Resistance is not Futile: It Shapes Insecticide Discovery
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
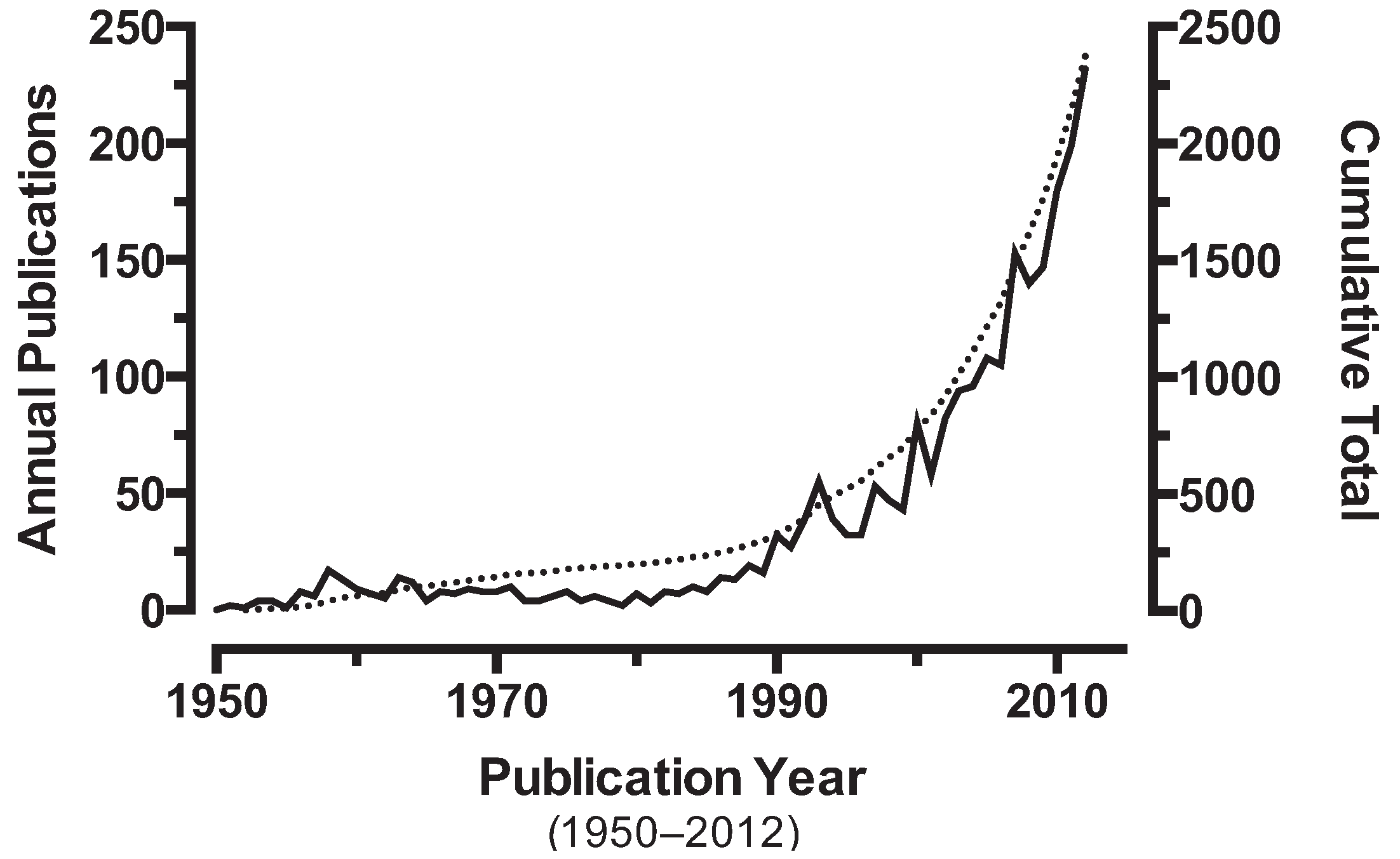
3.1. Insecticides in Context
| Organization | Standard | Year Enacted |
|---|---|---|
| European Union | Council Directive 67/548/EEC | 1967 |
| US Environmental Protection Agency | Federal Insecticide, Fungicide, and Rodenticide Act (FIRFA) | 1972 |
| World Health Organization | Recommended Classification of Pesticides by Hazard | 1975 |
| IOBC 1 | Pesticides Side-Effects Standards | 1985 |
| IRAC 2 | Mode of Action Classification Scheme | 2001 |
| United Nations | Globally Harmonized System of Classification and Labeling of Chemicals | 2002 |
| By Molecular Target | By IRAC Category |
|---|---|
| Acetylcholinesterase | 1 |
| Chloride Channels | 2, 6 |
| Sodium Channels | 3, 22 |
| Nicotinic Acetylcholine Receptors | 4, 5, 14 |
| Growth/Chitin Disruptors | 7, 10, 15, 16, 17, 18, 23 |
| Mitochondrial Complex Electron Transport Inhibitors | 12, 13, 20, 21, 24, 25 |
| Feeding Disruption | 9, 11 |
| Ryanodine Receptors | 28 |
| Octopamine Receptors | 19 |
| Miscellaneous or Unknown | 8, UN |
3.2. Insecticide Resistance and Cross Resistance

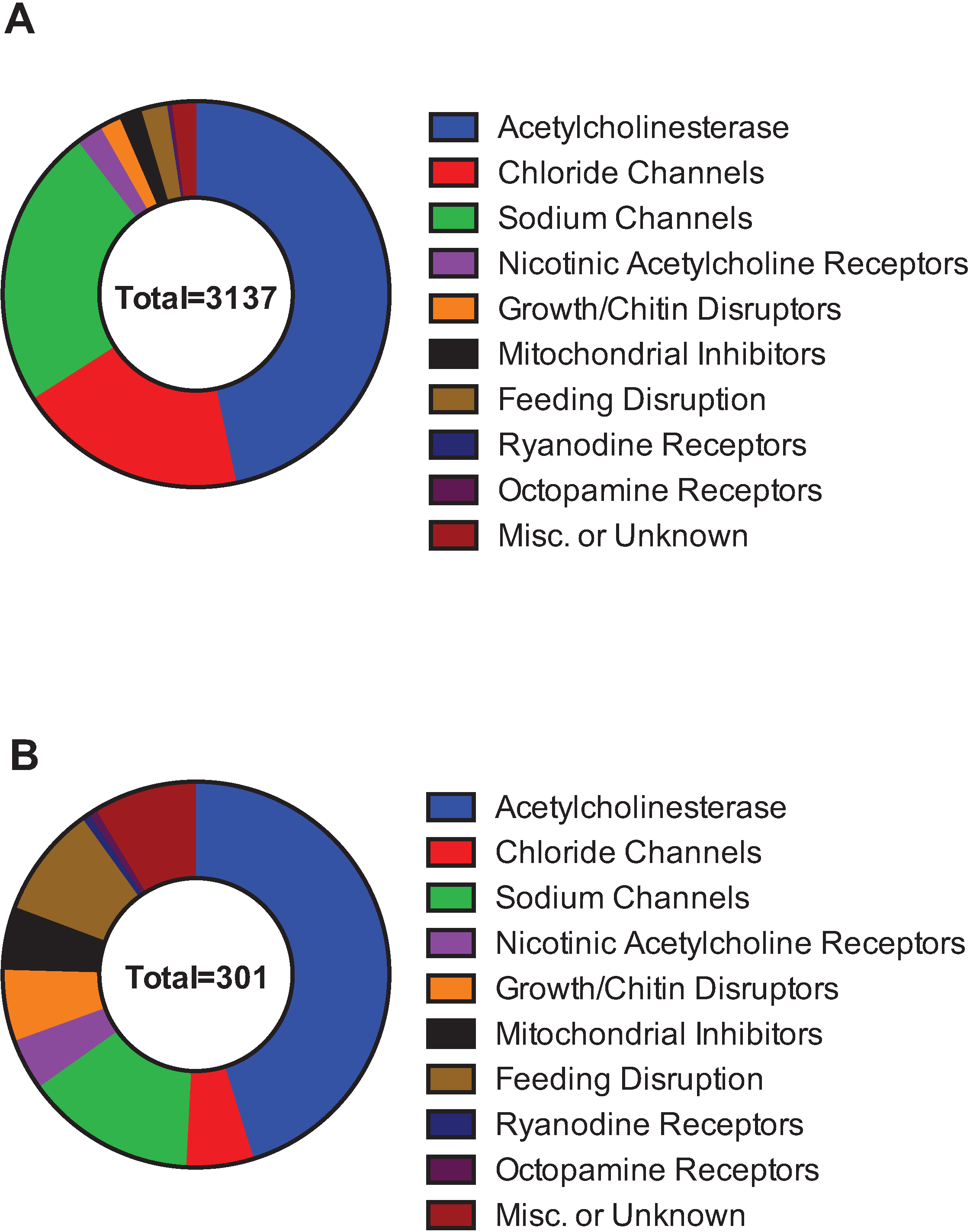
3.2.1. Human Health

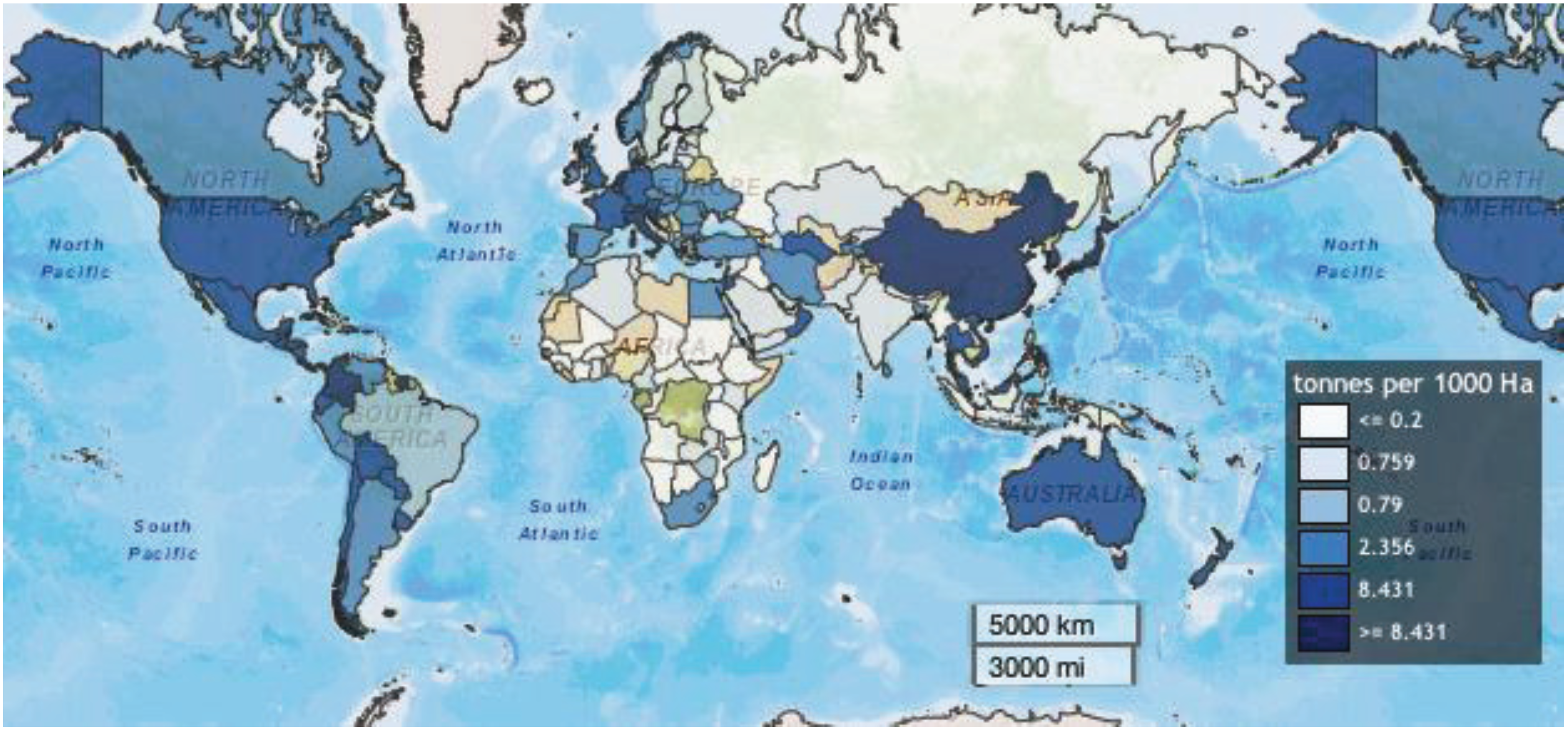
3.2.2. Global Food Supply


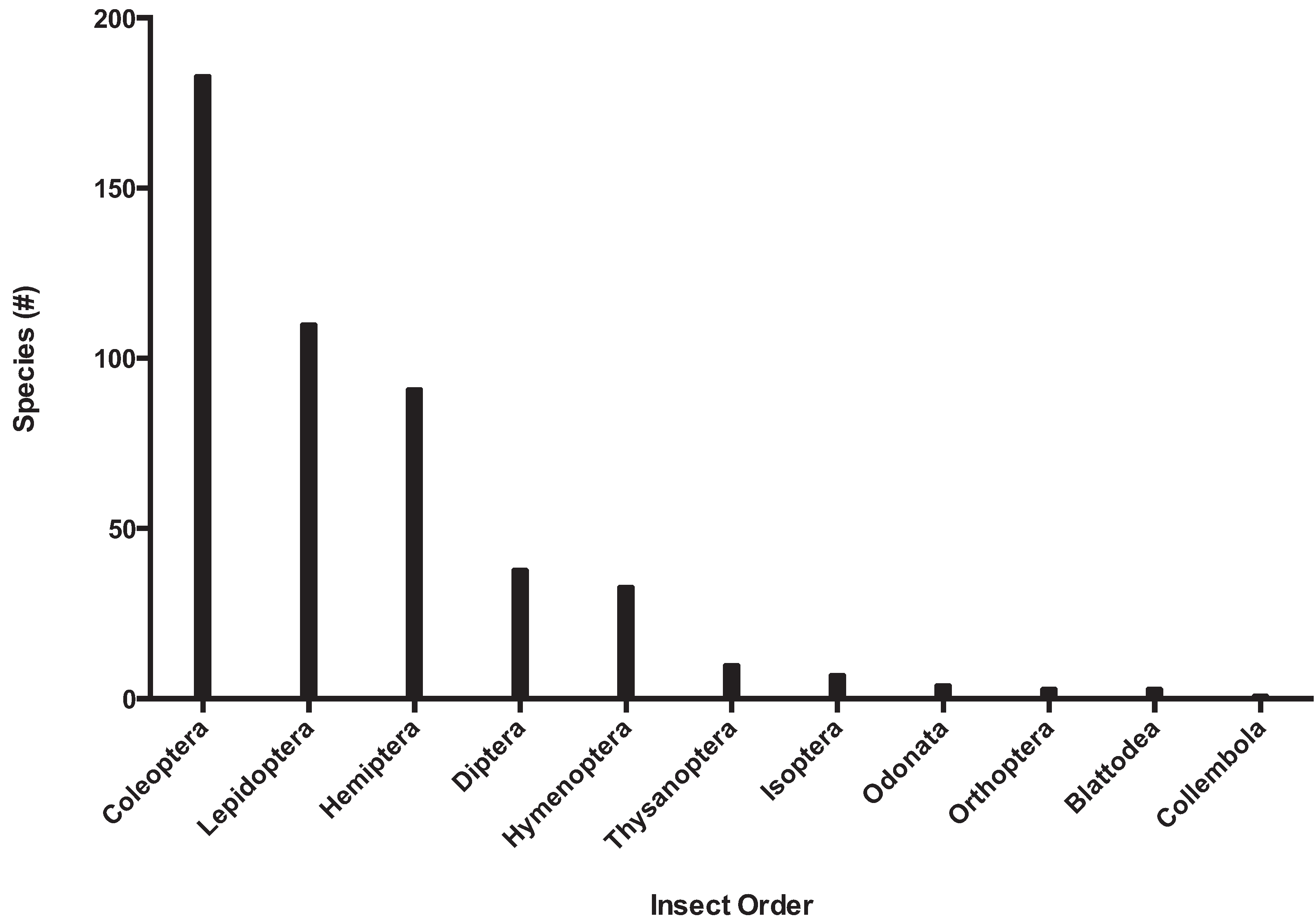
3.2.3. Biosecurity and Conservation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- WHO Global Malaria Programme. World Malaria Report; WHO: Geneva, Switzerland, 2012; pp. 1–88. [Google Scholar]
- OERKE, E.-C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. Bioscience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Hardy, M.C. Using selective insecticides in sustainable IPM. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 7. [Google Scholar]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Kennedy, G.G. Integration of Insect-Resistant Genetically Modified Crops within IPM Programs. In Integration of Insect-Resistant Genetically Modified Crops within IPM Programs; Romeis, J., Shelton, A.M., Kennedy, G.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 5, pp. 1–26. [Google Scholar]
- Romeis, J.; Bartsch, D.; Bigler, F.; Candolfi, M.P.; Gielkens, M.M.C.; Hartley, S.E.; Hellmich, R.L.; Huesing, J.E.; Jepson, P.C.; Layton, R.; Quemada, H.; Raybould, A.; Rose, R.I.; Schiemann, J.; Sears, M.K.; Shelton, A.M.; Sweet, J.; Vaituzis, Z.; Wolt, J.D. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotechnol. 2008, 26, 203–208. [Google Scholar] [CrossRef]
- Bradford, K.J.; Van Deynze, A.; Gutterson, N.; Parrott, W.; Strauss, S.H. Regulating transgenic crops sensibly: Lessons from plant breeding, biotechnology and genomics. Nat. Biotechnol. 2005, 23, 439–444. [Google Scholar] [CrossRef]
- Wise de Valdez, M.R.; Nimmo, D.; Betz, J.; Gong, H.-F.; James, A.A.; Alphey, L.; Black, W.C. Genetic elimination of dengue vector mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 4772–4775. [Google Scholar]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Dekkers, S.; Noordam, M.Y.; Hagens, W.I.; Bulder, A.S.; de Heer, C.; ten Voorde, S.E.C.G.; Wijnhoven, S.W.P.; Marvin, H.J.P.; Sips, A.J.A.M. Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharmacol. 2009, 53, 52–62. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Seder, A.; Sarwar, N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; Manoj, A.; Li, M.; Ruben, A.J.; Li, T.; Abraham, G.; Stafford, R.E.; Plummer, S.H.; Cynthia, S.; Novik, L.; Costner, P.J.M.; Mendoza, F.H.; Saunders, J.G.; Nason, C.; Richardson, J.H.; Davidson, S.A.; Richie, T.L.; Sutamihardja, A.; Fahle, G.A.; Kirsten, E.; Laurens, M.B.; Tewari, K.; Epstein, J.E.; Sim, B.K.L.; Ledgerwood, J.E. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.-C.; Miao, X.-X. Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci. 2013, 20, 15–30. [Google Scholar] [CrossRef]
- Louda, S.M.; Russell, F.L. Invasiveness of some biological control insects and adequacy of their ecological risk assessment and regulation. Conserv. Biol. 2003, 17, 73–82. [Google Scholar] [CrossRef]
- Drogui, P.; Lafrance, P. Pesticides and Sustainable Agriculture. In Farming for Food and Water Security; Lichtfouse, E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; Volume 10, pp. 23–55. [Google Scholar]
- McKenzie, J.A. The character or the variation: the genetic analysis of the insecticide-resistance phenotype. Bull. Entomol. Res. 2000, 90, 3–7. [Google Scholar] [CrossRef]
- ffrench-Constant, R.H. Which came first: insecticides or resistance? Trends Genet. 2007, 23, 1–4. [Google Scholar] [CrossRef]
- Toda, S.; Komazaki, S.; Tomita, T.; Kono, Y. Two amino acid substitutions in acetylcholinesterase associated with pirimicarb and organophosphorous insecticide resistance in the cotton aphid, Aphis gossypii Glover (Homoptera: Aphididae). Insect Mol. Biol. 2004, 13, 549–553. [Google Scholar] [CrossRef]
- ffrench-Constant, R.H.; Pittendrigh, B.; Vaughan, A.; Anthony, N. Why are there so few resistance-associated mutations in insecticide target genes? Philos. Trans. R. Soc. London B Biol. Sci. 1998, 353, 1685–1693. [Google Scholar] [CrossRef]
- Clark, J.K.; Scott, J.G.; Campos, F.; Bloomquist, J.R. Resistance to avermectins: extent, mechanisms, and management implications. Annu. Rev. Entomol. 1995, 40, 1–30. [Google Scholar] [CrossRef]
- Bloomquist, J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996, 41, 163–190. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007, 63, 628–633. [Google Scholar] [CrossRef]
- Enayati, A.; Hemingway, J. Malaria management: past, present, and future. Annu. Rev. Entomol. 2010, 55, 569–591. [Google Scholar] [CrossRef]
- Kelly-Hope, L.; Ranson, H.; Hemingway, J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect. Dis. 2008, 8, 387–389. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Brogdon, W.G.; McAllister, J.C. Insecticide resistance and vector control. Emerg. Infect. Dis. 1998, 4, 605–613. [Google Scholar] [CrossRef]
- Hoy, M.A. Myths, models and mitigation of resistance to pesticides. Philos. Trans. R. Soc. London B Biol. Sci. 1998, 353, 1787–1795. [Google Scholar] [CrossRef]
- Carrière, Y.; Ellers-Kirk, C.; Hartfield, K.; Larocque, G.; Degain, B.; Dutilleul, P.; Dennehy, T.J.; Marsh, S.E.; Crowder, D.W.; Li, X.; Ellsworth, P.C.; Naranjo, S.E.; Palumbo, J.C.; Fournier, A.; Antilla, L.; Tabashnik, B.E. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 775–780. [Google Scholar] [CrossRef]
- Bloomquist, J.R. Cyclodiene resistance at the insect GABA receptor/chloride channel complex confers broad cross resistance to convulsants and experimental phenylpyrazole insecticides. Arch. Insect Biochem. Physiol. 1994, 26, 69–79. [Google Scholar] [CrossRef]
- Martin, T.; Chandre, F.; Ochou, O.G.; Vaissayre, M.; Fournier, D. Pyrethroid resistance mechanisms in the cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) from West Africa. Pestic. Biochem. Physiol. 2002, 74, 17–26. [Google Scholar] [CrossRef]
- George, J.E.; Pound, J.M.; Davey, R.B. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 2004, 129, S353–S366. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- IRAC. Available online: http://www.irac-online.org/about/irac/ (accessed on 20 August 2013).
- Nauen, R.; Elbert, A.; Mccaffery, A.; Slater, R. Modern Crop Protection Compounds, Volumes 1-3, 2nd ed.; Krämer, W., Schirmer, U., Jeschke, P., Witschel, M., Eds.; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2012; pp. 935–955. [Google Scholar]
- IRAC. Available online: http://www.irac-online.org/eClassification/# (accessed on 2 July 2013).
- PubMed. Available online: http://www.ncbi.nlm.nih.gov/pubmed (accessed on 6 August 2013).
- Arthropod Pesticide Resistance Database. Available online: http://www.pesticideresistance.com/ (accessed on 2 July 2013).
- WHOPES. Available online: http://www.who.int/whopes/en/ (accessed on 20 August 2013).
- IR Mapper. Available online: http://www.irmapper.com/ (accessed on 2 July 2013).
- Invasive.org. Available online: http://www.invasive.org (accessed on 20 August 2013).
- FAOSTAT. Available online: http://faostat3.fao.org/ (accessed on 26 August 2013).
- Renear, A.; Palmer, C. Strategic reading, ontologies, and the future of scientific publishing. Science 2009, 325, 828–832. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitin synthesis inhibitors: old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Singh, N. Pesticide use and application: an Indian scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef]
- Enayati, A.; Hemingway, J. Malaria management: past, present, and future. Annu. Rev. Entomol. 2010, 55, 569–591. [Google Scholar] [CrossRef]
- Oduola, A.O.; Idowu, E.T.; Oyebola, M.K.; Adeogun, A.O.; Olojede, J.B.; Otubanjo, O.A.; Awolola, T.S. Evidence of carbamate resistance in urban populations of Anopheles gambiae s.s. mosquitoes resistant to DDT and deltamethrin insecticides in Lagos, South-Western Nigeria. Parasit. Vectors 2012, 5, 116. [Google Scholar] [CrossRef]
- Hemingway, J.; Vontas, J.; Poupardin, R.; Raman, J.; Lines, J.; Schwabe, C.; Matias, A.; Kleinschmidt, I. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc. Natl. Acad. Sci. USA 2013, 110, 9397–402. [Google Scholar] [CrossRef]
- Farenhorst, M.; Mouatcho, J.C.; Kikankie, C.K.; Brooke, B.D.; Hunt, R.H.; Thomas, M.B.; Koekemoer, L.L.; Knols, B.G.J.; Coetzee, M. Fungal infection counters insecticide resistance in African malaria mosquitoes. Proc. Natl. Acad. Sci. 2009, 106, 17443–17447. [Google Scholar] [CrossRef]
- Negrisoli, A.S.; Garcia, M.S.; Barbosa Negrisoli, C.R.C. Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) with registered insecticides for Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae) under laboratory conditions. Crop Prot. 2010, 29, 545–549. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E. Synergistic interaction between Beauveria bassiana- and Bacillus thuringiensis tenebrionis-based biopesticides applied against field populations of Colorado potato beetle larvae. J. Invertebr. Pathol. 2005, 90, 139–150. [Google Scholar] [CrossRef]
- Lu, D.; Pava-Ripoll, M.; Li, Z.; Wang, C. Insecticidal evaluation of Beauveria bassiana engineered to express a scorpion neurotoxin and a cuticle degrading protease. Appl. Microbiol. Biotechnol. 2008, 81, 515–522. [Google Scholar] [CrossRef]
- St. Leger, R.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 2010, 85, 901–907. [Google Scholar] [CrossRef]
- Wang, C.; St Leger, R.J. A scorpion neurotoxin increases the potency of a fungal insecticide. Nat. Biotechnol. 2007, 25, 1455–1456. [Google Scholar] [CrossRef]
- Fang, W.; Vega-Rodriguez, J.; Ghosh, A.K.; Jacobs-Lorena, M.; Kang, A.; St Leger, R.J. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 2011, 331, 1074–1077. [Google Scholar] [CrossRef]
- Fitches, E.C.; Pyati, P.; King, G.F.; Gatehouse, J.A. Fusion to snowdrop lectin magnifies the oral activity of insecticidal ω-Hexatoxin-Hv1a peptide by enabling its delivery to the central nervous system. PLoS One 2012, 7, e39389. [Google Scholar]
- Fitches, E.C.; Bell, H.A.; Powell, M.E.; Back, E.; Sargiotti, C.; Weaver, R.J.; Gatehouse, J.A. Insecticidal activity of scorpion toxin (ButaIT) and snowdrop lectin (GNA) containing fusion proteins towards pest species of different orders. Pest Manag. Sci. 2010, 66, 74–83. [Google Scholar] [CrossRef]
- Fitches, E.; Wiles, D.; Douglas, A.E.; Hinchliffe, G.; Audsley, N.; Gatehouse, J.A. The insecticidal activity of recombinant garlic lectins towards aphids. Insect Biochem. Mol. Biol. 2008, 38, 905–915. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: the challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Shaaya, E. Advanced Methods for Controlling. Insect Pests in Dry Food. In Advanced Technologies for Managing Insect Pests SE-14; Ishaaya, I., Palli, S.R., Horowitz, A.R., Eds.; Springer Netherlands: Dordrecht, Netherlands, 2013; pp. 279–294. [Google Scholar]
- Self, M.; Turner, J.A. Market access for New Zealand forest products: An economic and environmental case for development of alternative phytosanitary treatments. New Zeal. J. For. Sci. 2009, 39, 15–27. [Google Scholar]
- Horne, P.A.; Page, J.; Nicholson, C. When will integrated pest management strategies be adopted? Example of the development and implementation of integrated pest management strategies in cropping systems in Victoria. Aust. J. Exp. Agric. 2008, 48, 1601. [Google Scholar] [CrossRef]
- Gentz, M.C. A review of chemical control options for invasive social insects in island ecosystems. J. Appl. Entomol. 2009, 133, 229–235. [Google Scholar] [CrossRef]
- Brockerhoff, E.; Liebhold, A.; Richardson, B.; Suckling, D. Eradication of invasive forest insects: Concepts, methods, costs and benefits. New Zeal. J. For. Sci. 2010, S40, S117–S135. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef]
- Peck, S.L. Antibiotic and insecticide resistance modeling—Is it time to start talking? Trends Microbiol. 2001, 9, 286–292. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hardy, M.C. Resistance is not Futile: It Shapes Insecticide Discovery. Insects 2014, 5, 227-242. https://doi.org/10.3390/insects5010227
Hardy MC. Resistance is not Futile: It Shapes Insecticide Discovery. Insects. 2014; 5(1):227-242. https://doi.org/10.3390/insects5010227
Chicago/Turabian StyleHardy, Margaret C. 2014. "Resistance is not Futile: It Shapes Insecticide Discovery" Insects 5, no. 1: 227-242. https://doi.org/10.3390/insects5010227
APA StyleHardy, M. C. (2014). Resistance is not Futile: It Shapes Insecticide Discovery. Insects, 5(1), 227-242. https://doi.org/10.3390/insects5010227





