Repellency of Plant Extracts against the Legume Flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae)
Abstract
:1. Introduction
2. Experimental Section
2.1. Insect Culture and Rearing
2.2. Olfactometer Bioassay
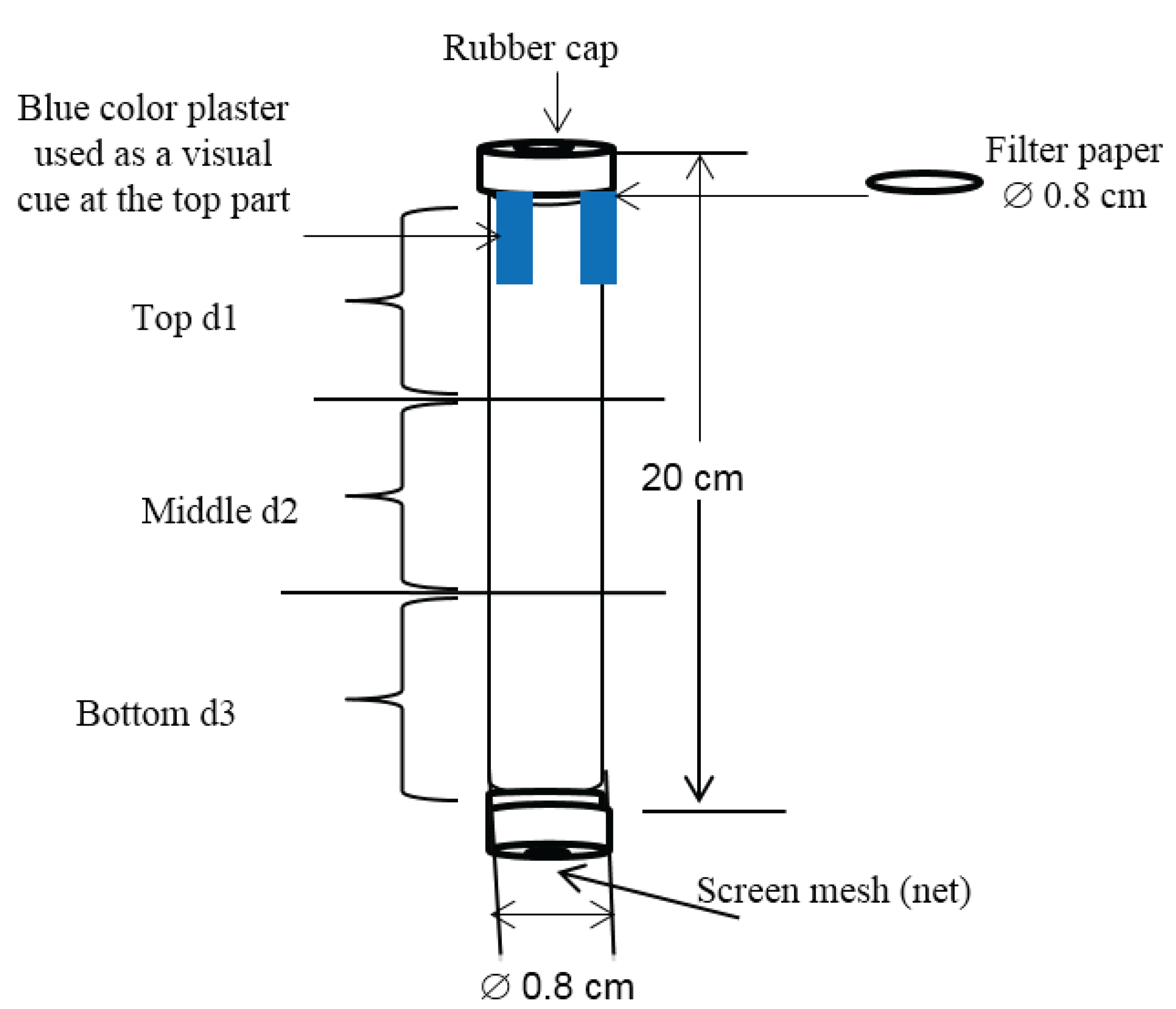
2.3. Data Collection
2.4. GC-MS Analysis
| No | Common Name | Scientific Name | Family | Extract Type, Plant Part Used | Supplier, Country |
|---|---|---|---|---|---|
| 1 | African blue basil | Ocimum kilimandscharicum | Lamiaceae | Essential oil, leaf | icipe—Bioprospecting unit, Kenya |
| 2 | Black pepper | Piper nigrum | Piperaceae | Essential oil, seed | IBMM 1, France |
| 3 | Ceylon cinnamomum | Cinnamomum zeylanicum | Lauraceae | Essential oil, inner bark | Nactis, France |
| 4 | Chinese cinnamomum | Cinnamomum cassia | Lauraceae | Essential oil, bark | Huiles & Sens, France |
| 5 | Citronella | Cymbopogon nardus | Poaceae | Essential oil, leaf | Burgess & Finch, South Africa |
| 6 | Conyza | Conyza newii | Asteraceae | Essential oil, leaf | icipe—Bioprospecting unit, Kenya |
| 7 | Coriander | Coriandrum sativum | Umbelliferae | Essential oil, seed | Fabster, France |
| 8 | Dill | Anethum graveolens | Apiaceae | Essential oil, seed | IBMM, France |
| 9 | Eucalyptus | Eucalyptus globulus | Myrtaceae | Essential oil, leaf | Huiles & Sens, France |
| 10 | Geranium | Pelargonium graveolens | Geraniaceae | Essential oil, leaf | IBMM, France |
| 11 | Ginger | Zingiber officinale | Zingiberaceae | Essential oil, root | Burgess & Finch, South Africa |
| 12 | Lemon | Citrus limon | Rutaceae | Essential oil, fruit | Capua, Italy |
| 13 | Lemon grass | Cymbopogon citratus | Poaceae | Essential oil, leaf | Burgess & finch, South Africa |
| 14 | Lemon savory | Satureja biflora | Lamiaceae | Essential oil, leaf | icipe—Bioprospecting unit, Kenya |
| 15 | Marjoram | Origanum majorana | Labiatae | Essential oil, leaf | Burgess & Finch, South Africa |
| 16 | May chang | Litsea cubeba | Lauraceae | Essential oil, fruit | IBMM, France |
| 17 | Myrrha | Commiphora myrrha | Burseraceae | Essential oil, oleoresin-gum | Burgess & Finch, South Africa |
| 18 | Neem | Melia azadirachta | Meliaceae | Vegetable oil, seed | Huiles & Sens, France |
| 19 | Pennyroyal | Mentha pulegium | Lamiaceae | Essential oil, leaf | IBMM, France |
| 20 | Rosemary | Rosmalinus officinalis | Lamiaceae | Organic floral water , leaf | Huiles & Sens, France |
| 21 | Savory | Satureja abyssinica | Lamiaceae | Essential oil, leaf | icipe—Bioprospecting unit, Kenya |
| 22 | Solidage | Solidago canadensis | Asteraceae | Essential oil, flower | Huiles & Sens, France |
| 23 | Thyme (wild) | Thymus satureioides | Lamiaceae | Essential oil, flower | Huiles & Sens, France |
| 24 | Thyme (common) | Thymus vulgaris | Lamiaceae | Essential oil, leaf | Burgess & Finch, South Africa |
2.5. Quantification of Terpenes
2.6. Data Analysis
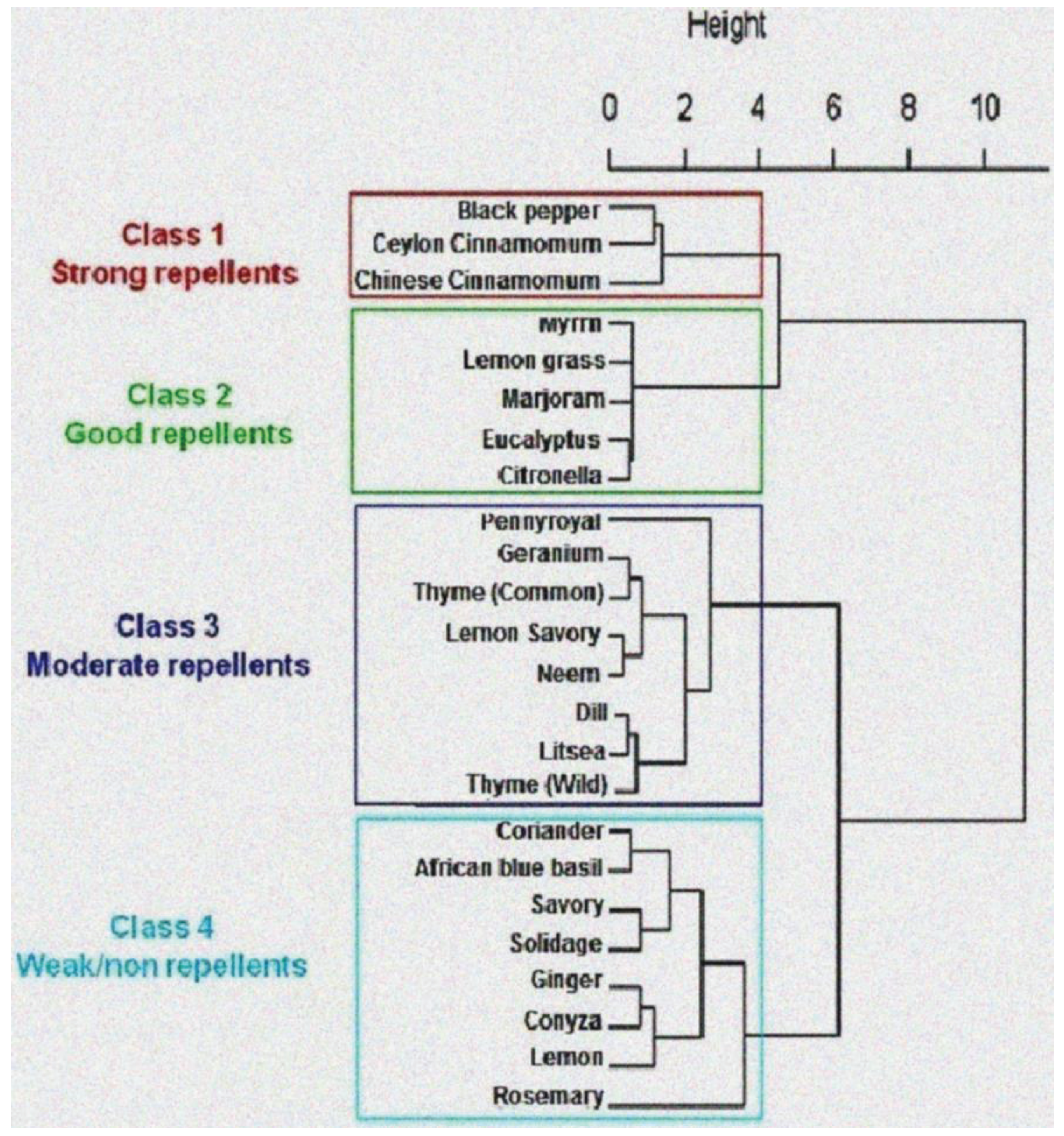
3. Results
3.1. Olfactometer Bioassays
| S.N | Extract | Concentration (%) | SE of Mean | |||
|---|---|---|---|---|---|---|
| 0 (control) | 0.01 | 0. 1 | 1 | |||
| 6.9 | ||||||
| 1 | Black pepper | 10.3 * | 11.3 * | 10.1 * | ±0.59 | |
| 2 | Ceylon Cinnamomum | 9.4 * | 9.7 * | 10.5 * | ±0.35 | |
| 3 | Chinese Cinnamomum | 9.5 * | 9.3 * | 11.8 * | ±0.41 | |
| 4 | Myrrh | 8.7 | 9.9 * | 9.9 * | ±0.38 | |
| 5 | Lemongrass | 9.2 * | 9.0 | 9.8 * | ±0.41 | |
| 6 | Marjoram | 8.9 | 8.9 | 9.2 * | ±0.43 NS | |
| 7 | Eucalyptus | 8.6 | 9.7 * | 9.5 * | ±0.46 | |
| 8 | Citronella | 8.4 | 9.2 | 9.8 * | ±0.43 | |
| 9 | Pennyroyal | 6.8 | 9.0 | 11.1 * | ±0.35 | |
| 10 | Geranium | 7.6 | 9.3 * | 9.8 * | ±0.36 | |
| 11 | Thyme (Common) | 7.9 | 10.1 * | 9.4 * | ±0.49 | |
| 12 | Lemon savory | 8.1 | 8.9 | 9.7 * | ±0.42 | |
| 13 | Neem | 7.7 | 8.8 | 9.6 * | ±0.28 | |
| 14 | Dill | 7.3 | 9.0 | 9.4 * | ±0.38 | |
| 15 | Litsea | 8.5 | 7.2 | 9.3 * | ±0.39 | |
| 16 | Thyme (wild) | 6.9 | 8.9 | 8.9 | ±0.43 | |
| 17 | Coriander | 8.5 | 8.3 | 9.3 * | ±0.36 | |
| 18 | African blue basil | 8.3 | 9.1 | 6.6 | ±0.36 | |
| 19 | Savory | 7.7 | 7.5 | 9.1 * | ±0.38 | |
| 20 | Solidago | 7.7 | 7.5 | 8.3 | ±0.34 NS | |
| 21 | Ginger | 7.6 | 9.3 * | 8.4 | ±0.37 | |
| 22 | Conyza | 7.4 | 8.6 | 8.5 | ±0.39 NS | |
| 23 | Lemon | 8.1 | 8.4 | 7.6 | ±0.45 NS | |
| 24 | Rosemary | 6.6 | 6.6 | 7.7 | ±0.38 NS | |
| SE of mean | ±0.43 | ±0.46 | ±0.48 | ±0.59 | ||
3.2. Chemical Characterization
4. Disscussion
| No | Name | Retention Time (min) | Types of Essential Oils and Relative Percentage (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P. nigrum | C. zeylanicum | C. cassia | C. myrrha | C. citratus | O. marjorana | E. globulus | |||
| 1 ◊ | α-Phellandrene | 9.69 | – | – | – | – | – | 1.34 | – |
| 2 * | α-Pinene | 9.83 | – | – | – | – | – | 0.74 | 1.71 |
| 3 * | Camphene | 10.21 | – | – | – | – | 1.44 | – | 0.02 |
| 4 ◊ | Sabinene | 10.68 | 0.08 | – | – | – | – | 8.95 | – |
| 5 * | Myrcene | 11.04 | – | – | – | – | 1.15 | 1.79 | – |
| 6 * | δ-3-Carene | 11.40 | 0.37 | – | – | 0.94 | – | – | – |
| 7 * | δ-2-Carene | 11.53 | – | – | – | 0.57 | – | 8.45 | – |
| 8 * | Limonene | 11.75 | 3.43 | – | – | 2.10 | 0.72 | 3.09 | 3.09 |
| 9 * | β-Pinene | 11.76 | – | – | – | – | – | 4.38 | – |
| 10 * | 1,8-Cineole | 11.80 | – | – | – | – | – | – | 93.43 |
| 11 * | (Z)-Ocimene | 11.91 | – | – | – | – | 0.40 | – | – |
| 12 ◊ | γ-Terpinene | 12.31 | – | – | – | – | – | 12.7 | 1.75 |
| 13 ◊ | Sabinene hydrate=cis-> | 12.47 | – | – | – | – | – | 4.26 | – |
| 14 ◊ | Terpinolene | 12.83 | – | – | – | – | – | 3.22 | – |
| 15 * | Linalool | 13.01 | – | – | – | – | 2.06 | – | – |
| 16 ◊ | Sabinene hydrate=trans-> | 13.03 | – | – | – | – | – | 18.59 | – |
| 17 ◊ | Menth-2-en-1-ol=cis-para-> | 13.39 | – | – | – | – | – | 1.58 | – |
| 18 ◊ | (E)-Isocitral | 14.33 | – | – | – | – | 2.78 | – | – |
| 19 * | Terpinen-4-ol | 14.33 | – | – | – | – | – | 20.79 | – |
| 20 * | α-Terpineol | 14.51 | – | – | – | – | 0.55 | 4.87 | – |
| 21 ◊ | Neral | 15.29 | – | – | – | – | 33.66 | – | – |
| 22 * | Linalool acetate | 15.41 | – | – | – | – | – | 2.29 | – |
| 23 * | Geraniol | 15.43 | – | – | – | – | 5.98 | – | – |
| 24 ◊ | Geranial | 15.72 | – | – | – | – | 38.32 | – | – |
| 25 * | (E)-Cinnamaldehyde | 15.79 | – | 79.6 | 76.7 | – | – | – | – |
| 26 ◊ | δ-Elemene | 16.64 | 7.0 | – | – | – | – | – | – |
| 27 ◊ | α-Cubebene | 16.82 | 1.00 | – | – | – | – | – | – |
| 28 ◊ | Geranyl propanoate | 17.18 | – | – | – | – | 6.4 | – | – |
| 29 ◊ | α-Copaene | 17.20 | 12.3 | 1.49 | 0.7 | – | – | – | – |
| 30 ◊ | β-Elemene | 17.40 | 2.5 | – | – | 13.52 | 1.16 | – | – |
| 31 * | β-Caryophyllene | 17.80 | 45.9 | – | – | – | 3.78 | 3.07 | – |
| 32 ◊ | Sesquithujene | 17.94 | 1.65 | – | – | – | – | – | – |
| 33 ◊ | (E)-Cinnamyl acetate | 18.05 | – | – | 0.74 | – | – | – | – |
| 34 * | α-Humulene | 18.25 | 3.05 | – | – | – | 0.95 | – | – |
| 35 * | allo- Aromadendrene | 18.34 | – | – | 0.55 | – | – | – | – |
| 36 ◊ | γ-Muurolene | 18.50 | – | 2.80 | – | – | – | – | – |
| 37 ◊ | β-Selinene | 18.68 | 1.73 | – | – | 7.60 | – | – | – |
| 38 ◊ | Curzerene | 18.74 | – | – | – | 75.27 | – | – | – |
| 39 ◊ | α-Muurolene | 18.79 | 1.76 | 0.54 | – | – | – | – | – |
| 40 ◊ | Bicyclogermacrene | 19.79 | – | – | – | – | – | 1.10 | – |
| 41 ◊ | γ- Cadinene | 18.99 | – | 0.7 | – | – | – | – | – |
| 42 ◊ | δ- Cadinene | 19.08 | 3.35 | – | – | – | – | – | – |
| 43 ◊ | trans- Cadina-1(6),4-diene | 19.08 | – | 13.24 | – | – | – | – | – |
| 44 ◊ | (E)-Methoxy cinnamaldehyde | 19.21 | – | – | 16.1 | – | – | – | – |
| 45 * | Caryophyllene oxide | 19.86 | 12.95 | – | – | – | – | – | – |
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, S.R.; Allen, D.J. Pests, diseases, resistance and protection in cowpea. In Advances in Legumes Science; Summerfield, R.J., Bunting, A.H., Eds.; Her Majestys Stationery Office: London, UK, 1980; pp. 419–443. [Google Scholar]
- Jackai, L.E.; Adalla, C.B. Pest management practices in cowpea: A review. In Advances in Cowpea Research; Singh, B.B., Raj, D.R.M., Dashiell, K.E., Jackai, L.E.N., Eds.; Sayce Publishing: Devon, UK, 1997; pp. 240–258. [Google Scholar]
- Ekesi, S.; Maniania, N.K.; Onu, I. Antibiosis and antixenosis of two cowpea varieties to the legume flower thrips, Megalurothrips sjostedti (Trybom) (Thysanoptera: Thripidae). J. Afr. Crop. Sci. 1998, 6, 49–59. [Google Scholar]
- Karungi, J.; Adipala, E.; Nampala, P.; Ogenga-Latigo, M.W.; Kyamanywa, S. Pest management in cowpea. Part 3. Quantifying the effect of field pests on grain yields in eastern Uganda. Crop. Prot. 2000, 19, 343–347. [Google Scholar] [CrossRef]
- Abudulai, M.; Salifu, A.B.; Haruna, M. Screening of cowpeas for resistance to the flower bud thrips, Megalurothrips sjostedti Trybom (Thysanoptera: Thripidae). J. Appl. Sci. 2006, 6, 1621–1624. [Google Scholar]
- Omo-Ikerodah, E.E.; Fatokun, C.A.; Fawol, I. Genetic analysis of resistance to flower bud thrips (Megalurothrips sjostedti) in cowpea (Vigna unguiculata [L.] Walp.). Euphytica 2009, 165, 145–154. [Google Scholar] [CrossRef]
- Oparaeke, A.M. The sensitivity of flower bud thrips, Megalurothrips sjostedti Trybom (Thysanoptera: Thripidae), on cowpea to three concentrations and spraying schedules of Piper guineense Schum. & Thonn. extracts. Plant Protect. Sci. 2006, 42, 106–111. [Google Scholar]
- Bediako, A. Inheritance of resistance to flower bud thrips (Megalurothrips sjostedti) in cowpea. M.Sc. Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2012. [Google Scholar]
- Muthomi, J.W.; Otieno, P.E.; Chemining’wa, G.N.; Nderitu, J.H.; Wagacha, J.M. Effect of chemical spray on insect pests and yield quality of food grain legumes. J. Entomol. 2008, 3, 156–163. [Google Scholar]
- Egho, E.O. Management of major field insect pests and yield of cowpea (Vigna unguiculata (L) Walp) under calendar and monitored application of synthetic chemicals in Asaba, Southern Nigeria. Am. J. Sci. Ind. Res. 2011, 4, 592–602. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.M.; Moore, J.S. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, R.W.; Jame, D.E.; de Kogel, W.J.; Teulon, D.A.J. Plant odours with potential for a push-pull strategy to control the onion thrips, Thrips tabaci. Entomol. Exp. Appl. 2006, 12, 69–76. [Google Scholar] [CrossRef]
- Agostini-Costa, T.S.; Vieira, R.F.; Bizzo, H.R.; Silveira, D.; Gimenes, M.A. Chromatography and its applications. In Plant secondary metabolites; Dhanarasu, S., Ed.; In Tech Publisher: Rijeka, Croatia, 2012; pp. 131–164. [Google Scholar]
- Plimmer, J.R. Regulatory problems associated with natural products and biopesticides. Pesticide Sci. 1993, 39, 103–108. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. 1997, 2, 25–34. [Google Scholar] [CrossRef]
- Koschier, E.H. Plant allelochemicals in thrips control strategies. In Naturally Occurring Bioactive Compounds; Series: Advances in Phytomedicine Vol. 3; Rai, M., Carpinella, C.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 221–249. [Google Scholar]
- Reitz, S.R.; Maiorino, G.; Olson, S.; Sprenkel, R.; Crescenzi, A.; Momol, M.T. Integrating plant essential oils and kaolin for the sustainable management of thrips and tomato spotted wilt on tomato. Plant Dis. 2008, 92, 878–886. [Google Scholar] [CrossRef]
- Picard, I.; Hollingsworth, G.R.; Salmieri, S.; Lacroix, M. Repellency of essential oils to Frankliniella occidentalis (Thysanoptera: Thripidae) as affected by type of oil and polymer release. J. Econ. Entom. 2012, 105, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Den Beider, E.; Elderson, J.; Agca, I. Is olfactory orientation of Thrips tabaci disrupted by a non-host plant? Proc. Exp. Appl. Entomol. N.E.V. Amsterdam 2001, 12, 61–64. [Google Scholar]
- Peterson, C.; Coats, J. Insect repellents—Past, present and future. Pestic. Outlook 2001, 12, 154–158. [Google Scholar] [CrossRef]
- Deletre, E.; Chandre, F.; Barkman, B.; Menut, C.; Martin, T. Naturally occurring bioactive compounds from four repellent essential oils against Bemisia tabaci whiteflies. Pest Manag. Sci. 2015. [Google Scholar] [CrossRef]
- Deletre, E.; Mallent, M.; Menut, C.; Chandre, F.; Martin, T. Behavioral response of Bemisia tabaci to 20 plant extracts. J. Econ. Entomol. 2015. [Google Scholar] [CrossRef]
- Teulon, D.A.; Penman, D.R.; Ramakers, P.M. Volatile chemicals for thrips (Thysanoptera: Thripidae) host finding and application for thrips pest management. J. Econ. Entomol. 1993, 86, 1405–1415. [Google Scholar] [CrossRef]
- Terry, L.I. Host selection, communication and reproductive behavior. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 435–475. [Google Scholar]
- Muvea, A.M.; Waiganjo, M.M.; Kutim, H.L.; Osiemo, Z.; Nyasani, J.O.; Subramanian, S. Attraction of pest thrips (Thysanoptera: Thripidae) infesting French beans to coloured sticky traps with Lurem-TR and its utility for monitoring thrips populations. Int. J. Trop. Insect Sci. 2014, 34, 197–206. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Matteson, N.A.; Terry, L.I. Response to color by male and female Frankliniella occidentalis during swarming and non-swarming behavior. Entomol. Exp. Appl. 1992, 63, 187–201. [Google Scholar] [CrossRef]
- Vernon, R.S.; Gillespie, D.R. Spectral responsiveness of Frankliniella occidentalis (Thysanoptera: Thripidae) determined by trap catches in greenhouses. Environ. Entomol. 1990, 19, 1229–1241. [Google Scholar] [CrossRef]
- Ebssa, L.; Borgemeister, C.; Berndt, O.; Poehling, H.M. Impact of entomopathogenic nematodes on different soil-dwelling stages of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), in the laboratory and under semi-field conditions. Biocontrol. Sci. Techn. 2001, 4, 515–525. [Google Scholar] [CrossRef]
- Holtmann, H. Untersuchungen zur Biologie der Getreide-Thysanopteren. Z. Angew. Entomol. 1962, 51, 285–299. [Google Scholar] [CrossRef]
- Pow, E.M.; Bennison, J.A.; Birkett, M.A.; Luszniak, M.L.; Manjunata, M.; Pickett, P.A.; Segers, I.S.; Wadhams, L.J.; Wardlow, L.R.; Woodcock, C.M. Behavioural responses of western flower thrips (Frankliniella occidentalis) to host plant volatiles. In Proceedings International Symposium on VI Thysanoptera, Akdeniz University, Antalya, Turkey, 27 April–1 May 1998; pp. 121–128.
- Koschier, E.H.; de Kogel, W.J.; Visser, J.H. Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. J. Chem. Ecol. 2000, 26, 2643–2655. [Google Scholar] [CrossRef]
- Koschier, E.H.; Sedy, K.A.; Novak, J. Influence of plant volatiles on feeding damage caused by the onion thrips Thrips tabaci. Crop. Prot. 2002, 21, 419–425. [Google Scholar] [CrossRef]
- Delétré, E.; Martin, T.; Campagne, P.; Bourguet, D.; Cadin, A.; Menut, C.; Bonafos, R.; Chandre, F. Repellent, irritant and toxic effects of 20 plant extracts on adults of the malaria vector Anopheles gambiae mosquito. PLoS ONE 2013, 12, e82103. [Google Scholar] [CrossRef] [PubMed]
- Samarasekera, R.; Kosmulalage, S.K.; Indira, S.W. Insecticidal activity of essential oils of ceylon Cinnamomum and Cymbopogon species against Musca domestica. J. Essent. Oil Res. 2006, 18, 352–354. [Google Scholar] [CrossRef]
- Ishii, T.; Matsuzawa, H.; Vairappan, C.S. Repellent activity of common spices against the rice weevil, Sitophilus zeamais Motsch (Coleoptera, Curculionidae). J. Trop. Biol. Conserv. 2010, 7, 75–80. [Google Scholar]
- Ratnasekera, D.; Rajapakse, R.H.S. Repellent properties of plant oil vapours on pulse beetle (Callasobruchus maculatus l.) (Coleoptera: Bruchidae) in stored green gram (Vigna radiata walp.). Trop. Agric. Res. Ext. 2009, 12, 13–16. [Google Scholar] [CrossRef]
- Prajapati, V.; Tripathi, A.; Aggarwal, K.; Khanuja, S.P. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresou. Technol. 2005, 96, 1749–1757. [Google Scholar]
- Chang, K.S.; Tak, J.H.; Kim, S.I.; Lee, W.J.; Ahn, Y.J. Repellency of Cinnamomum cassia bark compounds and cream containing cassia oil to Aedes aegypti (Diptera: Culicidae) under laboratory and indoor conditions. Pest Manag. Sci. 2006, 62, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Lwande, W.; Hassanali, A.; Mcdowell, P.G.; Moreka, L.; Nokoe, S.K.; Waterman, P.G. Constituents of Commiphora rostrata and some of their analogs as maize weevil, Sitophilus zeamais repellents. Insect Sci. Appl. 1992, 13, 679–683. [Google Scholar]
- Birkett, M.A.; Abassi, S.A.; Krober, T.; Chamberlain, K.; Hooper, A.M.; Guerin, P.M.; Pettersson, J.; Pickett, J.A.; Slade, R.; Wadhams, L.J. Antiectoparasitic activity of the gum resin, gum haggar, from the East African plant, Commiphora holtziana. Phytochemistry 2008, 69, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, T.; Jebanesan, A.; Govindarajan, M. Larvicidal, ovicidal and repellent activities of Cymbopogan citratus Stapf (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera: Culicidae). Trop. Biomed. 2006, 23, 208–212. [Google Scholar] [PubMed]
- Olivero-Verbel, J.; Nerio, L.S.; Stashenko, E.E. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag. Sci. 2010, 66, 664–668. [Google Scholar] [PubMed]
- Franz, A.R.; Knaak, N.; Fiuza, L.M. Toxic effects of essential plant oils in adult Sitophilus oryzae (Linnaeus) (Coleoptera, Curculionidae). Rev. Bras. Entomolo. São Paulo 2011, 55, 116–120. [Google Scholar] [CrossRef]
- Costa, A.V.; Pinheiro, P.F.; Rondelli, V.M.; de Queiroz, V.T.; Tuler, A.C.; Brito, K.B.; Stinguel, P.; Pratissoli, D. Cymbopogon citratus (Poaceae) essential oil on Frankliniella schultzei (Thysanoptera: Thripidae) and Myzus persicae (Hemiptera: Aphididae). Biosci. J. Uberlând. 2013, 29, 1840–1847. [Google Scholar]
- Yi, C.G.; Choi, B.R.; Park, H.M.; Park, C.G.; Ahn, Y.J. Fumigant toxicity of plant essential oils to Thrips palmi (Thysanoptera: Thripidae) and Orius strigicollis (Heteroptera: Anthocoridae). J. Econ. Entomol. 2006, 99, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Oparaeke, A.M.; Dike, M.C.; Amatobi, C.I. Botanical pesticide mixtures for insect pest management on cowpea, Vigna unguiculata (L.) walp plants–The legume flower bud thrips, Megalurothrips sjostedti Trybom. J. Sust. Agric. 2006, 29, 5–13. [Google Scholar] [CrossRef]
- Koschier, E.H.; Sedy, K.A. Labiate essential oils affecting host selection and acceptance of Thrips tabaci Lindeman. Crop. Prot. 2003, 22, 929–934. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Queiroz, T.V.; Rondelli, M.V.; Costa, V.A.; Marcelino, T.P.; Pratissoli, D. Insecticidal activity of citronella grass essential oil on Frankliniella schultzei and Myzus persicae. Ciênc. Agrotec. Lavras 2013, 37, 138–144. [Google Scholar] [CrossRef]
- Mayeku, W.P.; Omollo, N.I.; Odalo, O.J.; Hassanali, A. Chemical composition and mosquito repellency of essential oil of Conyza newii propagated in different geographical locations of Kenya. Med. Vet. Entomol. 2013, 28, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Katerinopoulos, H.E.; Georgia, P.; Athanasios, A.; Nicolaos, S.; Nikolaos, R. Composition and Insect Attracting Activity of the Essential Oil of Rosmarinus officinalis. J. Chem. Ecol. 2005, 31, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, H.; Belanger, A.; Bostanian, N.; Vincent, C.; Poliquin, A. Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J. Econ. Entomol. 2001, 94, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Lee, E.H.; Choi, B.R.; Park, H.M.; Ahn, Y.J. Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera: Aleyrodidae). J. Econ. Entomol. 2003, 96, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.F. Industrial use of Eucalyptus oil. In Proceedings, the Oil Mallee Profitable Landcare Seminar; Oil Mallee Association of Western Australia: Perth, Western Australia, Australia, 1999; pp. 43–54. [Google Scholar]
- Isman, M.B. Pesticides based on plant essential oils for management of plant pests and diseases. In Proceedings of the International Symposium on Development of Natural Pesticides from Forest Resources, Seoul, Korea, 8–10 October 2001; Forest Research Institute: Seoul, Korea, 2001; pp. 1–9. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abtew, A.; Subramanian, S.; Cheseto, X.; Kreiter, S.; Garzia, G.T.; Martin, T. Repellency of Plant Extracts against the Legume Flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae). Insects 2015, 6, 608-625. https://doi.org/10.3390/insects6030608
Abtew A, Subramanian S, Cheseto X, Kreiter S, Garzia GT, Martin T. Repellency of Plant Extracts against the Legume Flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae). Insects. 2015; 6(3):608-625. https://doi.org/10.3390/insects6030608
Chicago/Turabian StyleAbtew, Andnet, Sevgan Subramanian, Xavier Cheseto, Serge Kreiter, Giovanna Tropea Garzia, and Thibaud Martin. 2015. "Repellency of Plant Extracts against the Legume Flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae)" Insects 6, no. 3: 608-625. https://doi.org/10.3390/insects6030608
APA StyleAbtew, A., Subramanian, S., Cheseto, X., Kreiter, S., Garzia, G. T., & Martin, T. (2015). Repellency of Plant Extracts against the Legume Flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae). Insects, 6(3), 608-625. https://doi.org/10.3390/insects6030608





