribution and Heat Coma Temperature of Oceanic Skaters, Halobates (Insecta, Heteroptera: Gerridae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Treatment of Specimens for Heat Coma Experiment
2.3. Heat Coma Experiment
2.4. Statistical Analysis
3. Results
3.1. Distribution
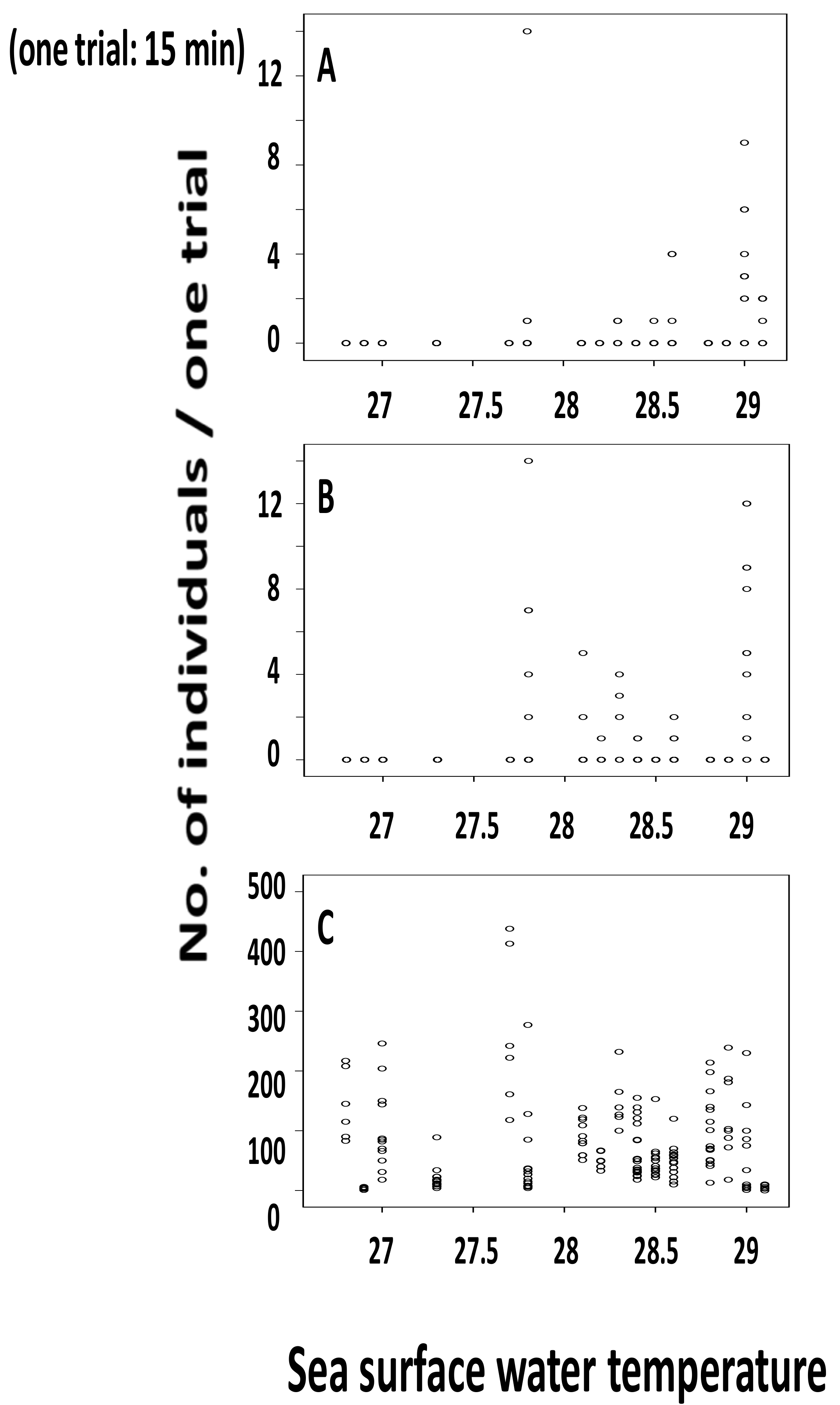
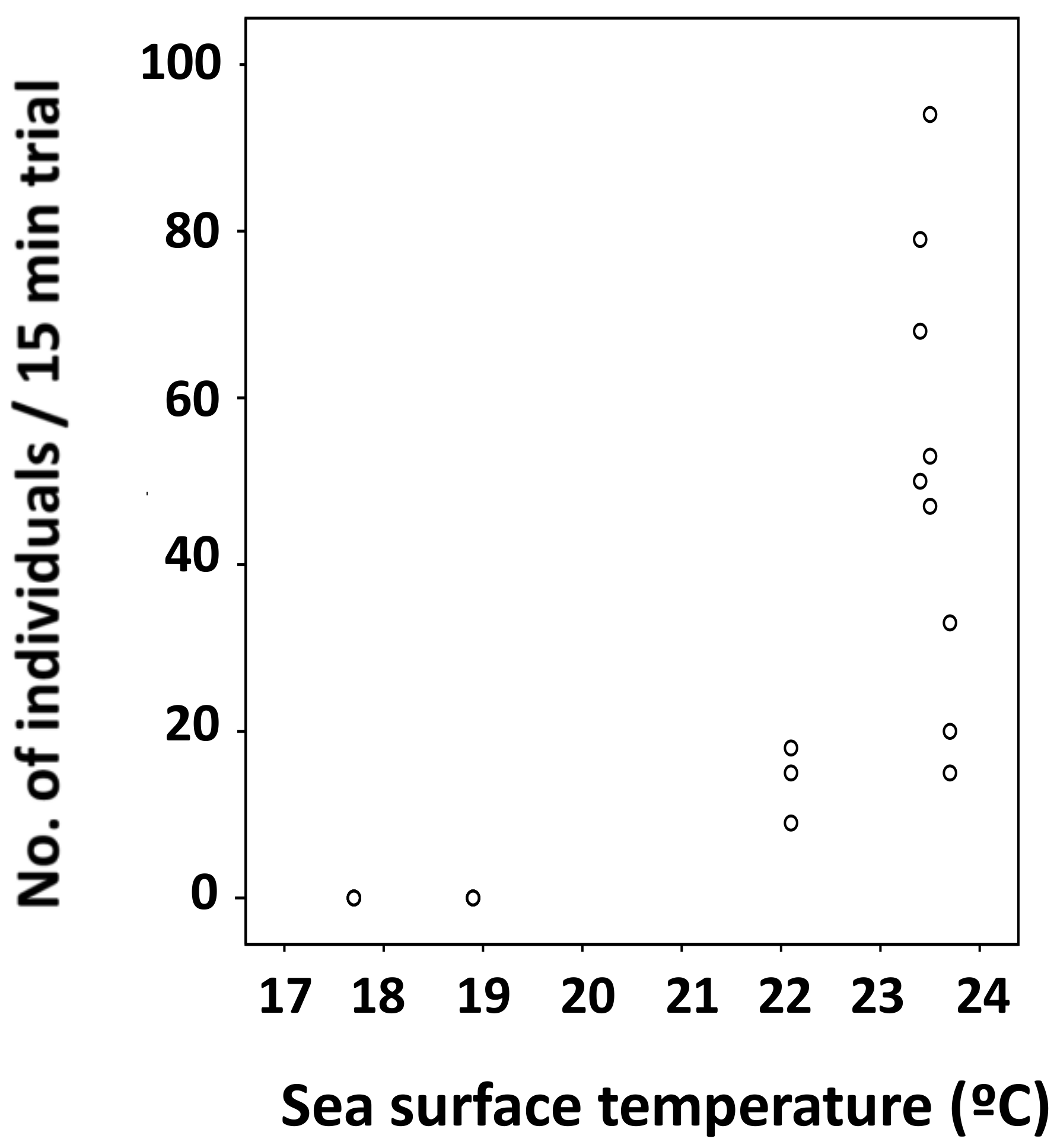
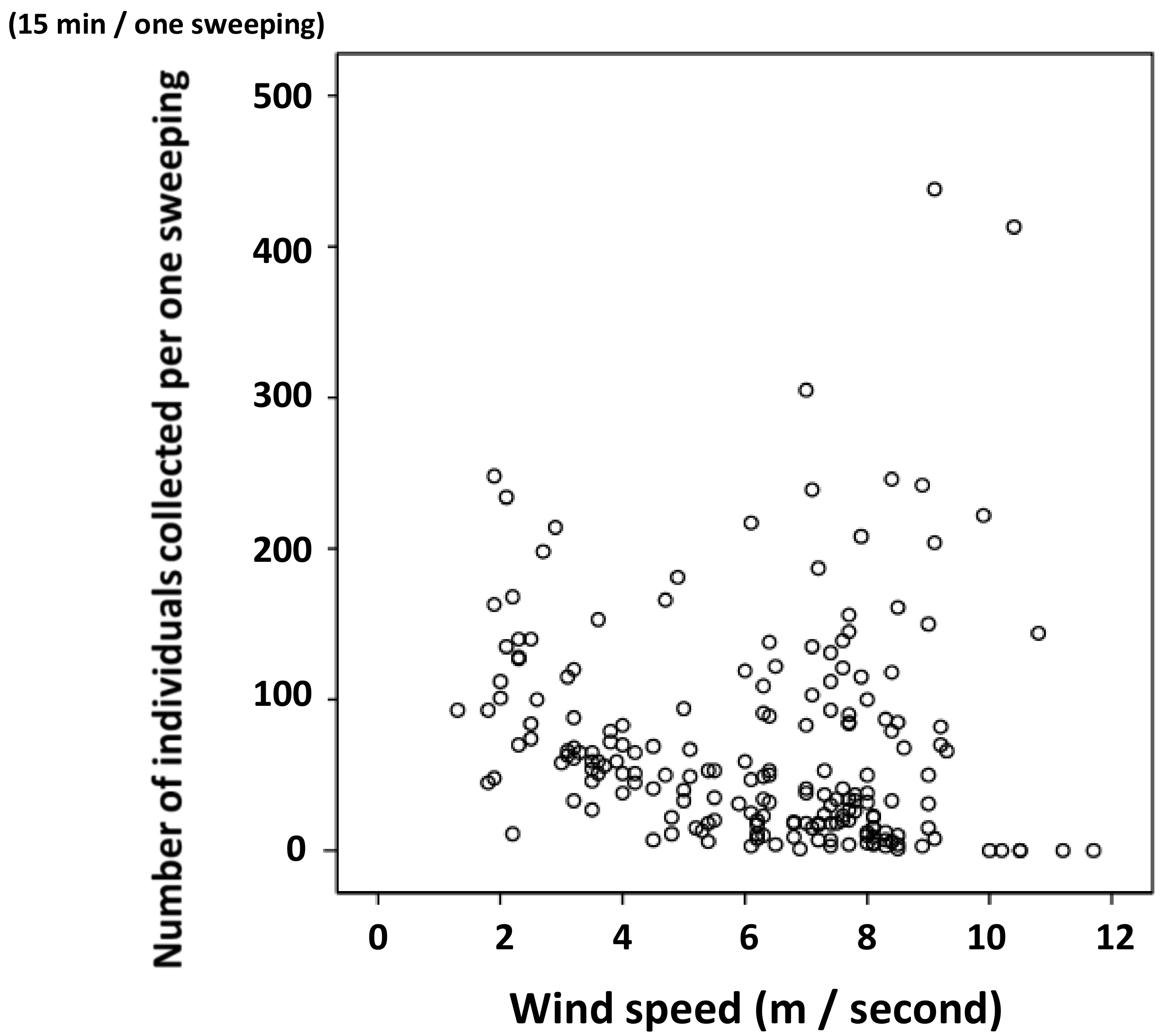
3.2. Relationship between Water Temperature and the Number of Individuals Collected
3.3. Statistical Analysis on Number of Individuals Collected and Surface Water Temperature
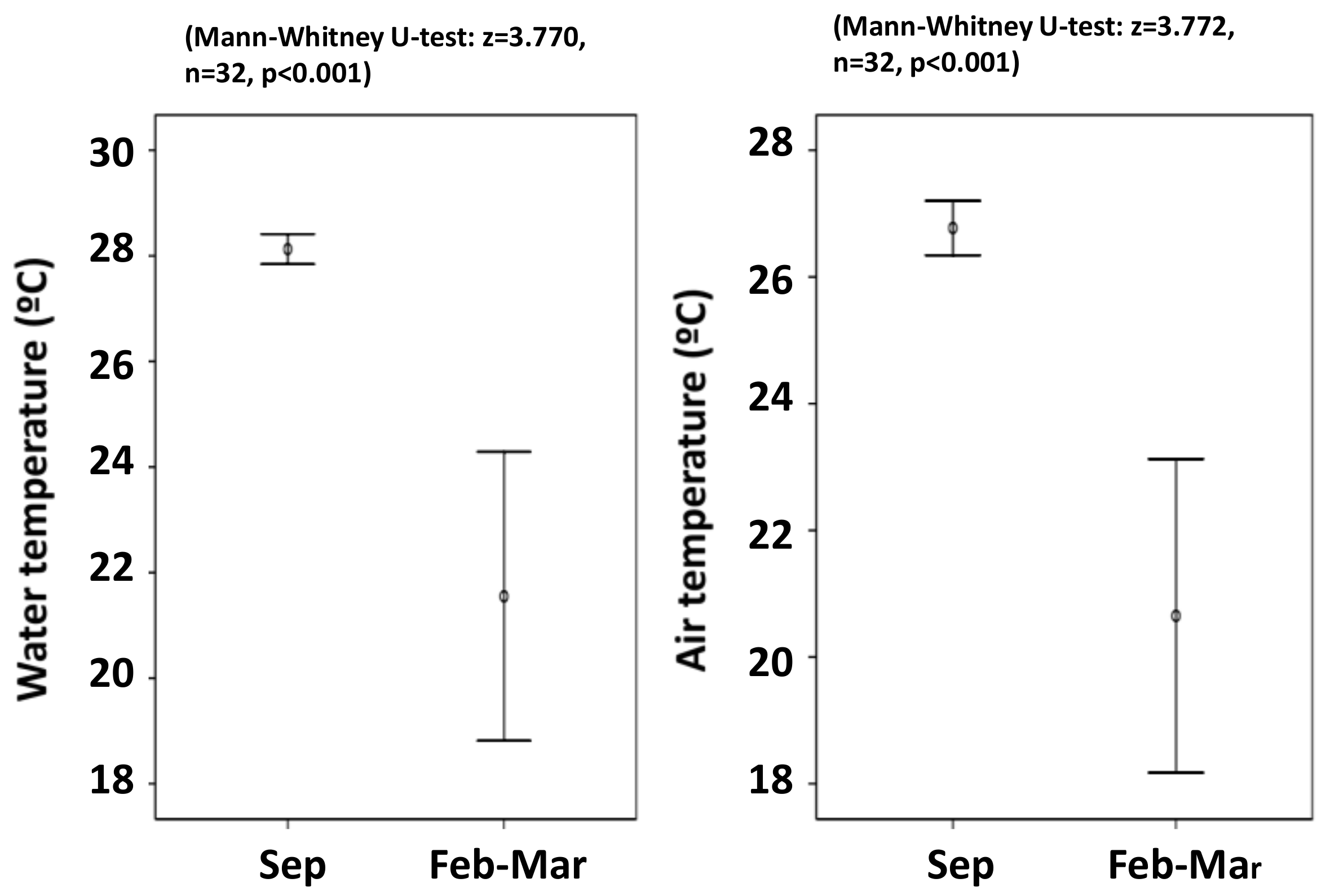
3.4. Semi-Heat Coma Temperatures (SHCTs) and Heat Coma Temperatures (HCTs)
4. Discussion
4.1. Distribution and Habitat Temperature
4.2. Seasonal Change of Heat Tolerance as a Possible Temperature Acclimation Phenomenon
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreadis, S.S.; Athanassiou, C.G. A review of insect cold hardiness and its potential in stored products insect control. Crop Prot. 2007, 91, 93–99. [Google Scholar] [CrossRef]
- Harada, T. Hardiness to low temperature and drought in a water strider, Aquarius paludum in comparison with other insect groups. Trends Entomol. 2003, 3, 29–41. [Google Scholar]
- Cheng, L. Biology of Halobates (Heteroptera: Gerridae). Annu. Rev. Entomol. 1985, 30, 111–135. [Google Scholar] [CrossRef]
- Andersen, N.M.; Cheng, L. The marine insect Halobates (Heteroptera: Gerridae): Biology, adaptations distribution, and phylogeny. Oceanogr. Mar. Biol. 2004, 42, 119–180. [Google Scholar]
- Harada, T. Geographical distribution of three oceanic Halobates spp. and an account of the behaviour of H. sericeus (Heteroptera: Gerridae). Eur. J. Entomol. 2005, 102, 299–302. [Google Scholar]
- Harada, T.; Takenaka, S.; Sekimoto, T.; Nakajyo, M.; Inoue, T.; Ishibashi, T.; Katagiri, C. Heat coma as an indicator of resistance to environmental stress and its relationship to ocean dynamics in the sea skaters, Halobates (Heteroptera: Gerridae). Insect Sci. 2011, 18, 703–711. [Google Scholar] [CrossRef]
- Andersen, N.M. The Semiaquatic Bugs (Hemiptera: Gerromorpha) Phylogeny, Adaptations, Biogeography and Classification; Scandinavian Science Press Ltd.: Klampenborg, Denmark, 1982; p. 455. [Google Scholar]
- Pörtner, H.O. Physiological basis of temperature-dependent biogeography: Trade-offs in muscle design and performance in polar ectotherms. J. Exp. Biol. 2002, 205, 2217–2230. [Google Scholar] [PubMed]
- Peck, L.S.; Morley, S.A.; Clark, M.S. Poor acclimation capacities in Antarctic marine ectotherms. Mar. Biol. 2010, 157, 2051–2059. [Google Scholar] [CrossRef]
- Lagerspetz, K.Y.H. What is thermal acclimation? J. Therm. Biol. 2006, 31, 332–336. [Google Scholar] [CrossRef]
- Leroi, A.M.; Bennett, A.F.; Lenski, R.E. Temperature acclimation and competitive fitness: An experimental test of the beneficial acclimation assumption. Proc. Natl. Acad. Sci. USA 1994, 91, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Morley, S.A.; Hirse, T.; Thorne, M.A.S.; Pörtner, H.O.; Peck, L.S. Physiological plasticity, long term resistance or acclimation to temperature, in the Antarctic bivalve, Laternulae lliptica. Comp. Biochem. Physiol. Part A 2012, 162, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.R. Some principles in the thermal requirements of fishes. Q. J. Biol. 1956, 31, 75–87. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment, 4th ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Jumbam, K.R.; Jackson, S.; Terblanche, J.S.; McGeoch, M.A.; Chown, L. Acclimation effects on critical and lethal thermal limits of workers of the Argentine ant, Linepithema humile. J. Insect Physiol. 2008, 54, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Heneghan, A.F.; Haymet, A.D.J. Ice nucleation in nature: Super cooling point (SCP) measurements and the role of heterogeneous nucleation. Cryobiology 2003, 46, 88–98. [Google Scholar] [CrossRef]
- Harada, T.; Takenaka, S.; Sekimoto, T.; Ohsumi, Y.; Nakajyo, M.; Katagiri, C. Heatcoma and its relationship to ocean dynamics in the oceanic sea skaters of Halobates (Heteroptera: Gerridae) inhabiting Indian and Pacific Oceans. J. Therm. Biol. 2011, 36, 299–305. [Google Scholar] [CrossRef]
- Harada, T.; Takenaka, S.; Sekimoto, T.; Osumi, Y.; Iyota, K.; Furutani, T.; Shiraki, T.; Nakajo, M.; Katagiri, C.; Moku, M.; et al. Correlation analysis of heat hardiness and super-cooling point in the oceanic sea skaters, Halobates. Trends Entomol. 2012, 10, 115–124. [Google Scholar]
- Harada, T.; Takenaka, S.; Iyota, K.; Shiraki, T.; Moku, M.; Katagiri, C.; Koštál, V. Supercooling points and heat coma temperatures in four species of oceanic sea skaters of the genus Halobates (Heteroptera: Gerridae: Halobatinae). J. Asia-Pac. Entomol. 2013, 16, 219–222. [Google Scholar] [CrossRef]
- Cheng, L.; Shulenberger, E. Distribution and abundance of Halobates species (Insecta: Heteroptera) in the eastern tropical Pacific. Fish. Bull. 1980, 78, 579–591. [Google Scholar]
- Furuki, T.; Umamoto, N.; Nakajo, M.; Sekimoto, T.; Moku, M.; Katagiri, C.; Harada, T. Comparative study of cool coma temperature between two populations of oceanic sea skaters, Halobates sericeus (Heteroptera: Gerridae), located at 24–25°N and 138°E or 160°E in the Pacific Ocean. Trends Entomol. 2015, 11, 55–61. [Google Scholar]
- Harada, T.; Sekimoto, T.; Iyota, K.; Shiraki, T.; Takenaka, S.; Nakajyo, M.; Osumi, Y.; Katagiri, C. Comparison of the population density of oceanic sea skater of Halobates (Heteroptera: Gerridae) among several areas in the tropical Pacific Ocean and the tropical Indian ocean. Formos. Entomol. 2010, 30, 307–316. [Google Scholar]
- Nakajo, M.; Sekimoto, T.; Emi, K.; Ide, R.; Wada, K.; Inoue, T.; Moku, M.; Koštál, V.; Katagiri, C.; Harada, T. Comparison of temperature preference for habitat among three species of oceanic sea skaters, Halobates micans, Hgermanus and H. sericeus. Nat. Sci. 2013, 5, 9–15. [Google Scholar]
- Harada, T.; Osumi, Y.; Sekimtoto, T.; Iyota, K.; Shiraki, T.; Takenaka, S.; Takeuchi, H.; Nakajo, M.; Tamura, T. The distribution of the oceanic sea skaters, Halobates inside and outside the Kuroshio. Open J. Mar. Sci. 2017, 7, 433–444. [Google Scholar] [CrossRef]
- Holmstrup, M.; Zachariassen, K.E. Physiology of cold hardiness in earthworms. Comp. Biochem. Physiol. Part A 1996, 115, 91–101. [Google Scholar] [CrossRef]
- Richard, J.; Morley, S.A.; Deloffre, J.; Pecl, L.S. Thermal acclimation capacity for four Arctic marine benthic species. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 38–43. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C.-S. Climate warming may increase aphids’ dropping probabilities in response to high temperatures. J. Insect Physiol. 2012, 58, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.S.; Williams, B.H.; Angilletta, M.J. Unifying indices of heat tolerance in ectotherms. J. Therm. Biol. 2008, 33, 320–323. [Google Scholar] [CrossRef]
- Hazell, S.P.; Neve, B.P.; Groutides, C.; Douglas, A.E.; Blackburn, T.M.; Bale, J.S. Hyperthermic aphids: Insights into behaviour and mortality. J. Insect Physiol. 2010, 56, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.A.; Hoffmann, A.A. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 2010, 24, 694–700. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Deere, J.A.; Clusella-Trullas, S.; Janion, C.; Chown, S.L. Critical thermal limits depend on methodological context. Proc. R. Soc. Lond. B 2007, 274, 2935–2942. [Google Scholar] [CrossRef] [PubMed]

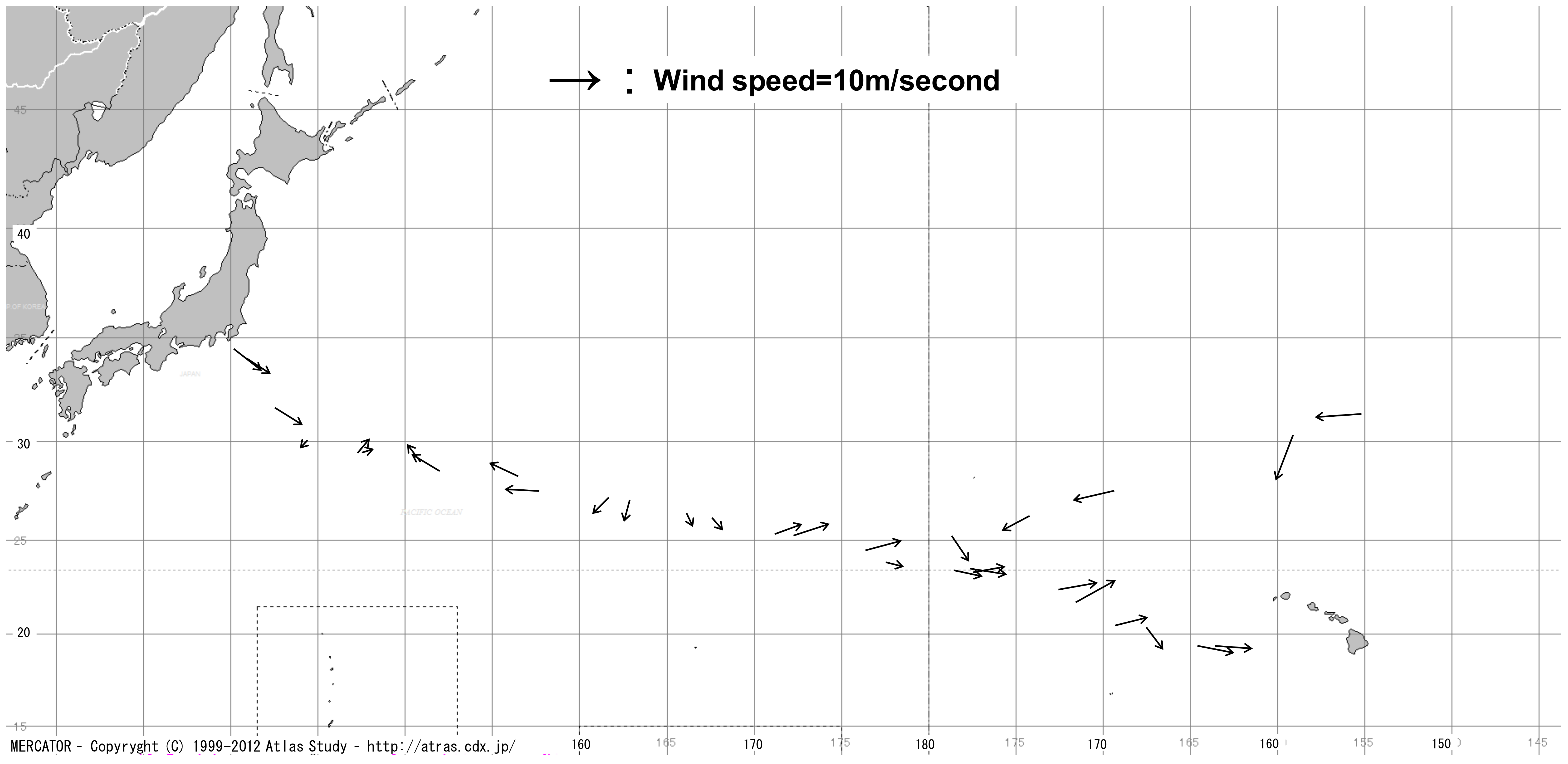
| Cruise No. | Sampling Sites (Sample No.) | T | L | A | H.m. | H.g. | H.s. | H. spp. | EG | E | WT | AT | Time | Date | SAT | PD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KH-10-04 | 34°43–45′N, 140°14–24′E (1) | 645 | 239 | 406 | 16 | 34 | 595 | 0 | 0 | 3 | 27.8 | 29.8 | 20:02–22:00 | 1-Sep | 5109 | 126,248 |
| KH-10-04 | 34°05–06′N, 141°21–26′E (2) | 54 | 14 | 40 | 9 | 12 | 32 | 1 | 0 | 5 | 29.1 | 26.8 | 02:09–04:00 | 2-Sep | 6012 | 8982 |
| KH-10-04 | 31°11–12′N, 143°41–46′E (3) | 91 | 37 | 54 | 7 | 0 | 42 | 42 | 0 | 0 | 29.1 | 27.3 | 18:45–20:57 | 2-Sep | 6518 | 13,961 |
| KH-10-04 | 30°5–8′N, 144° E30–32′E (4) | 754 | 140 | 614 | 29 | 55 | 668 | 2 | 0 | 0 | 29 | 26.8 | 02:07–03:55 | 3-Sep | 6506 | 115,893 |
| KH-10-04 | 29°38′N, 147°37–40′E (5) | 418 | 147 | 271 | 1 | 0 | 408 | 9 | 0 | 0 | 28.5 | 26.3 | 18:22–20:26 | 3-Sep | 5316 | 78,631 |
| KH-10-04 | 29°36′N, 147°48E (6) | 897 | 109 | 788 | 2 | 9 | 886 | 0 | 0 | 0 | 28.3 | 26.3 | 01:32–03:15 | 4-Sep | 6081 | 147,509 |
| KH-10-04 | 29°03′N, 151°0–4′E (7) | 326 | 65 | 261 | 8 | 4 | 314 | 0 | 0 | 0 | 28.6 | 26.2 | 17:42–20:04 | 4-Sep | 7167 | 45,486 |
| KH-10-04 | 28°49–51′N–152°18–21′E (8) | 638 | 128 | 510 | 0 | 0 | 638 | 0 | 0 | 0 | 28.1 | 24.9 | 02:07–03:47 | 5-Sep | 5132 | 124,318 |
| KH-10-04 | 28°08′N, 156°19–24′E (9) | 397 | 29 | 368 | 0 | 0 | 397 | 0 | 0 | 0 | 28.4 | 27.2 | 18:40–21:04 | 5-Sep | 5906 | 67,220 |
| KH-10-04 | 27°55′N, 157°40–43′E (10) | 217 | 7 | 210 | 0 | 1 | 216 | 0 | 0 | 0 | 28.4 | 26.5 | 02:05–03:52 | 6-Sep | 4862 | 44,632 |
| KH-10-04 | 27°17–18′N, 161°13–17′E (11) | 407 | 39 | 368 | 0 | 0 | 407 | 0 | 0 | 0 | 28.8 | 27.8 | 18:12–20:44 | 6-Sep | 6360 | 63,994 |
| KH-10-04 | 27°0–2′N, 162°45–47′E (12) | 287 | 10 | 277 | 0 | 1 | 286 | 0 | 0 | 0 | 28.2 | 28.4 | 02:59–04:42 | 7-Sep | 5295 | 54,202 |
| KH-10-04 | 26°25–6′N, 166°9–12′E (13) | 1145 | 253 | 892 | 0 | 0 | 1145 | 0 | 0 | 0 | 28.8 | 26.6 | 18:41–21:04 | 7-Sep | 6878 | 166,473 |
| KH-10-04 | 26°10–11′N, 167°39–41′E (14) | 383 | 29 | 354 | 1 | 0 | 382 | 0 | 0 | 0 | 28.6 | 28.7 | 02:32–04:20 | 8-Sep | 5292 | 72,373 |
| KH-10-04 | 25°33–5′N, 171°E–19–24E (15) | 988 | 335 | 653 | 0 | 0 | 988 | 0 | 0 | 0 | 28.9 | 26.3 | 18:41–21:06 | 8-Sep | 6981 | 141,527 |
| KH-10-04 | 25°18–9′N, 172°41–4′E (16) | 689 | 140 | 549 | 0 | 1 | 688 | 0 | 0 | 0 | 28.4 | 27.8 | 02:01–03:42 | 9-Sep | 5312 | 129,706 |
| KH-10-04 | 24°38–42′N, 176°25–9′E (17) | 279 | 81 | 198 | 0 | 0 | 279 | 0 | 0 | 0 | 28.5 | 27.2 | 18:12–20:34 | 9-Sep | 6967 | 40,046 |
| KH-10-04 | 24°23–4′N, 177°E46–9′E (18) | 417 | 173 | 244 | 0 | 7 | 410 | 0 | 0 | 0 | 28.1 | 26.9 | 01:31–03:11 | 10A-Sep | 4484 | 92,997 |
| KH-10-04 | 23°36–8°N, 178°E34–8′E (19) | 99 | 37 | 62 | 0 | 0 | 99 | 0 | 0 | 0 | 27.8 | 24.2 | 18:43–21:05 | 10A-Sep | 7215 | 13,721 |
| KH-10-04 | 23°10–1°N, 177°E14–6′W (20) | 1594 | 685 | 909 | 0 | 0 | 1594 | 0 | 0 | 0 | 27.7 | 27.1 | 02:29–04:09 | 10B-Sep | 5128 | 310,842 |
| KH-10-04 | 22°11–5′N, 173°48–51′E (21) | 115 | 51 | 64 | 0 | 0 | 115 | 0 | 0 | 0 | 27.3 | 26.3 | 18:42–20:03 | 10B-Sep | 7352 | 15,642 |
| KH-10-04 | 21°48–9′N, 173°48–51′E (22) | 538 | 214 | 324 | 0 | 0 | 538 | 0 | 0 | 0 | 27 | 25.9 | 02:03–03:43 | 11-Sep | 5484 | 98,104 |
| KH-10-04 | 20°49–53′N, 169°12–6′W (23) | 200 | 118 | 82 | 0 | 0 | 200 | 0 | 0 | 0 | 27.3 | 26.3 | 18:11–20:34 | 11-Sep | 7188 | 27,824 |
| KH-10-04 | 20°20–23′N, 167°56′W (24) | 695 | 406 | 289 | 0 | 0 | 695 | 0 | 0 | 0 | 27 | 25.7 | 02:52–04:36 | 12-Sep | 5248 | 132,431 |
| KH-10-04 | 19°33–5′N, 164°39–45′W (25) | 26 | 8 | 18 | 0 | 0 | 26 | 0 | 0 | 0 | 26.9 | 26.8 | 18:38–21:00 | 12-Sep | 6608 | 3935 |
| KH-10-04 | 19°39–40′N, 163°48–52′W (26) | 858 | 338 | 520 | 0 | 0 | 858 | 0 | 0 | 0 | 26.6 | 26.4 | 03:15–05:04 | 13-Sep | 5050 | 169,901 |
| KH-10-04 | In total | 13,157 | 3832 | 9325 | 73 | 124 | 12,906 | 54 | 8 | 155,451 | 84,638 | |||||
| Cruise No. | Sampling sites | N | L | A | H.m. | H.g. | H.s. | H. spp. | EG | E | WT | AT | Time | Date | SAT | PD |
| KH-12-1 | 24°30′N, 177°31–2′W (27) | 197 | 123 | 74 | 0 | 0 | 197 | 0 | 0 | 2 | 23.4 | 22 | 02:08–02:59 | 25-Feb | 2466 | 79,886 |
| KH-12-1 | 25°17′N, 178°57–8′E (28) | 68 | 22 | 46 | 0 | 0 | 68 | 0 | 0 | 0 | 23.7 | 22.4 | 20:04–20:55 | 26-Feb | 2235 | 30,425 |
| KH-12-1 | 26°27–8′N, 174°14–5′E (29) | 194 | 43 | 151 | 0 | 0 | 194 | 0 | 0 | 1 | 23.5 | 23.3 | 19:31–20:23 | 27-Feb | 2568 | 75,545 |
| KH-12-1 | 27°41–2′N, 169°23–4E (30) | 42 | 9 | 33 | 0 | 0 | 42 | 0 | 0 | 2 | 22.1 | 20.5 | 18:41–19:32 | 29-Feb | 2486 | 16,895 |
| KH-12-1 | 30°15′N, 159°04–5′E (31) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.9 | 18.2 | 19:34–20:25 | 2-Mar | 2420 | 0 |
| KH-12-1 | 31°26–7′N, 154°06–7′E (32) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17.7 | 17.5 | 18:42–19:33 | 3-Mar | 2323 | 0 |
| KH-12-1 | In total | 501 | 197 | 304 | 0 | 0 | 501 | 0 | 0 | 0 | 14,498 | 34,556 |
| SHCT | Summer | Winter |
|---|---|---|
| Females | 36.1 ± 1.9 (62) | 33.7 ± 2.3 (18) |
| Males | 35.8 ± 2.2 (66) | 33.7 ± 1.6 (17) |
| Total | 36.0 ± 2.1(128) | 33.7 ± 2.0 (35) |
| HCT | Summer | Winter |
| Females | 36.8 ± 1.2 (62) | 35.5 ± 0.9 (18) |
| Males | 36.4 ± 1.4 (66) | 34.6 ± 0.8 (17) |
| Total | 36.6 ± 1.3 (128) | 35.1 ± 0.9 (35) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, T.; Nakajo, M.; Furuki, T.; Umamoto, N.; Moku, M.; Sekimoto, T.; Katagiri, C. Seasonal Change in Distribution and Heat Coma Temperature of Oceanic Skaters, Halobates (Insecta, Heteroptera: Gerridae). Insects 2018, 9, 133. https://doi.org/10.3390/insects9040133
Harada T, Nakajo M, Furuki T, Umamoto N, Moku M, Sekimoto T, Katagiri C. Seasonal Change in Distribution and Heat Coma Temperature of Oceanic Skaters, Halobates (Insecta, Heteroptera: Gerridae). Insects. 2018; 9(4):133. https://doi.org/10.3390/insects9040133
Chicago/Turabian StyleHarada, Tetsuo, Mitsuru Nakajo, Takahiro Furuki, Noritomo Umamoto, Masatoshi Moku, Takero Sekimoto, and Chihiro Katagiri. 2018. "Seasonal Change in Distribution and Heat Coma Temperature of Oceanic Skaters, Halobates (Insecta, Heteroptera: Gerridae)" Insects 9, no. 4: 133. https://doi.org/10.3390/insects9040133
APA StyleHarada, T., Nakajo, M., Furuki, T., Umamoto, N., Moku, M., Sekimoto, T., & Katagiri, C. (2018). Seasonal Change in Distribution and Heat Coma Temperature of Oceanic Skaters, Halobates (Insecta, Heteroptera: Gerridae). Insects, 9(4), 133. https://doi.org/10.3390/insects9040133





