Wolbachia Affects Reproduction and Population Dynamics of the Coffee Berry Borer (Hypothenemus hampei): Implications for Biological Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Antibiotics Treatment
2.3. Detection of Wolbachia
2.3.1. Phylogenetic Classification in Wolbachia Supergroups
2.3.2. Relative Proportion of Wolbachia in the CBB Microbiota
2.4. Effect of Antibiotic Treatments on CBB Reproduction and Sex Ratio
2.5. Effect of Antibiotic Treatment on Life Table Parameters of the CBB
2.6. Data Analysis
3. Results
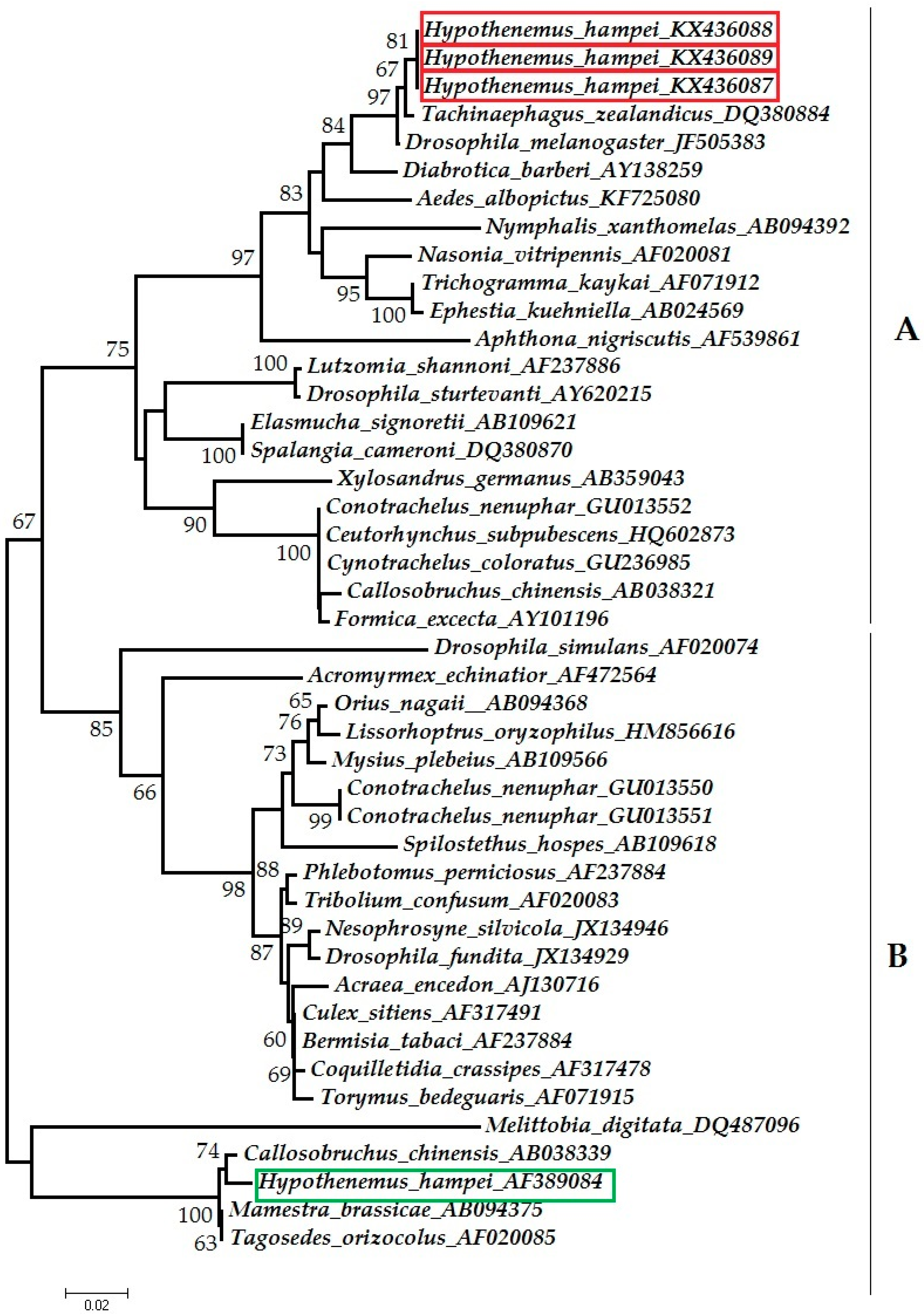
3.1. Detection of Wolbachia
3.2. Effect of Antibiotic Treatment on Wolbachia Infection and CBB Reproduction
3.3. Effect of Antibiotic Treatment on Life Table Parameters of the CBB
3.4. Formatting of Mathematical Components
4. Discussion
4.1. Wolbachia in Insects and Status in the CBB
4.2. Placement of CBB Symbionts in Wolbachia Supergroups
4.3. Antibiotic Treatments and Their Effect on CBB Reproduction and Fitness
4.4. Sex Ratio
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Host Insect Species | Order | Wolbachia Supergroup | GenBank Accession No. |
|---|---|---|---|
| Aphthona nigriscutis | Coleoptera | A | AF539861 |
| Callosobruchus chinensis | Coleoptera | A | AB038321 |
| Ceutorhynchus subpubescens | Coleoptera | A | HQ602873 |
| Conotrachelus nenuphar | Coleoptera | A | GU013552 |
| Cynotrachelus coloratus | Coleoptera | A | GU236985 |
| Diabrotica barberi | Coleoptera | A | AY138259 |
| Hypothenemus hampei | Coleoptera | A | KX436087 |
| Hypothenemus hampei | Coleoptera | A | KX436088 |
| Hypothenemus hampei | Coleoptera | A | KX436089 |
| Xylosandrus germanus | Coleoptera | A | AB359043 |
| Aedes albopictus | Diptera | A | KF725080 |
| Drosophila melanogaster | Diptera | A | JF505383 |
| Drosophila sturtevanti | Diptera | A | AY620215 |
| Lutzomia shannoni | Diptera | A | AF237886 |
| Elasmucha signoretii | Heteroptera | A | AB109621 |
| Formica excecta | Hymenoptera | A | AY101196 |
| Nasonia vitripennis | Hymenoptera | A | AF020081 |
| Spalangia cameroni | Hymenoptera | A | DQ380870 |
| Tachinaephagus zealandicus | Hymenoptera | A | DQ380884 |
| Trichogramma kaykai | Hymenoptera | A | AF071912 |
| Ephesia kuehniella | Lepidoptera | A | AB024569 |
| Nymphalis xanthomelas | Lepidoptera | A | AB094392 |
| Callosobruchus chinensis | Coleoptera | B | AB038339 |
| Conotrachelus nenuphar | Coleoptera | B | GU013550 |
| Conotrachelus nenuphar | Coleoptera | B | GU013551 |
| Hypothenemus hampei | Coleoptera | B | AF389084 |
| Lissorhotrus oryzophilus | Coleoptera | B | HM856616 |
| Tribolium confusum | Coleoptera | B | AF020083 |
| Culex sitiens | Diptera | B | AF317491 |
| Coquilletidia crassipes | Diptera | B | AF317478 |
| Drosophila fundita | Diptera | B | JX134929 |
| Drosophila simulans | Diptera | B | AF020074 |
| Phlebotomus perniciosus | Diptera | B | AF237884 |
| Bermisia tabaci | Hemiptera | B | AF237884 |
| Nesophrosyne silvicola | Hemiptera | B | JX134946 |
| Orius nagaii | Hemiptera | B | AB094368 |
| Tagosedes orizocolus | Hemiptera | B | AF020085 |
| Mysius plebeius | Heteroptera | B | AB109566 |
| Spilostethus hospes | Heteroptera | B | AB109618 |
| Acromyrmex echinatior | Hymenoptera | B | AF472564 |
| Melittobia digitata | Hymenoptera | B | DQ487096 |
| Torymus bedeguaris | Hymenoptera | B | AF071915 |
| Acraea encedon | Lepidoptera | B | AJ130716 |
| Mamestra brassicae | Lepidoptera | B | AB094375 |
| Effects | Estimate | Standard Error | Z-Value | p |
|---|---|---|---|---|
| Intercept | 4.108 | 0.0177 | 231.6 | <0.0001 *** |
| Type of diet (control vs. penicillin) | 0.0871 | 0.0109 | 7.93 | 0.0670 |
| Type of diet (control vs. tetracycline) | −0.0804 | 0.0114 | −7.02 | <0.0001 *** |
| Generation (F1 vs. F2) | 0.7764 | 0.0199 | 38.91 | <0.0001 *** |
| Generation (F1 vs. F3) | 0.8587 | 0.0197 | 43.58 | <0.0001 *** |
| Generation (F1 vs. F4) | 0.8285 | 0.0197 | 41.86 | <0.0001 *** |
| Generation (F1 vs. F5) | 0.6950 | 0.0202 | 34.38 | <0.0001 *** |
| Generation (F1 vs. F10) | 1.172 | 0.0188 | 62.07 | <0.0001 *** |
References
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef] [PubMed]
- Brelsfoard, C.L.; Dobson, S.L. Wolbachia-based strategies to control insect pests and disease vectors. Asia Pac. J. Mol. Biol. Biotechnol. 2009, 17, 55–63. [Google Scholar]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Casiraghi, M.; Salati, E.; Bazzocchi, C.; Bandi, C. How many Wolbachia supergroups exist? Mol. Biol. Evol. 2002, 19, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rousset, F.; O’Neill, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, A.; Hoy, M. Long PCR improves Wolbachia DNA amplification: Wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000, 9, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Palmer, M.; Rand, D. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 2004, 93, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Wang, J.-J.; Zhao, Z.-M. Wolbachia infection and its influence on the reproduction of stored-product psocid, Liposcelis tricolor (Psocoptera: Liposcelididae). J. Insect Sci. 2006. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.X.; Yang, M.S.; Yang, W.J.; Wang, J.J. Influence of continuous high temperature conditions on Wolbachia infection frequency and the fitness of Liposcelis tricolor (Psocoptera: Liposcelididae). Environ. Entomol. 2009, 38, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Borad, C.; Harari, A.R. Oogenesis in the date stone beetle, Coccotrypes dactyliperda, depends on symbiotic bacteria. Physiol. Entomol. 2006, 31, 164–169. [Google Scholar] [CrossRef]
- Engelstädter, J.; Telschow, A. Cytoplasmic incompatibility and host population structure. Heredity 2009, 103, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Lu, F.; Cheng, J.-A.; Jiang, M.-X.; Way, M.O. Identification and biological role of the endosymbionts Wolbachia in rice water weevil (Coleoptera: Curculionidae). Environ. Entomol. 2012, 41, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Dedeine, F.; Vavre, F.; Fleury, F.; Loppin, B.; Hochberg, M.E.; Boulétreau, M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 2001, 98, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Zabalou, S.; Riegler, M.; Theodorakopoulou, M.; Stauffer, C.; Savakis, C.; Bourtzis, K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 2004, 101, 15042–15045. [Google Scholar] [CrossRef] [PubMed]
- Damon, A. A review of the biology and control of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 2000, 90, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pinto, L.; Perfecto, I.; Caballero-Nieto, J. Shade over coffee: Its effects on berry borer, leaf rust and spontaneous herbs in Chiapas, Mexico. Agrofor. Syst. 2002, 55, 37–45. [Google Scholar] [CrossRef]
- Jaramillo, J.; Borgemeister, C.; Baker, P. Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): Searching for sustainable control strategies. Bull. Entomol. Res. 2006, 96, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Infante, F.; Castillo, A.; Jaramillo, J. The coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthropod Rev. 2009, 2, 129–147. [Google Scholar]
- Vega, F.E.; Benavides, P.; Stuart, J.A.; O’Neill, S.L. Wolbachia infection in the coffee berry borer (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 2002, 95, 374–378. [Google Scholar] [CrossRef]
- Portilla, M. Mass rearing technique for Cephalonomia stephanoderis (Hymenoptera: Bethylidae) on Hypothenemus hampei (Coleoptera: Scolytidae) developed using cenibroca artificial diet. Rev. Colomb. Entomol. 1999, 25, 57–66. [Google Scholar]
- Werren, J.H.; Beukeboom, L.W. Sex determination, sex ratios, and genetic conflict. Annu. Rev. Ecol. Syst. 1998, 29, 233–261. [Google Scholar] [CrossRef]
- Ballard, J.; Melvin, R. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol. Biol. 2007, 16, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Maurin, M.; Raoult, D. Wolbachia pipientis growth kinetics and susceptibilities to 13 antibiotics determined by immunofluorescence staining and real-time PCR. Antimicrob. Agents Chemother. 2003, 47, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Bandi, C.; Hoerauf, A.; Lazdins, J. Wolbachia bacteria of filarial nematodes: A target for control? Parasitol. Today 2000, 16, 179–180. [Google Scholar] [CrossRef]
- Kondo, N.; Ijichi, N.; Shimada, M.; Fukatsu, T. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol. Ecol. 2002, 11, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D. Mesquite: A Modular System for Evolutionary Analysis. Available online: http://mesquiteproject.org (accessed on 12 August 2016).
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-high-throughput microbial community analysis on the illumina hiseq and miseq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Mariño, Y. Reproduction, sex ratio and bacterial communities of the coffee berry borer Hypothenemus hampei F. (Coleoptera: Curculionidae). Ph.D. Thesis, University of Puerto Rico, Río Piedras, Puerto Rico, 2015. [Google Scholar]
- Bergamin, J. Contribuição para o conhecimento da biologia da broca do café Hypothenemus hampei (Ferrari, 1867) (Col. Ipidae). Arq. Inst. Biol. 1943, 14, 31–72. (In Portuguese) [Google Scholar]
- Ruiz-Cárdenas, R.; Baker, P. Life table of Hypothenemus hampei (Ferrari) in relation to coffee berry phenology under colombian field conditions. Sci. Agricola 2010, 67, 658–668. [Google Scholar] [CrossRef]
- Romero, J.; Cortina, H. Tablas de vida de Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) sobre tres introducciones de café. Rev. Colomb. Entomol. 2007, 33, 10–16. (In Spanish) [Google Scholar]
- Caswell, H. Matrix Population Models: Construction, Analysis and Interpretation, 2nd ed.; Sinauer: Sunderland, MA, USA, 2001. [Google Scholar]
- Stubben, C.; Milligan, B. Estimating and analyzing demographic models using the popbio package in R. J. Stat. Softw. 2007, 22, 1–23. [Google Scholar] [CrossRef]
- R Core Team. A Languaje and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kyei-Poku, G.; Giladi, M.; Coghlin, P.; Mokady, O.; Zchori-Fein, E.; Floate, K. Wolbachia in wasps parasitic on filth flies with emphasis on Spalangia cameroni. Entomol. Exp. Appl. 2006, 121, 123–135. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, J.L.; Scott, T.W. An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2004, 41, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Tagami, Y.; Miura, K. Distribution and prevalence of Wolbachia in Japanese populations of Lepidoptera. Insect Mol. Biol. 2004, 13, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Marshall, M.L.; Fry, A.J.; Kim, U.; Wernegreen, J.J. The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathog. 2006, 2, e43. [Google Scholar]
- Ahantarig, A.; Trinachartvanit, W.; Kittayapong, P. Relative Wolbachia density of field-collected Aedes albopictus mosquitoes in Thailand. J. Vector Ecol. 2008, 33, 173–177. [Google Scholar] [CrossRef]
- Wolfgang, A.; Markus, R.; Dimitrios, N.; Christian, S. Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, scolytinae). Environ. Microbiol. 2009, 11, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Paraskevopoulos, C.; Bourtzis, K.; O’Neill, S.; Werren, J.; Bordenstein, S.; Bandi, C. Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int. J. Syst. Evol. Microbiol. 2007, 57, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Ros, V.I.; Fleming, V.M.; Feil, E.J.; Breeuwer, J.A. How diverse is the genus wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl. Environ. Microbiol. 2009, 75, 1036–1043. [Google Scholar] [CrossRef]
- Baldo, L.; Hotopp, J.C.D.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
- Baldo, L.; Werren, J.H. Revisiting Wolbachia supergroup typing based on wsp: Spurious lineages and discordance with mlst. Curr. Microbiol. 2007, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Santos-Garcia, D.; Dionyssopoulou, E.; Moreira, M.; Papapanagiotou, A.; Scarvelakis, M.; Doudoumis, V.; Ramos, S.; Aguiar, A.F.; Borges, P.A. Detection and characterization of Wolbachia infections in natural populations of aphids: Is the hidden diversity fully unraveled? PLoS ONE 2011, 6, e28695. [Google Scholar] [CrossRef]
- Srivastava, P.; Auclair, J. Effects of antibiotics on feeding and development of the pea aphid, Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Can. J. Zool. 1976, 54, 1025–1029. [Google Scholar] [CrossRef]
- Büyükgüzel, K.; Yazgan, Ş. Effects of antimicrobial agents on the survival and development of larvae of Pimpla turionellae L. (Hymenoptera: Ichneumonidae) reared on an artificial diet. Turk. J. Zool. 2001, 26, 111–119. [Google Scholar]
- Dale, C.; Welburn, S. The endosymbionts of tsetse flies: Manipulating host–parasite interactions. Int. J. Parasitol. 2001, 31, 628–631. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, D.; Traut, W. Opposite sex–specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 251–258. [Google Scholar] [CrossRef]
- Charlat, S.; Davies, N.; Roderick, G.K.; Hurst, G.D. Disrupting the timing of Wolbachia-induced male-killing. Biol. Lett. 2007, 3, 154–156. [Google Scholar] [CrossRef]
- Breeuwer, J. Wolbachia and cytoplasmic incompatibility in the spider mites Tetranychus urticae and T. turkestani. Heredity 1997, 79, 41–47. [Google Scholar] [CrossRef]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect–Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.C.; Sommer, U.; Viant, M.R.; Sinkins, S.P. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol. 2016, 82, 3109–3120. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a new sex–ratio–distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. Lond. Biol. 2003, 270, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Jiggins, F.M.; Hurst, G.D.; Majerus, M.E. Sex-ratio distorting Wolbachia causes sex-role reversal in its butterfly host. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Dyson, E.A.; Hurst, G.D. Persistence of an extreme sex-ratio bias in a natural population. Proc. Nat. Acad. Sci. USA 2004, 101, 6520–6523. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, M. Induction of males in thelytokous populations of Encarsia meritoria and Encarsia protransvena: A systematic tool. BioControl 2001, 46, 427–438. [Google Scholar] [CrossRef]
- Pijls, J.; Steenbergen, H.J.V.; Alphen, J.J.V. Asexuality cured: The relations and differences between sexual and asexual Apoanagyrus diversicornis. Heredity 1996, 76, 506–513. [Google Scholar] [CrossRef]
- Hurst, G.D.; Johnson, A.P.; vd Schulenburg, J.H.G.; Fuyama, Y. Male-killing Wolbachia in Drosophila: A temperature-sensitive trait with a threshold bacterial density. Genetics 2000, 156, 699–709. [Google Scholar] [PubMed]
- Stouthamer, R.; Luck, R.F.; Hamilton, W. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera: Trichogrammatidae) to revert to sex. Proc. Nat. Acad. Sci. USA 1990, 87, 2424–2427. [Google Scholar] [CrossRef]
- Majerus, M.; Knowles, B.; Bertrand, D.; Hurst, G.D. Extreme variation in the prevalence of inherited male-killing microorganisms between three populations of Harmonia axyridis (Coleoptera: Coccinellidae). Heredity 1998, 81, 683–691. [Google Scholar] [CrossRef]
- Pannebakker, B.A.; Beukeboom, L.W.; van Alphen, J.J.; Brakefield, P.M.; Zwaan, B.J. The genetic basis of male fertility in relation to haplodiploid reproduction in Leptopilina clavipes (Hymenoptera: Figitidae). Genetics 2004, 168, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.; Pen, I.; Shuker, D.M. Genomic conflict in scale insects: The causes and consequences of bizarre genetic systems. Biol. Rev. 2010, 85, 807–828. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.O.; Stuart, J.; Gaudichon, V.; Aronstein, K.; French-Constant, R. Functional haplodiploidy: A mechanism for the spread of insecticide resistance in an important international insect pest. Proc. Natl. Acad. Sci. USA 1995, 92, 9861–9865. [Google Scholar] [CrossRef] [PubMed]
- Stouthamer, R.; Mark, F. Influence of antibiotics on the offspring production of the Wolbachia-infested parthenogenetic parasitoid Encarsia formosa. J. Invertebr. Pathol. 2002, 80, 41–45. [Google Scholar] [CrossRef]
- Caswell, H. Life table response experiment analysis of the stochastic growth rate. J. Ecol. 2010, 98, 324–333. [Google Scholar] [CrossRef]

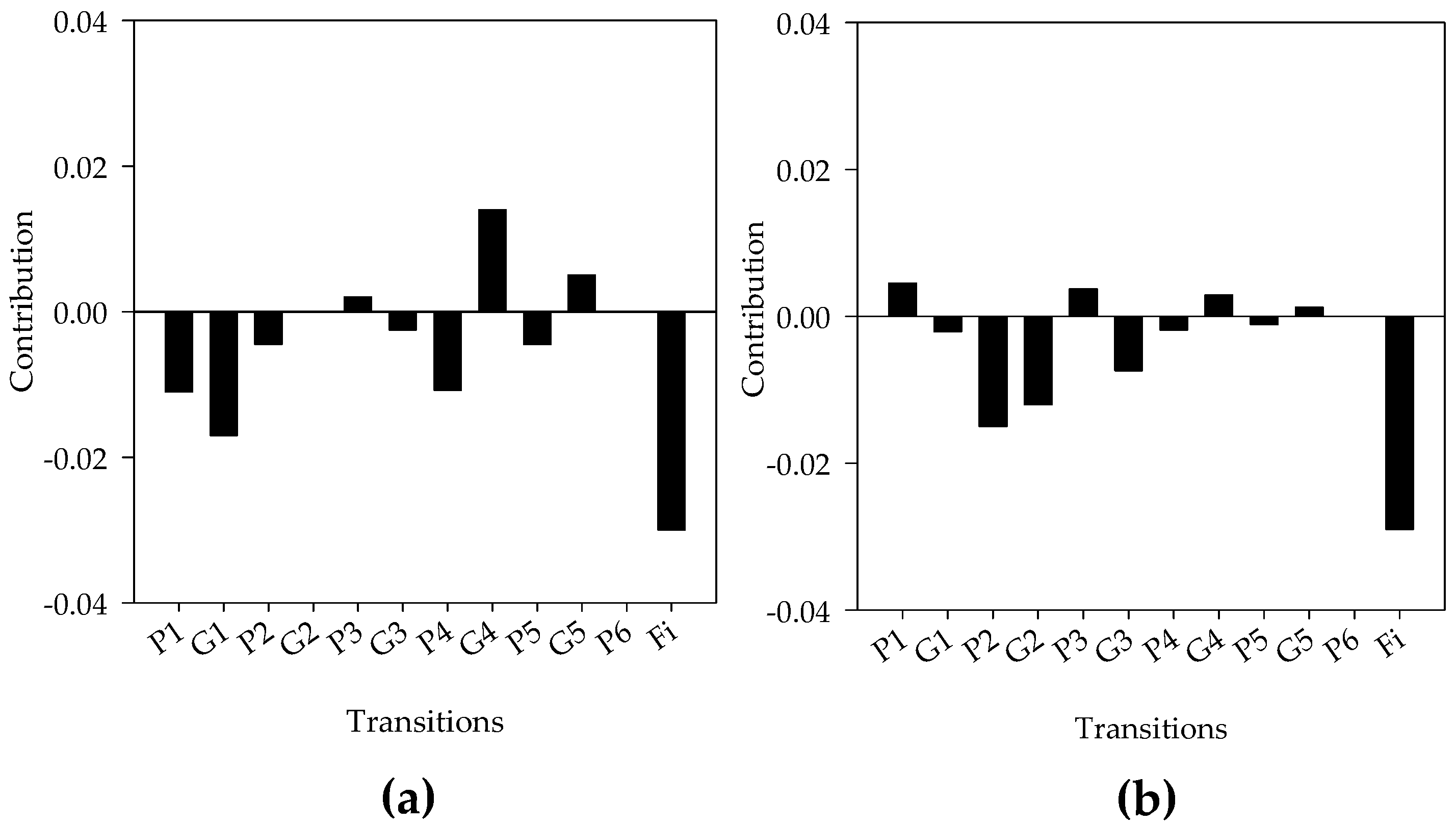
| Diet | Generation (F1) | ||
| Females | Males | Sex Ratio | |
| control | 20.8 ± 1.8 | 2.4 ± 0.3 | 10.2 ± 0.8 a |
| penicillin | 20.6 ± 3.4 | 2.8 ± 0.4 | 8.80 ± 1.2 a |
| tetracycline | 17.5 ± 2.5 | 3.5 ± 0.4 | 10.3 ± 1.7 a |
| Statistics | Z = 61.0/p = 0.04 a * | Z = 7.29/p < 0.77 a | F = 0.4/p = 0.66 b |
| Generation (F5) | |||
| Females | Males | Sex Ratio | |
| control | 62.0 ± 4.9 | 9.4 ± 0.6 | 31.0 ± 2.4 b |
| penicillin | 39.1 ± 4.8 | 5.5 ± 0.7 | 19.5 ± 2.4 a |
| tetracycline | 27.1 ± 3.5 | 6.2 ± 0.7 | 13.5 ± 1.7 a |
| Statistics | Z = 1.05/p < 0.001 a *** | Z = 30.5/p < 0.001 a *** | F = 16.13/p < 0.001 b *** |
| Generation (F10) | |||
| Females | Males | Sex Ratio | |
| control | 84.2 ± 5.1 | 11.3 ± 0.8 | 42.1 ± 2.5 b |
| penicillin | 98.3 ± 8.6 | 10.9 ± 0.8 | 49.2 ± 4.3 b |
| tetracycline | 52.5 ± 7.2 | 9.1 ± 1.1 | 26.5 ± 3.6 a |
| Statistics | Z = 182.0/p < 0.001 a *** | Z = 36.45/p = 0.74 a | F = 10.81/p = 0.0001 b *** |
| Diet | Development Time (Days) | |||||
|---|---|---|---|---|---|---|
| Egg | Larva | Pre-Pupa | Pupa | Juvenile | Egg to Adult | |
| control | 6.08 ± 0.22 a | 15.22 ± 0.75 a | 1.94 ± 0.11 a | 6.37 ± 0.15 a | 4.05 ± 0.12 a | 34.41 ± 0.75 a |
| penicillin | 6.07 ± 0.31 a | 15.45 ± 0.59 a | 1.87 ± 0.07 a | 6.78 ± 0.18 a | 4.09 ± 0.19 a | 34.26 ± 0.61 a |
| tetracycline | 6.10 ± 0.21 a | 15.52 ± 0.74 a | 2.03 ± 0.09 a | 6.82 ± 0.26 a | 4.67 ± 0.24 b | 35.13 ± 0.94 a |
| Statistics | F = 1.91/p = 0.15 | F = 0.89/p = 0.41 | F = 0.86/p = 0.42 | F = 1.59/p = 0.20 | F = 3.34/p = 0.01 * | F = 0.95/p = 0.38 |
| Diet | Life Table Parameters | ||
|---|---|---|---|
| λ | R0 | T | |
| control | 1.11 ± 0.01 a | 51.51 ± 5.72 b | 39.01 ± 3.13 a |
| penicillin | 1.10 ± 0.01 a | 45.20 ± 8.88 ab | 39.19 ± 5.39 a |
| tetracycline | 1.07 ± 0.01 a | 23.40 ± 3.43 a | 45.19 ± 3.05 a |
| Statistics | F = 3.13/p = 0.08 | F = 5.29/p = 0.02 * | F = 0.77/p = 0.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariño, Y.A.; Verle Rodrigues, J.C.; Bayman, P. Wolbachia Affects Reproduction and Population Dynamics of the Coffee Berry Borer (Hypothenemus hampei): Implications for Biological Control. Insects 2017, 8, 8. https://doi.org/10.3390/insects8010008
Mariño YA, Verle Rodrigues JC, Bayman P. Wolbachia Affects Reproduction and Population Dynamics of the Coffee Berry Borer (Hypothenemus hampei): Implications for Biological Control. Insects. 2017; 8(1):8. https://doi.org/10.3390/insects8010008
Chicago/Turabian StyleMariño, Yobana A., José C. Verle Rodrigues, and Paul Bayman. 2017. "Wolbachia Affects Reproduction and Population Dynamics of the Coffee Berry Borer (Hypothenemus hampei): Implications for Biological Control" Insects 8, no. 1: 8. https://doi.org/10.3390/insects8010008
APA StyleMariño, Y. A., Verle Rodrigues, J. C., & Bayman, P. (2017). Wolbachia Affects Reproduction and Population Dynamics of the Coffee Berry Borer (Hypothenemus hampei): Implications for Biological Control. Insects, 8(1), 8. https://doi.org/10.3390/insects8010008







