Dispersal in Host–Parasitoid Interactions: Crop Colonization by Pests and Specialist Enemies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. General Design of Field Studies
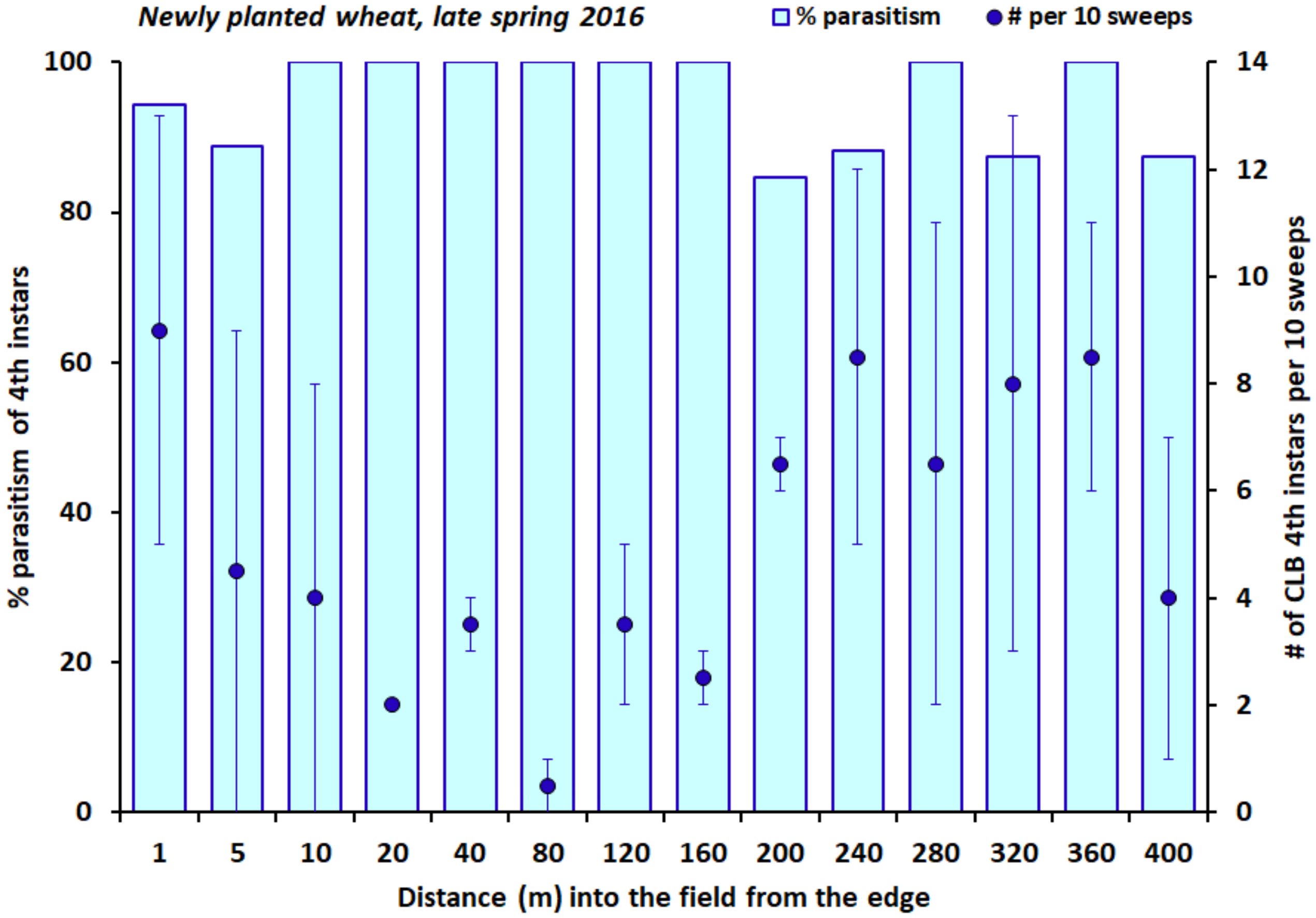
2.3. Field Study in Small Grains
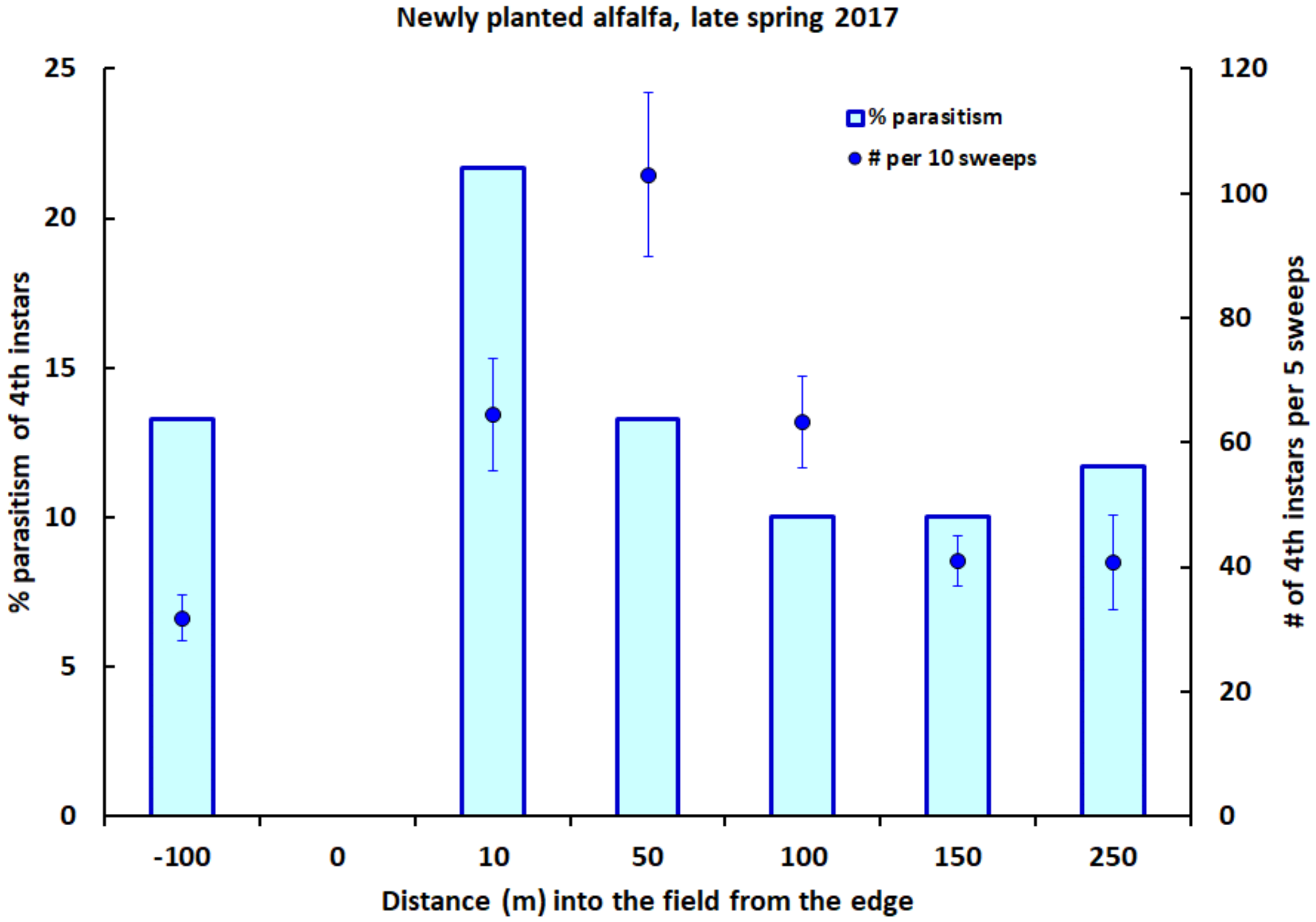
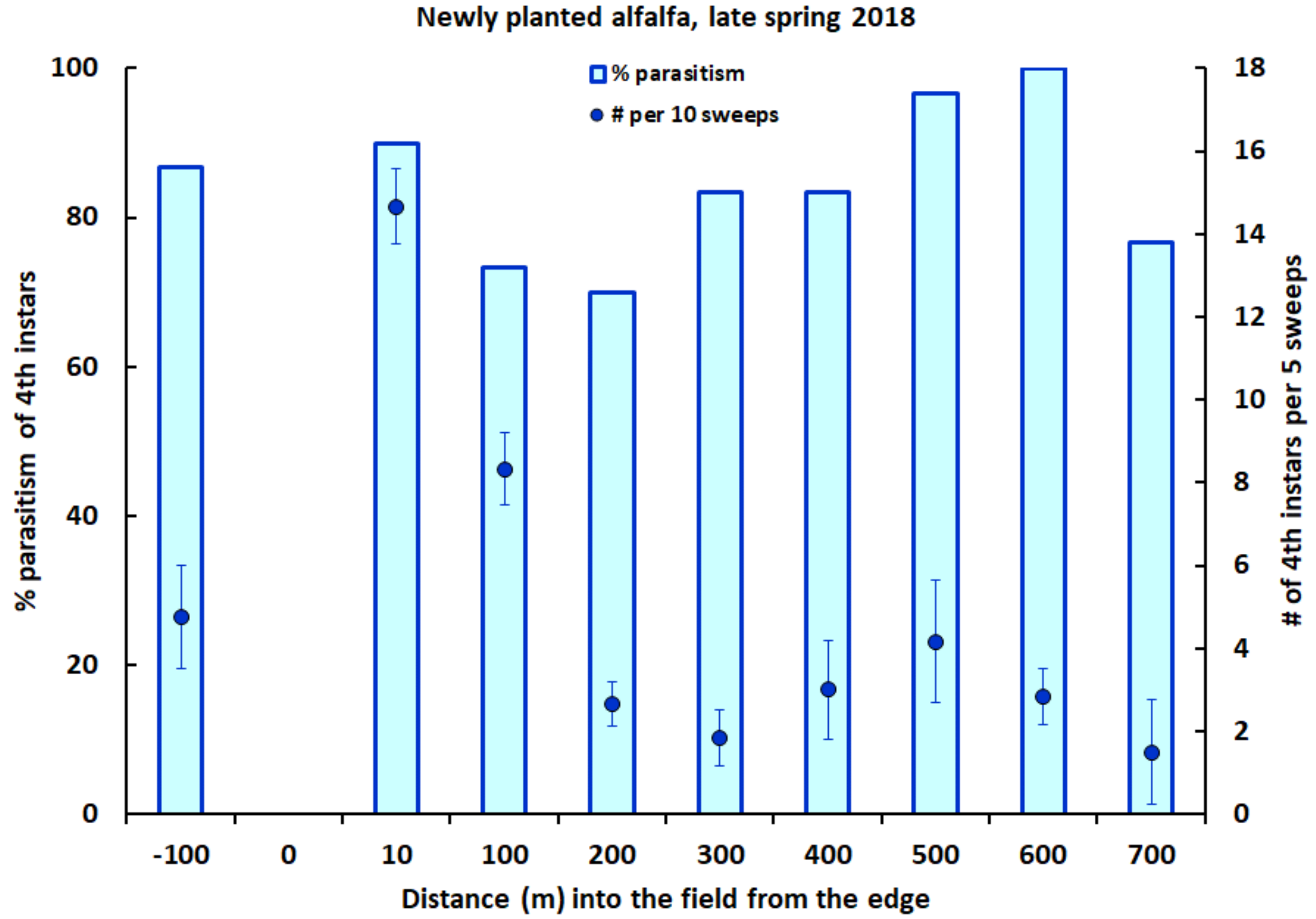
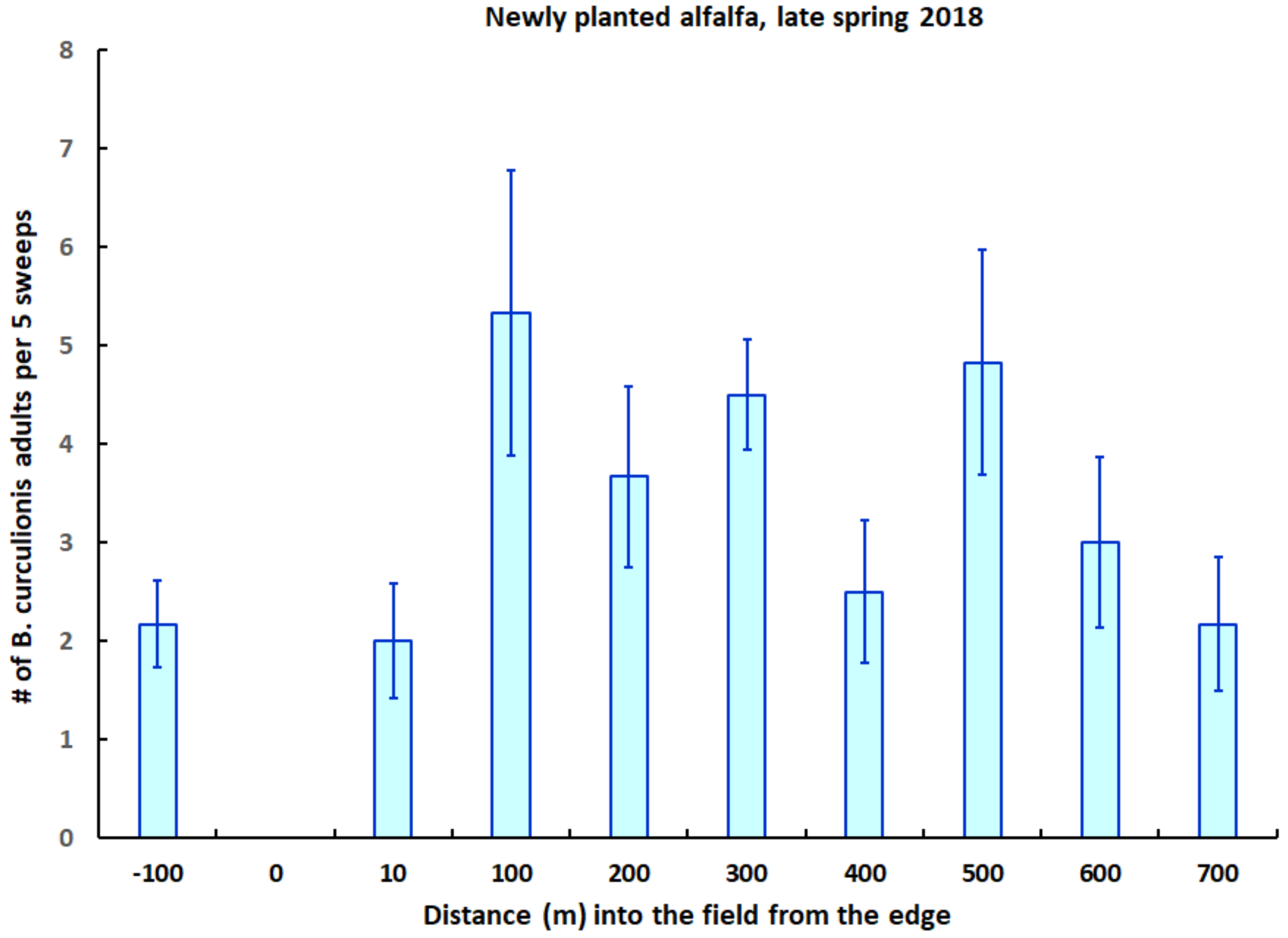
2.4. Field Studies in Alfalfa
2.5. Analyses
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kareiva, P. The spatial dimension in pest-enemy interactions. In Critical Issues in Biological Control; Mackauer, M., Ehler, L.E., Roland, J., Eds.; Intercept: Andover, Hants, UK, 1990; pp. 213–227. [Google Scholar]
- Thies, C.; Tscharntke, T. Landscape structure and biological control in agroecosystems. Science 1999, 285, 983–895. [Google Scholar] [CrossRef]
- Roland, J. Landscape ecology of parasitism. In Parasitoid Population Biology; Hochberg, M.E., Ives, A.R., Eds.; Princeton University Press: Princeton, NJ, USA, 2000; pp. 81–99. [Google Scholar]
- Cronin, J.T.; Reeve, J.D. Host-parasitoid spatial ecology: A plea for a landscape-level synthesis. P. R. Soc. B 2005, 271, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.T.; Reeve, J.D. An integrative approach to understanding host-parasitoid dynamics in real landscapes. Basic Appl. Ecol. 2014, 15, 101–113. [Google Scholar] [CrossRef]
- Rand, T.A.; Tylianakis, J.M.; Tscharntke, T. Spillover edge effects: The dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 2006, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Bommarco, R.; Clough, Y.; Crist, T.O.; Kleijn, D.; Rand, T.A.; Tylianakis, J.M.; Van Nouhuys, S.; Vidal, S. Conservation biological control and enemy diversity on a landscape scale. Biol. Control 2007, 43, 294–309. [Google Scholar] [CrossRef]
- Tscharntke, T.; Karp, D.S.; Chaplin-Cramer, R.; Batáry, P.; DeClerck, F.; Gratton, C.; Hunt, L.; Ives, A.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control—five hypotheses. Biol. Conserv. 2016, 204, 449–458. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Bianchi, F.J.J.A.; Hsu, C.L. Movement of entomophagous arthropods in agricultural landscapes: Links to pest suppression. Annu. Rev. Entomol. 2014, 59, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.M.; Peralta, G.; Rand, T.A.; Didham, R.K.; Varsani, A.; Tylianakis, J.M. Apparent competition drives community-wide parasitism rates and changes in host abundance across ecosystem boundaries. Nat. Comm. 2016, 7, 12644. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, C.B.; Messenger, P.S.; DeBach, P. The natural enemy component in natural control and the theory of biological control. In Biological Control; Huffaker, C.B., Ed.; Plenum: New York, NY, USA, 1971; pp. 16–67. [Google Scholar]
- Huffaker, C.B.; Simmonds, F.J.; Laing, J.E. The theoretical and empirical basis of biological control. In Theory and Practice of Biological Control; Huffaker, C.B., Messenger, P.S., Eds.; Academic Press: New York, NY, USA, 1976; pp. 41–77. [Google Scholar]
- Murdoch, W.W.; Briggs, C.J.; Swarbrick, S. Host suppression and stability in a parasitoid-host system: Experimental demonstration. Science 2005, 309, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.J. The balance of animal populations. J. Anim. Ecol. 1933, 2, 132–178. [Google Scholar] [CrossRef]
- Huffaker, C.B. Experimental studies on predation: Dispersion factors and predator-prey oscillations. Hilgardia 1958, 27, 343–383. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Chesson, J.; Chesson, P.L. Biological control in theory and practice. Am. Nat. 1985, 125, 344–366. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Briggs, C.J.; Nisbet, R.M. Consumer-Resource Dynamics; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Hassell, M.P. The Spatial and Temporal Dynamics of Host-Parasitoid Interactions; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Briggs, C.J.; Hoopes, M.F. Stabilizing effects in spatial parasitoid-host and predator-prey models: A review. Theor. Popul. Biol. 2004, 65, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Heimpel, G.E.; Mills, N.J. Biological Control: Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- McEvoy, P.B. Theoretical contributions to biological control success. BioControl 2018, 63, 87–103. [Google Scholar] [CrossRef]
- Price, P.W. Colonization of crops by arthropods: Non-equilibrium communities in soybean fields. Environ. Entomol. 1976, 5, 605–611. [Google Scholar] [CrossRef]
- Risch, S.J. Agricultural ecology and insect outbreaks. In Insect Outbreaks; Barbosa, P., Schultz, J.C., Eds.; Academic Press: San Diego, CA, USA, 1987; pp. 217–239. [Google Scholar]
- Kogan, M.D.; Gerling, D.; Maddox, J.V. Enhancement of biological control in annual agricultural environments. In Handbook of Biological Control. Principles and Applications of Biological Control; Bellows, T.S., Fisher, T.W., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 789–818. [Google Scholar]
- Van Driesche, R.; Hoddle, M.; Center, T. Control of Pests and Weeds by Natural Enemies. An Introduction to Biological Control; Blackwell: Oxford, UK, 2008. [Google Scholar]
- Letourneau, D.K.; Bothwell Allen, S.G.; Stireman III, J.O. Perennial habitat fragments, parasitoid diversity and parasitism in ephemeral crops. J. Appl. Ecol. 2012, 49, 1405–1416. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Thomas, M.B.; Hochberg, M.E. Refuge theory and biological control. Science 1993, 262, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.D. Metapopulations, dispersal, and predator prey dynamics: An overview. Ecology 1990, 71, 429–436. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Stiling, P.; Cornelissen, T. What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol. Control 2005, 34, 236–246. [Google Scholar] [CrossRef]
- Gillespie, M.A.K.; Gurr, G.M.; Wratten, S.D. Beyond nectar provision: The other resource requirements of parasitoid biological control agents. Entomol. Exp. Appl. 2016, 159, 207–221. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids. Behavioral and Evolutionary Ecolog; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Zappalà, L.; Campolo, O.; Grande, S.B.; Saraceno, F.; Biondi, A.; Siscaro, G.; Palmeri, B. Dispersal of Aphytis melinus (Hymenoptera: Aphelinidae) after augmentative releases in citrus orchards. Eur. J. Entomol. 2012, 109, 561–568. [Google Scholar] [CrossRef]
- Avila, G.A.; Berndt, L.A.; Holwell, G.I. Dispersal behavior of the parasitic wasp Cotesia urabae (Hymenoptera: Braconidae): A recently introduced biocontrol agent for the control of Uraba lugens (Lepidoptera: Nolidae) in New Zealand. Biol. Control 2013, 66, 166–172. [Google Scholar] [CrossRef]
- Quicke, D.L.J. The Braconid and Ichneumonid Wasps: Biology, Systematics, Evolution and Ecology; Wiley-Blackwell: Oxford, UK, 2015. [Google Scholar]
- Hamlin, J.C.; Lieberman, F.V.; Bunn, R.W.; McDuffie, W.C.; Newton, R.C.; Jones, L.J. Field studies of the alfalfa weevil and its environment. USDA Tech. Bull. 1949, 975, 1–84. [Google Scholar]
- Haynes, D.L.; Gage, S.H. The cereal leaf beetle in North America. Annu. Rev. Entomol. 1981, 26, 259–287. [Google Scholar] [CrossRef]
- Dosdall, L.M.; Cárcamo, H.; Olfert, O.; Meers, S.; Hartley, S.; Gavloski, J. Insect invasions of agroecosystems in the western Canadian prairies: Case histories, patterns, and implications for ecosystem function. Biol. Invasions 2011, 13, 1135–1149. [Google Scholar] [CrossRef]
- Böttger, J.A.A.; Bundy, C.S.; Oesterle, N.; Hanson, S.F. Phylogenetic analysis of the alfalfa weevil complex (Coleoptera: Curculionidae) in North America. J. Econ. Entomol. 2013, 106, 426–426. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, M.E.; Nelson, Z.; Jabbour, R. Ecology and management of the alfalfa weevil (Coleoptera: Curculionidae) in western United States alfalfa. J. Integr. Pest Manag. 2017, 8, 1. [Google Scholar] [CrossRef]
- Davis, D.W.; Bohart, G.E.; Griffin, G.D.; Haws, B.A.; Knowlton, G.F.; Nye, W.P. Insects and nematodes associated with alfalfa in Utah. Utah Sci. 1976, 494, 1–59. [Google Scholar]
- Casagrande, R.A.; Ruesink, W.E.; Haynes, D.L. The behavior and survival of adult cereal leaf beetles. Ann. Entomol. Soc. Amer. 1977, 70, 19–30. [Google Scholar] [CrossRef]
- Honěk, A. Crop density and abundance of cereal leaf beetles (Oulema spp.) in winter wheat (Coleoptera, Chrysomelidae). J. Plant Dis. Prot. 1991, 98, 174–178. [Google Scholar]
- Stehr, F.W. Establishment in the United States of Tetrastichus julis, a larval parasite of the cereal leaf beetle. J. Econ. Entomol. 1970, 63, 1968–1969. [Google Scholar] [CrossRef]
- Dysart, R.; Maltby, H.L.; Brunson, M.H. Larval parasites of Oulema melanopus in Europe and their colonization in the United States. Entomophaga 1973, 18, 133–167. [Google Scholar] [CrossRef]
- Chamberlin, T.R. The introduction and establishment of the alfalfa weevil parasite, Bathyplectes curculionis (Thoms.), in the United States. J. Econ. Entomol. 1926, 63, 119–125. [Google Scholar] [CrossRef]
- Gage, S.H.; Haynes, D.L. Emergence under natural and manipulated conditions of Tetrastichus julis, an introduced larval parasite of the cereal leaf beetle, with reference to regional population management. Environ. Entomol. 1975, 4, 425–434. [Google Scholar] [CrossRef]
- Evans, E.W.; Karren, J.B.; Israelsen, C.E. Interactions over time between cereal leaf beetle (Coleoptera: Chrysomelidae) and larval parasitoid Tetrastichus julis (Hymenoptera: Eulophidae) in Utah. J. Econ. Entomol. 2006, 99, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.W.; England, S. Indirect interactions in biological control of insects: Pests and natural enemies in alfalfa. Ecol. Appl. 1996, 63, 920–930. [Google Scholar] [CrossRef]
- Evans, E.W.; Carlile, N.R.; Innes, M.B.; Pitigala, N. Warm springs reduce parasitism of the cereal leaf beetle through phenological mismatch. J. Appl. Entomol. 2013, 137, 383–391. [Google Scholar] [CrossRef]
- Evans, E.W.; Bolshakova, V.L.J.; Carlile, N.R. Parasitoid dispersal and colonization lag in disturbed habitats: Biological control of cereal leaf beetle metapopulations. J. Appl. Entomol. 2015, 139, 529–538. [Google Scholar] [CrossRef]
- SAS Institute. The SAS System for Windows; Version 9.2; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Hougardy, E.; Mills, N.J. The influence of host deprivation and egg expenditure on the rate of dispersal of a parasitoid following field release. Biol. Control 2006, 37, 206–213. [Google Scholar] [CrossRef]
- Corbett, A.; Rosenheim, J.A. Quantifying movement of a minute parasitoid, Anagrus epos (Hymenoptera: Mymaridae), using fluorescent dust marking and recapture. Biol. Control 1996, 6, 35–44. [Google Scholar] [CrossRef]
- Weisser, W.W.; Völkl, W.; Hassell, M.P. The importance of adverse weather conditions for behaviour and population ecology of an aphid parasitoid. J. Anim. Ecol. 1997, 66, 386–400. [Google Scholar] [CrossRef]
- Fournier, F.; Boivin, G. Comparative dispersal of Trichogramma evanescens and Trichogramma pretiosum (Hymenoptera: Trichogrammatide) in relation to environmental conditions. Environ. Entomol. 2000, 29, 55–63. [Google Scholar] [CrossRef]
- Sallam, M.N.; Overholt, W.A.; Kairu, E. Dispersal of the exotic parasitoid Cotesia flavipes in a new ecosystem. Entomol. Exp. Appl. 2001, 98, 211–217. [Google Scholar] [CrossRef]
- Lei, G.-C.; Camara, M.D. Behaviour of a specialist parasitoid, Cotesia melitaearum: From individual behaviour to metapopulation processes. Ecol. Entomol. 1999, 24, 59–72. [Google Scholar] [CrossRef]
- Rauch, G.; Weisser, W.W. Local and spatial dynamics of a host-parasitoid system in a field experiment. Basic Appl. Ecol. 2007, 8, 89–95. [Google Scholar] [CrossRef]
- Bueno, R.C.O.F.; Parra, J.R.P.; Bueno, A.F. Trichogramma pretiosum parasitism and dispersal capacity: A basis for developing biological control programs for soybean caterpillars. Bull. Entomol. Res. 2012, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, S.J.; Lelito, J.P.; Blaedow, K.; Heimpel, G.E.; Aukema, B.H. Factors affecting the flight capacity of Tetrastichus planipennisi (Hymenoptera: Eulophidae), a classical biological control agent of Agrilus planipennis (Coleoptera: Buprestidae). Environ. Entomol. 2014, 43, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Irvin, N.A.; Hagler, J.R.; Hoddle, M.S. Laboratory investigation of triple marking the parasitoid Gonatocerus ashmeadi with a fluorescent dye and two animal proteins. Entomol. Exp. Appl. 2012, 143, 1–12. [Google Scholar] [CrossRef]
- Kristensen, N.P.; Schellhorn, N.A.; Hulthen, A.D.; Howie, L.J.; De Barro, P.J. Wind-borne dispersal of a parasitoid: The process, the model, and its validation. Environ. Entomol. 2013, 42, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Van Nouhuys, S. Diversity, population structure, and individual behaviour of parasitoids as seen using molecular markers. Curr. Opin. Insect Sci. 2016, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Pickett, C.H.; Pitcairn, M.J. Classical biological control of ash whitefly: Factors contributing to its success in California. BioControl 1999, 44, 143–158. [Google Scholar] [CrossRef]
- Tylianakis, J.; Didham, R.K.; Wratten, S.D. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 2004, 85, 658–666. [Google Scholar] [CrossRef]
- Darrouzet-Nardi, A.; Hoopes, M.F.; Walker, J.D.; Briggs, C.J. Dispersal and foraging behaviour of Platygaster californica: Hosts can’t run, but they can hide. Ecol. Entomol. 2006, 31, 298–306. [Google Scholar] [CrossRef]
- Antolin, M.F.; Strong, D.R. Long-distance dispersal by a parasitoid (Anagrus delicatus, Mymaridae) and its host. Oecologia 1987, 7, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Roland, J.; Taylor, P.D. Herbivore-natural enemy interactions in fragmented and continuous forests. In Population Dynamics. New Approaches and Synthesis; Cappuccino, N., Price, P.W., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 195–227. [Google Scholar]
- Brodmann, P.; Wilcox, C.V.; Harrison, S. Mobile parasitoids may restrict the spatial spread of an insect outbreak. J. Anim. Ecol. 1997, 66, 65–72. [Google Scholar] [CrossRef]
- Van Nouhuys, S.; Hanki, I. Colonization rates and distances of a host butterfly and two specific parasitoids in a fragmented landscape. J. Anim. Ecol. 2002, 71, 639–650. [Google Scholar] [CrossRef]
- Elzinga, J.A.; Van Nouhuys, S.; Van Leeuwen, D.-J.; Biere, A. Distribution and colonization ability of three parasitoids and their herbivorous host in a fragmented landscape. Basic Appl. Ecol. 2007, 8, 75–88. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Bellati, J.; Paull, C.A.; Maratos, L. Parasitoid and moth movement from refuge to crop. Basic Appl. Ecol. 2008, 9, 691–700. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.J.; Mills, N.J. Dispersal of Trichogramma platneri Nagarkatti (Hym., Trichogrammatidae) from point-source releases in an apple orchard in California. J. Appl. Entomol. 1997, 121, 205–209. [Google Scholar] [CrossRef]
- Wright, M.G.; Hoffmann, M.P.; Chenus, S.A.; Gardner, J. Dispersal behavior of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) in sweet corn fields: Implications for augmentative releases against Ostrinia nubilalis (Lepidoptera: Crambidae). Biol. Control 2001, 22, 29–37. [Google Scholar] [CrossRef]
- Gardner, J.; Wright, M.G.; Kuhar, T.P.; Pitcher, S.A.; Hoffmann, M.P. Dispersal of Trichogramma ostriniae in field corn. Biocontrol Sci. Tech. 2012, 22, 1221–1233. [Google Scholar] [CrossRef]
- Cronin, J.T. Patch structure, oviposition behavior, and the distribution of parasitism risk. Ecol. Monogr. 2003, 73, 283–300. [Google Scholar] [CrossRef]
- Day, W.H.; Tilmon, K.J.; Romig, R.F.; Eaton, A.T.; Murray, K.D. Recent range expansions of Peristenus digoneutis (Hymenoptera: Braconidae), a parasite of the tarnished plant bug (Hemiptera: Miridae), and high temperatures limiting its geographic distribution in North America. J. N. Y. Entomol. Soc. 2000, 108, 326–331. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Pickett, C.H.; Nieto, D.J.; Bryer, J.A.; Swezey, S.L.; Stadtherr, M.; Wisheropp, D.; Erlandson, M.; Pitcairn, M. Post-release dispersal of the introduced lygus bug parasitoid Peristenus relictus in California. Biocontrol Sci. Technol. 2013, 23, 861–871. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Habitat fragmentation, species loss, and biological control. Science 1994, 264, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.H.; Godfray, H.C.J.; Hassell, M.P. Relative movement patterns of a tephritid fly and its parasitoid wasps. Oecologia 1996, 106, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Roland, J.; Taylor, P.D. Insect parasitoid species respond to forest structure at different spatial scales. Science 1997, 386, 710–713. [Google Scholar] [CrossRef]
- Thies, C.; Roschewitz, I.; Tscharntke, T. The landscape context of cereal aphid-parastioid interactions. Proc. R. Soc. B 2005, 272, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Longley, M.; Jepson, P.C.; Izquierdo, J.; Sotherton, N. Temporal and spatial changes in aphid and parasitoid populations following applications of deltamethrin in winter wheat. Entomol. Exp. Appl. 1997, 83, 41–52. [Google Scholar] [CrossRef]
- Langhof, M.; Meyhöfer, R.; Poehling, H.-M.; Gathmann, A. Measuring the field dispersal of Aphidius colemani (Hymenoptera: Braconidae). Agric. Ecosyst. Environ. 2005, 107, 137–143. [Google Scholar] [CrossRef]
- Van Nouhuys, S. Effects of habitat fragmentation at different trophic levels in insect communities. Ann. Zool. Fenn. 2005, 42, 433–447. [Google Scholar]
- Sivakoff, F.S.; Rosenheim, J.A.; Hagler, J.R. Relative dispersal ability of a key agricultural pest and its predators in an annual agroecosytem. Biol. Control 2012, 63, 296–303. [Google Scholar] [CrossRef]
- Maron, J.L.; Harrison, S. Spatial pattern formation in an insect host-parasitoid system. Science 1997, 278, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I. Attracting carnivorous arthropods with plant volatiles: The future of biocontrol or playing with fire? Biol. Control 2012, 60, 77–89. [Google Scholar] [CrossRef]
- Harcourt, D.G.; Guppy, J.C.; Ellis, C.R. Establishment and spread of Tetrastichus julis (Hymenoptera: Eulophidae), a parasitoid of the cereal leaf beetle in Ontario. Can. Entomol. 1977, 109, 473–476. [Google Scholar] [CrossRef]
- Ehler, L.E.; Miller, J.C. Biological control in temporary agroecosystems. Entomophaga 1978, 23, 207–212. [Google Scholar] [CrossRef]
- Hirose, Y.; Takasu, K.; Takagi, M. Egg parasitoids of phytophagous bugs in soybean: Mobile natural enemies as naturally occurring biological control agents of mobile pests. Biol. Control 1996, 7, 84–94. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; Smith, J.W., Jr. Attributes of natural enemies in ephemeral crop habitats. Biol. Control 1997, 10, 16–22. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Habitat, the templet for ecological strategies? J. Anim. Ecol. 1977, 46, 337–365. [Google Scholar] [CrossRef]
- Simpson, R.G.; Cross, J.E.; Best, R.L.; Ellsbury, M.M.; Coseglia, A.F. Effects of hyperparasites on population levels of Bathyplectes curculionis in Colorado. Environ. Entomol. 1979, 8, 96–100. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, E.W. Dispersal in Host–Parasitoid Interactions: Crop Colonization by Pests and Specialist Enemies. Insects 2018, 9, 134. https://doi.org/10.3390/insects9040134
Evans EW. Dispersal in Host–Parasitoid Interactions: Crop Colonization by Pests and Specialist Enemies. Insects. 2018; 9(4):134. https://doi.org/10.3390/insects9040134
Chicago/Turabian StyleEvans, Edward W. 2018. "Dispersal in Host–Parasitoid Interactions: Crop Colonization by Pests and Specialist Enemies" Insects 9, no. 4: 134. https://doi.org/10.3390/insects9040134
APA StyleEvans, E. W. (2018). Dispersal in Host–Parasitoid Interactions: Crop Colonization by Pests and Specialist Enemies. Insects, 9(4), 134. https://doi.org/10.3390/insects9040134



