1. Introduction
The global expansion of urban areas reduces the extent of natural habitats, causing increasing fragmentation or replacement of natural areas by built environments. These changes affect both the availability and quality of habitats suitable for specific species [
1,
2,
3,
4]. As a result, some native species may become extinct from urban habitats due to heat or pollution stress or as a consequence of habitat management [
5,
6,
7]. Others expand their ranges into cities, responding to warmer microclimates [
8,
9] or tracking host plants absent from surrounding natural areas [
10]. Thus, urbanisation reshapes the distribution of plant and animal species across various scales, producing significant biogeographical effects—hosting isolated populations of some species while creating gaps in the occurrence of others [
11,
12].
In an urban landscape, habitat patches suitable for herbivorous insects are embedded within a matrix of buildings, roads and paved surfaces, covering up to 97% of the total area in downtown St. Petersburg [
13]. Knowledge of the spatial arrangement of these patches and the temporal dynamics of their occupancy by particular species is critical for understanding patterns of urban biodiversity and identifying the drivers behind them [
14]. However, long-term biogeographical studies in urban environments remain exceptionally rare. Most observational and experimental studies on within-city species distributions are restricted to a single year [
1,
15], leaving the temporal dynamics of local extinction and recolonisation poorly documented and insufficiently understood [
16].
These dynamic processes are especially important in the extremely small habitats in the urban matrix, such as enclosed courtyards—often only a few dozen square meters in size—found in historical city centres. The acute shortage of data on species occurrences in these habitat patches over multiple years hampers the application of metapopulation theory [
17] to describe how populations of species persist in a network of habitat patches within cities. Local populations with mutually independent within-patch dynamics may go extinct in some patches but can be recolonised from others, with connectivity, patch size, and habitat quality determining overall persistence, abundance, composition and distribution of biota under various urban development scenarios [
18]. This particularly concerns informal greenery—such as courtyard plantings and remnant natural patches—the extent and dynamics of which remain poorly documented despite growing appreciation of its importance for maintaining urban biodiversity [
19,
20]. This study, focussing on native birches and birch-mining
Eriocrania moths, seeks to address these research gaps.
Long-term datasets spanning several decades are widely regarded as the most effective for identifying factors that shape insect population dynamics [
21], yet many studies rely on much shorter time series [
22,
23] because extended monitoring records remain uncommon [
24]. Against this backdrop, our periodic surveys of
Eriocrania moths in St. Petersburg, Russia (1986–2025), represent an exceptionally rare dataset that combines nearly four decades of continuity with substantial spatial replication.
The earliest data on
Eriocrania distribution in St. Petersburg, Russia were collected in 1986 to classify urban areas by habitat suitability for these moths [
25]. A follow-up survey in 2000–2001 aimed to evaluate changes in distribution following urban expansion. Unexpectedly, comparison with the 1986 baseline revealed colonisation of habitat patches previously unoccupied by
Eriocrania—including in densely built downtown areas—rather than a decline in moth populations [
26]. However, drawing conclusions about the contraction of an urban distribution gap of
Eriocrania from only two time points proved problematic, as the observed patterns may have reflected natural cycles in habitat occupancy rather than a long-term directional trend.
Patch occupancy dynamics—a central concept in metapopulation theory [
17]—are closely tied to long-term fluctuations in species population density. For
Eriocrania, densities may vary 10- to 100-fold between consecutive years [
27,
28,
29], making detection of directional changes difficult without long-term datasets. To address this limitation, the study was expanded into a monitoring project, with annual surveys conducted from 2000 to 2012 and concluding surveys carried out in 2023–2025. In the final stage of the project, data on woody plant cover were collected from space photographs taken in 1984 and 2023. The overarching goal was to improve our understanding of how urbanisation drives gradual, cumulative changes in species distributions and ecological interactions.
Using data uniformly collected from 1986 to 2025 on habitat features, birch occupancy and abundance, and the occurrence and population density of Eriocrania leafminers, we tested the following hypotheses: (1) The quality of habitat patches suitable for birches and birch-feeding Eriocrania—quantified by the presence of undisturbed natural soil, artificial ground (asphalt, sand and gravel), and the woody plant (tree and shrub) cover—declines with proximity to the city centre and over time, reflecting habitat degradation with increasing urbanisation. (2) Both the proportion of birch-occupied patches and the number of birch trees per patch decline towards the city centre and over time. (3) The occurrence and density of Eriocrania vary among habitat patches containing birches, and their spatial and temporal variations can be predicted from patch characteristics. (4) The occurrence and population density of Eriocrania in birch-containing patches decline with proximity to the city centre and over time. In this way, we aimed to improve the current understanding of how progressing urbanisation influences the fine-scale biogeography of specialised insect herbivores and to identify the conditions that enable these insects to persist or collapse within urban environments.
2. Materials and Methods
2.1. Study Area
St. Petersburg, known as Leningrad from 1924 to 1991 (coordinates of city centre, the Palace Square: 59°56′ N, 30°19′ E), is the second largest city in Russia. It was established in 1703 A.D. on a previously uninhabited terrain covered by mires and boreal swamp forests. The study region exhibits a cool, humid continental climate with maritime influences. The mean temperature is −4.8 °C in January and 19.1 °C in July, and annual precipitation averages 660 mm. The frost-free period lasts approximately five months, while the summer season spans three to three and a half months. The mean annual temperature in St. Petersburg over the past decades was approximately 1.2 °C higher than in surrounding areas [
30] and increased from 5.7 °C to 6.7 °C between 1985 and 2024 [
31]. Despite this warming, birch leafing in 2018–2021 occurred in the same dates as in 1980–2009 [
32].
In the 1980s, the city’s administrative boundaries encompassed approximately 606 km
2, confining the urban footprint largely to its historical core and Soviet-era residential districts [
33]. From the mid-1980s to 2025 (the period covered by our study), St. Petersburg has experienced significant transformation, characterised by spatial expansion, demographic shifts, and evolving green infrastructure. New residential areas have emerged, expanding the city’s area to 1439 km
2 (
https://en.wikipedia.org/wiki/Saint_Petersburg; accessed on 19 December 2025). St. Petersburg’s official population has grown from approximately 4.9 million in 1986 to over 5.6 million by 2025 (
https://www.macrotrends.net/global-metrics/cities/22365/saint-petersburg/population; accessed on 19 May 2025). However, considering unregistered residents and labour migrants, the actual population was estimated to already have reached 7 million in 2019 (
https://www.sobaka.ru/city/society/138586; accessed on 19 May 2025).
During the late Soviet period, green infrastructure was centrally planned, resulting in numerous parks, public gardens and tree-lined streets. In 1986, when the study began, St. Petersburg maintained 14,922 hectares of green spaces, of which 4646 hectares were public [
34]. By the end of 1996, these values had increased to 18,569 and 5999 hectares, respectively [
35]. However, by 2022, public green spaces in the same area had shrunk to 3878 hectares (
https://iac.spb.ru/upload/medialibrary/023/0237165c7c33682451724a3a90588f3f.pdf; accessed on 19 December 2025), marking a reversal in the urban greenery development. Along with this reduction in area, the quality of green spaces in St. Petersburg suffered from fragmentation, pollution and overuse [
36].
2.2. Study System
White birches (
Betula pendula Roth and
B. pubescens Ehrhart) are deciduous, fast-growing, early successional broadleaved trees widespread across northern Eurasia. These iconic components of boreal forests, known for their distinctive white bark, serve as hosts to many herbivores, including eriocraniid moths—primarily
Eriocrania semipurpurella (Stephens) sensu lato and
E. sangii (Wood)—in northwestern Russia. The larvae of these small, metallic-coloured insects feed inside young birch leaves, forming large blotch mines (
Figure S1) that often lead to premature leaf abscission. These species can reach high population densities in certain years, both in natural forests [
29,
37] and urban plantings [
38], where they serve as a food source for birds and ants and support a diverse community of parasitic wasps [
28]. However,
E. semipurpurella and
E. sangii cannot be reliably distinguished on the basis of their vacated mines (
https://norfolkmoths.co.uk/micros.php; accessed on 4 December 2025).
Eriocrania prepupae and pupae spend nearly 11 months in the soil beneath their host trees [
39]; thus, soil quality and the absence of physical disturbance are critical for their survival. This plant–herbivore system provides a valuable model for ecological and environmental studies [
27,
28,
40].
2.3. Study Sites
The present study combines two data collection approaches that can conventionally be described as ecological and distributional. The first approach focused on understanding ecological processes and their drivers by selecting habitat patches replicated across space and studying the organisms within them. The second approach aimed to reveal spatial patterns across the urban landscape by dividing a representative part of it into a grid of uniform sampling units (cells hereafter), with no a priori knowledge of the occurrence of study objects in these cells, and surveying the selected groups of biota across this grid. These approaches are complementary, as the ecological method emphasises mechanisms, while the distributional method captures patterns. Their simultaneous use was intended to advance an understanding of how urban environments shape the occurrence of host plants (birches) and their specialist herbivores (Eriocrania) at low spatial scales.
An ecological approach was implemented by haphazardly selecting 173 habitat patches in 1986 to evenly represent varying levels of urbanisation (as quantified primarily by the extent of impervious cover) and different habitat types, including public parks, public gardens, roadsides, open courtyards, enclosed courtyards, and wastelands (
Figure S2). In 2000, we excluded 16 patches whose exact locations could not be reliably determined and merged patches situated 15–30 m apart. As a result, data were subsequently collected from 150 patches (
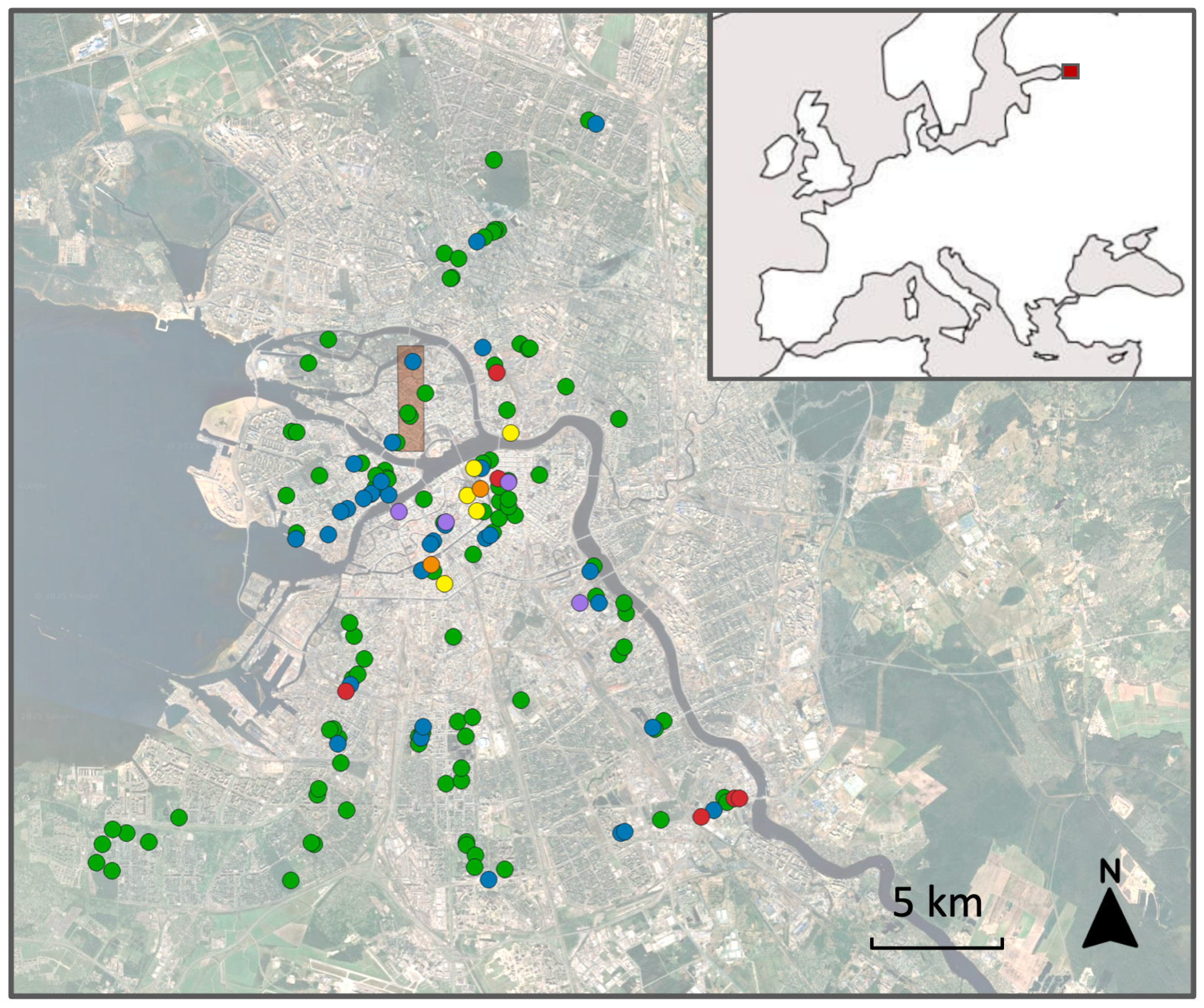
Figure 1;
Data S1). Seven of these patches were selected in historical public gardens that lacked birches in 1986 to assess whether birches would be planted later; the remaining 143 patches each contained one to more than 25 birches at that time.
A distributional approach was originally applied across four islands in the Neva River—Aptekarsky, Artillerysky, Petrogradsky and Zayachy—covering a total area of 8.75 km
2. Presence/absence data for birch and
Eriocrania were collected using a regular 160 × 160 m grid. In 1986, 379 grid cells, which covered an entire area of these islands, were surveyed. For feasibility reasons, surveys conducted in 2000, 2001, 2006–2012 and 2025 were each limited to 102 or fewer cells (
Figure 1;
Data S2), with the northeastern corner of the study area located at 59°58′43″ N, 30°18′39″ E, and the southwestern corner located at 59°56′58″ N, 30°17′38″ E (
Figure 1).
Following an approach widely used in urban ecology [
41,
42,
43], we used distance from the city centre as a proxy for the urbanisation gradient. Specifically, we measured, in Google Earth, the distance from each patch and grid cell to the urban centre of St. Petersburg (Palace Square). Distance from the urban core is correlated with multiple environmental factors associated with urbanization [
44] and, for St. Petersburg in particular, has been shown to relate to variation in vegetation cover and to characteristics of plant and insect populations [
2,
13].
2.4. Ground Surveys
All habitat patches—with a few exceptions due to logistical constraints—were surveyed from June to early July 1986 (by M.V.K.) and in 2000, 2012 (by M.V.K. and V.Z. in both years) and 2025 (mainly by A.A.E., with several patches assessed by M.V.K. and V.Z.). During each survey, we recorded all ground types present under birch canopies or, if birches were absent, at a representative site within the patch. The following ground types were distinguished: three natural or semi-natural types—undisturbed, loosened (e.g., by digging) and trampled (i.e., compacted by foot traffic)—and three artificial—asphalt, sand and gravel. In patches with clearly defined borders (e.g., enclosed courtyards), we counted all birch individuals, from juveniles over 50 cm tall to mature trees, whereas in patches with indefinite borders (e.g., in public parks), we categorised the size of surveyed birch tree groups as small (1–3 trees), medium (4–10 trees) or large (>10 trees). We also estimated the heights of the birch trees (minimal to maximal, if more than one birch was present in a patch), birch species (in 2025 only) and the density of Eriocrania mines. In some sites, mine density (but not patch features) was also recorded in 1990 (by M.V.K.), 2001–2011 (by M.V.K. and V.Z. in all years), 2023 (by M.V.K.) and 2024 (by M.V.K. and A.A.E.).
In the lower parts of birch crowns (up to 5 m above the ground), mines were searched for visually, bending down high branches whenever possible. If no mines were found there, the upper parts of birch crowns were examined using 8× binoculars (until 2012) or 25× optical magnification with a Canon PowerShot SX620 HS camera, Tokyo, Japan (in 2025). The Eriocrania population density was classified as ‘low’ if it took more than 1 min to find the first mine. If the first mine was found within 1 min, the search continued. If a second mine was also found within the next minute, the density was classified as ‘high’; otherwise, it remained ‘low’. The conclusion ‘no mines found’ was based on the following search durations: one large birch tree (10+ m high): 2–3 min; group of 2–5 birches: 5 min; group of 6–15 birches: 8–10 min; group of 16+ birches: 12–15 min. If a detailed inspection of the birch crown was not possible—due to restricted site access, for example—the data on leafminer occurrence were considered missing.
Data collection in grid cells followed a different protocol. We differentiated between cells ‘not visited’ in a given year and cells containing only birches that were ‘not accessed’ to check for the presence of mines. Cells without a single birch were marked as ‘unsuitable’ (for Eriocrania), while those containing birches were classified as ‘suitable’ and further categorised as ‘occupied’ if at least one Eriocrania mine was recorded or ‘vacant’ if no Eriocrania mines were found.
2.5. Remote Sensing
We used high-resolution satellite imagery for 1984 and 2023 to quantify the percentage of tree and shrub cover within a 30 m radius around habitat patch centres. For 1984, we complemented the satellite data with historical black-and-white aerial imagery obtained from the
www.retromap.ru (accessed on 1 September 2025), with a ground resolution of ~1.2 m/pixel. This dataset allowed us to capture fine-scale vegetation patterns not discernible in coarser satellite products available for that period. For 2023, we relied on very-high-resolution imagery from Google Earth (© Maxar Technologies, Westminster, CO, USA), with a native panchromatic resolution of ~0.3 m/pixel.
All imagery was first orthorectified and georeferenced to WGS84/UTM using stable control points identifiable in both periods (e.g., road intersections, isolated buildings). Within each 30 m buffer, tree and shrub cover was mapped by manually digitizing polygons corresponding to woody vegetation. Because classification relied exclusively on visual interpretation, polygons were delineated only from homogeneous patches that were visually unambiguous in both periods. To ensure consistency, the same annotator (E.V.-C.) produced all polygons following an internally standardised protocol defining minimum mapping units and class criteria.
We defined woody vegetation as any ligneous plant (tree or shrub) with a closed canopy visible from nadir. Individual crowns or crown clusters were included if their visible canopy diameter exceeded 1 m, reflecting the minimum resolvable object size across datasets. In cases where pixels or small patches contained mixed vegetation (e.g., shrubs interspersed with herbaceous cover), classification followed a majority rule: the polygons were assigned to the woody-vegetation class only if at least 50% of their area corresponded to woody canopy. Pixels for which woody cover could not be reliably assessed in the 1984 imagery were excluded; consequently, analyses were restricted to the 92 patches for which woody-cover delineation was feasible in both periods.
To assess classification consistency, 15% of the polygons (randomly selected) were re-examined by the same observer who performed the original delineation. This qualitative check showed generally high consistency, with discrepancies primarily linked to shadowed areas and heterogeneous shrub–grass mixtures in the 1984 imagery.
2.6. Data Analysis
Presence–absence data on ground types (Hypothesis 1), birches (Hypothesis 2) and
Eriocrania (Hypothesis 3) were analysed using Generalised Linear Mixed Models (GLMMs) implemented in the SAS 9.2 GLIMMIX procedure with type III sums of squares. A binomial error distribution with a logit link function was applied. For categorical data on birch group size (small, medium, large; Hypothesis 2) and
Eriocrania density (zero, low, high; Hypothesis 3), a multinomial error distribution with a cumulative logit (cumlogit) link function was used. All models included year as a fixed effect and patch or cell identifier as a random effect. The significance of random effects was evaluated using likelihood ratio tests [
45].
If GLMMs failed to converge or if the properties of the data did not allow their use, class-level differences were assessed using the non-parametric Kruskal–Wallis test (SAS NPAR1WAY procedure). Spatial and temporal trends in continuous variables (Hypotheses 1, 2 and 4) were examined using either linear or quadratic regression models (SAS REG procedure) or by Pearson correlation coefficients based on either original or standardised values of the study variables, or by point-biserial correlation (SAS CORR procedure). Associations between nominal variables (Hypotheses 1 to 4) were analysed with chi-square tests (SAS FREQ procedure). Differences in woody plant cover between 1984 and 2023 (Hypothesis 1) were compared using paired t-tests (SAS TTEST procedure [
45]).
4. Discussion
4.1. Observed Patterns and Alignment with Hypotheses
Birch presence, abundance, and ground quality declined toward the city centre and over time, supporting Hypothesis 1, whereas woody plant cover showed no clear spatial or temporal trend, contrary to this hypothesis. Consistent with Hypothesis 2, the proportion of patches containing birches decreased over time but increased with distance from the city centre, resulting in higher occupancy in peripheral areas. Support for Hypothesis 3 was mixed: Eriocrania occurrence within birch-containing patches was influenced by habitat type, artificial ground, and birch abundance, but these factors only moderately predicted local extinction risk. Distance from the city centre and year had no consistent effect on Eriocrania occurrence, providing no support for Hypothesis 4.
4.2. Soil Quality
Our long-term study reveals that multiple interacting environmental factors influence the dynamics of the birch–
Eriocrania interaction in urban landscapes. Among these, ground quality emerges as a potentially important—but often overlooked—component. Soil quality is especially critical for insects such as
Eriocrania, as their larvae—in common with many insect species—burrow into the soil and construct protective chambers lined with silk impregnated with soil particles [
46]. The larvae of
Eriocrania remain in the soil for an extended period—around 11 months—before emerging as adults [
39]. However, the relative importance of specific soil characteristics—such as type, texture, density, moisture and pH—for the survival of insects pupating in soil has only been explored in a limited number of species [
46,
47,
48,
49], and
Eriocrania is not among them. Our observations on the relationship between the ground quality beneath birch canopies and the occurrence and density of
Eriocrania mines in a patch provide preliminary insights into this issue.
Since Eriocrania larvae that drop from leaf mines cannot dig into asphalt or heavily compacted soil, they must crawl in search of a patch of undisturbed ground. These patches may lie several metres away from birch canopy projection, and the increased distance reduces larval survival due to desiccation and predation risk. This likely explains why Eriocrania mines were observed twice as frequently in habitat patches with natural undisturbed soil under birches compared to patches without it.
The occurrence of Eriocrania in patches lacking undisturbed soil may result from the migration of adults emerging in nearby habitat patches that offer a better environment for Eriocrania. As long as these migrants seem not to reliably discriminate between habitats with different soil suitability for safe overwintering, patches lacking undisturbed natural soil may act as death traps for the progenies of dispersing Eriocrania females. Alternatively, the occurrence of Eriocrania in these patches may indicate that larvae occasionally survive in seemingly unsuitable microsites, such as cracks in asphalt.
Soil quality for biota generally declines along urbanisation gradients due to disturbance (e.g., mowing, tilling), pollution, sealing and compaction [
50,
51,
52], and our data from St. Petersburg are consistent with these patterns. Specifically, increases in soil sealing towards the city centre and from 1986 to 2025 support our Hypothesis 1, namely, that the soil quality for the birch–
Eriocrania system declines both with proximity to the city centre and with the observation year, even though land management, in some patches, has resulted in the appearance of undisturbed soils where none previously existed.
Nonetheless, the modest decline in undisturbed soil occurrence does not appear to have had a fatal or severe impact on our study organisms, although it tended to increase the probability of birch extinction, in line with earlier studies that demonstrated adverse effects of soil sealing on tree vitality [
53,
54]. However, over a 40-year period, only six of 150 patches showed complete soil sealing that rendered habitats entirely unsuitable for birches and
Eriocrania. Therefore, we conclude that while changes in soil quality are important, their influence is weaker on
Eriocrania distribution and abundance in urban habitats than the effects of changes in birch populations.
4.3. Birch Population Dynamics
The proportion of birch-occupied patches and the number of birch trees per patch both declined with proximity to the city centre and over time. In contrast, woody plant cover showed no spatial or temporal changes. This discrepancy suggests that the availability of birches for specialised insect herbivores is shaped by factors distinct from those regulating overall urban greenery, namely, social and historical influences [
55].
The tree species composition in the green areas of St. Petersburg has undergone significant changes over decades and centuries. During the 1950s–1960s, poplars (
Populus spp.) were planted most intensively, and by the start of our study in the 1980s, they had become the most abundant tree species in the city [
56]. In the 1970s–1980s, limes (
Tilia spp.) accounted for 25% and maples (
Acer spp.) for 17% of all seedlings cultivated in city nurseries, whereas birches (
Betula spp.) comprised only about 5% [
57]. However, by the mid-1990s, the proportion of birches among newly planted trees increased to 25–30% [
26]. This trend is corroborated by our data showing a doubling in the proportion of grid cells that have a birch presence in the Petrogradsky district of St. Petersburg between 1986 and 2000 (
Figure 4).
A subsequent decrease in the number of birch-occupied cells aligns with previous findings of a temporal shift in birch population trends in St. Petersburg—from an increase in 1986–2000 to a decline over the past two decades [
13]. However, this decline did not reduce birch occurrence in the Petrogradsky district to the level observed in 1986. Contrary to our Hypothesis 2, grid-cell data indicate an overall increase in birch occurrence over the past 40 years (
Figure 4). This conclusion may be spatially limited, as our observations revealed local extinctions in 23% of the patches in which birches had been present in 1986 (
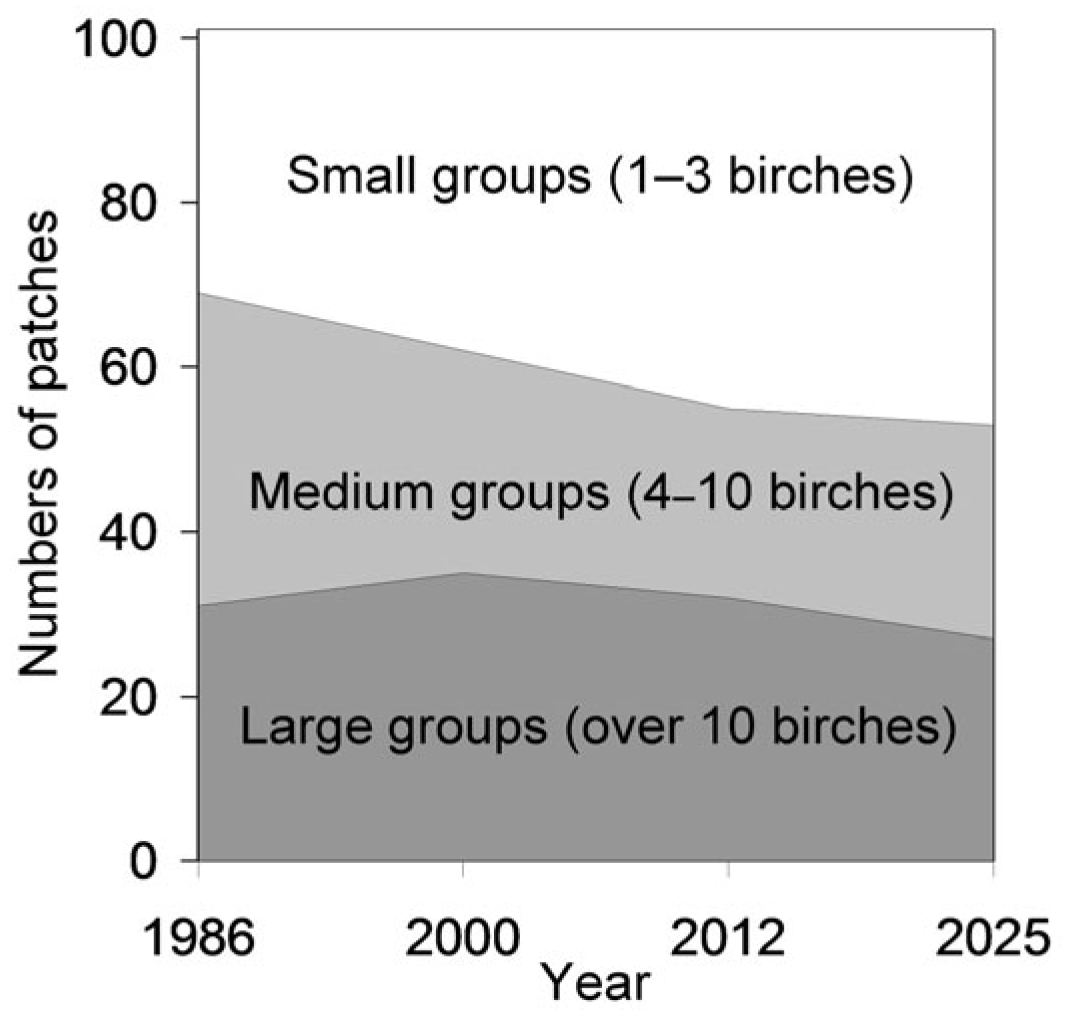
Figure 1). This trend is further reflected in the growing proportion of small birch groups (1–3 individuals), which increased from 32% in 1986 to 48% in 2025 (
Figure 2). The concurrent decline in both the number and size of birch-occupied patches suggests an increase in population fragmentation, which may reduce connectivity among birch groups [
57,
58] and compromise dispersal corridors for birch-feeding insects.
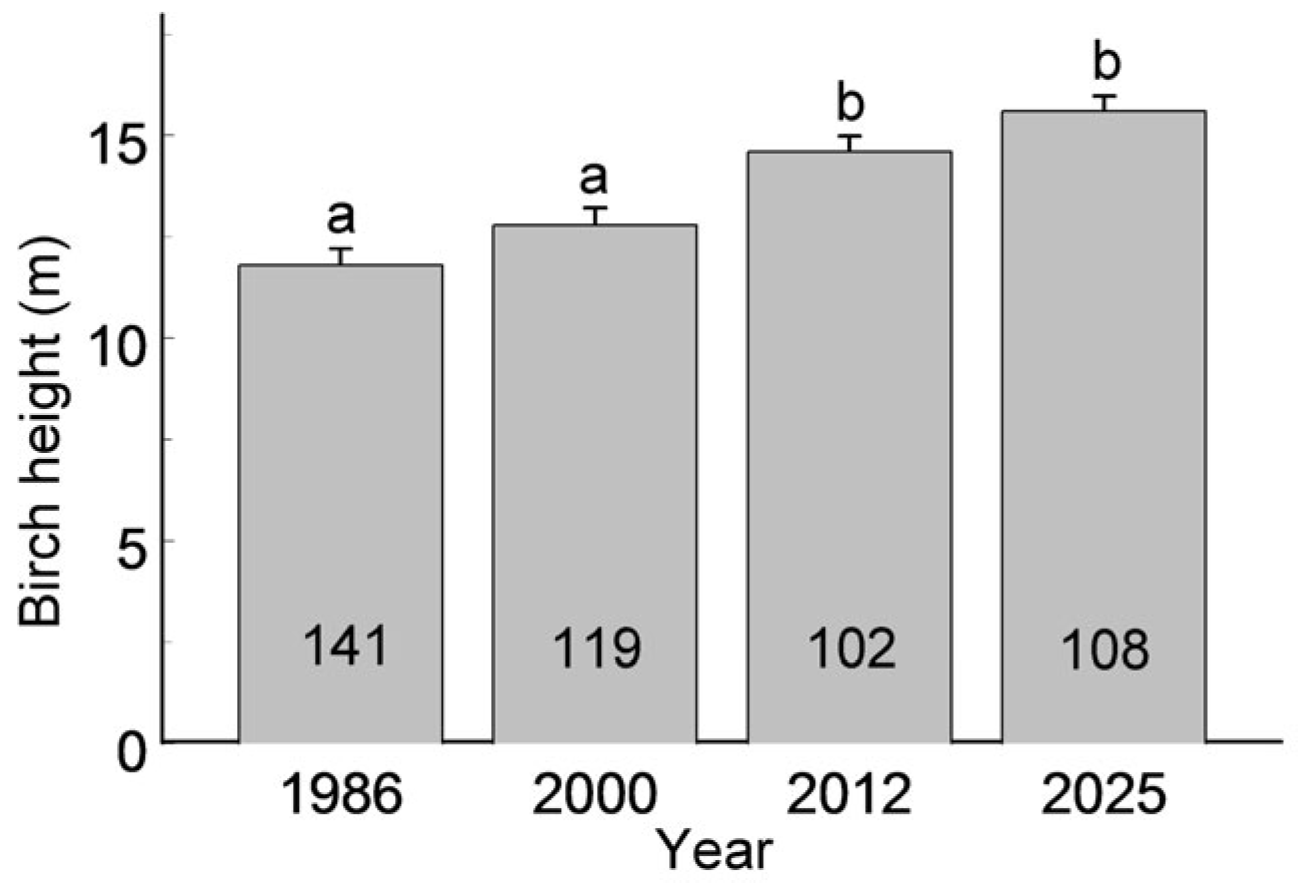
An increase in birch height (
Figure 3), which corresponds to a 50% rise in foliar biomass per average tree (based on equations provided in [
59]), may currently offset the decline in birch abundance, thereby maintaining relatively stable food resources for
Eriocrania. Specifically, in the historic city centre, smaller numbers of birches are offset by taller trees with higher foliar biomass, whereas in peripheral patches, birch numbers remain higher but biomass per tree shows more variable trends due to differences in recruitment and local management practices. Thus, our findings offer only partial support for Hypothesis 2. While birch occurrence declines towards the city centre, temporal trends are more nuanced and depend on the response variable (tree number vs. foliar biomass) and study approach (ecological vs. distributional). This discrepancy further challenges the reliability of using space-for-time substitution to predict temporal changes in ecosystem structure and functions [
60], particularly in urban ecological contexts [
13].
The continuous increase in average birch height across the city (
Figure 3) points to a lack of recruitment—either via planting or natural regeneration—and supports earlier findings on the ageing of birch populations in St. Petersburg [
13]. This trend suggests that the observed mismatch between spatial and temporal patterns in birch occurrence along the urbanisation gradient may soon be resolved through a rapid decline in birches in urban habitats. Birches typically begin to develop heartwood discolouration and rot at 60–70 years of age, which reduces their vitality and increases their vulnerability to windthrow [
61]. Unless tree planting is substantially increased in the coming years—as outlined in the St. Petersburg government’s plan to achieve 30% green coverage of the metropolitan area by 2030 (
https://gorod-plus.tv/news/141712; accessed on 19 December 2025)—a large-scale birch decline is likely, with significant implications for the composition of the urban tree community and the persistence of birch-associated biota.
4.4. Eriocrania Population Dynamics
The spatial population structure of any species depends on both the distribution of its habitat and the species’ ability to traverse the distances separating habitat patches [
62,
63]. Consistent with the classical metapopulation model [
17], we observed a dynamic balance of colonisation and extinction events for
Eriocrania among discrete habitat patches. Contrary to expectations rooted in the generally adverse effects of urbanisation on biota [
64,
65], none of the suitable patches—defined as those containing birches—remained persistently unoccupied by
Eriocrania for over a decade, even when the patch was limited to a single birch tree in the historic city centre.
The birch population density per unit area (including impervious surfaces) in downtown St. Petersburg averages 14% of that in the uptown [
13]. As a result, fine-scale dot maps based on single-year data [
25,
26] may suggest gaps in the distribution of both birches and
Eriocrania. However, as the observation period extends, the number of patches that have ever been colonised increases, challenging these interpretations. Given that the nearest neighbour distance between birch-inhabited patches in the downtown area rarely exceeds 150 m (M.V.K., pers. obs.), and that
Eriocrania occupancy is unrelated to proximity to the city centre, we conclude that the urban core does not represent a true lacuna in the
Eriocrania distribution within the St. Petersburg metropolitan area.
Likewise, no patch—except for large public parks—was continuously occupied by
Eriocrania throughout the study period. This suggests that local recruitment often fails to offset mortality and implies that
Eriocrania populations in small habitat patches function as demographic sinks (sensu [
66]). Thus, the key remaining questions are which populations act as demographic sources and how far they are from these sinks.
Most parks with substantial birch populations are located more than 5 km from the city centre, meaning that sink populations in the downtown areas are separated from potential sources by several kilometres of densely built terrain. The dispersal capacity of
Eriocrania females is unknown, but that of the similarly sized leafminer
Tischeria ekebladella (Bjerkander) does not exceed 100 m [
63]. Thus, direct colonisation of vacant patches in the urban core by
Eriocrania females emerging from distant sources is improbable, even during peak population years. Therefore, we suggest that colonisation involves multigenerational spread via stepping stones, as described by [
67], and that individual patches may act as either sinks or sources in different years.
Unsurprisingly, the occupancy and population density of
Eriocrania varied among both habitat patches and grid cells, supporting Hypothesis 3. Although our data confirm the island biogeography theory prediction [
68] that a larger patch size—as measured by birch abundance—increases the likelihood of
Eriocrania occupancy, the intercorrelations among patch characteristics hinder the identification of a primary driver of this occupancy based on observational data alone.
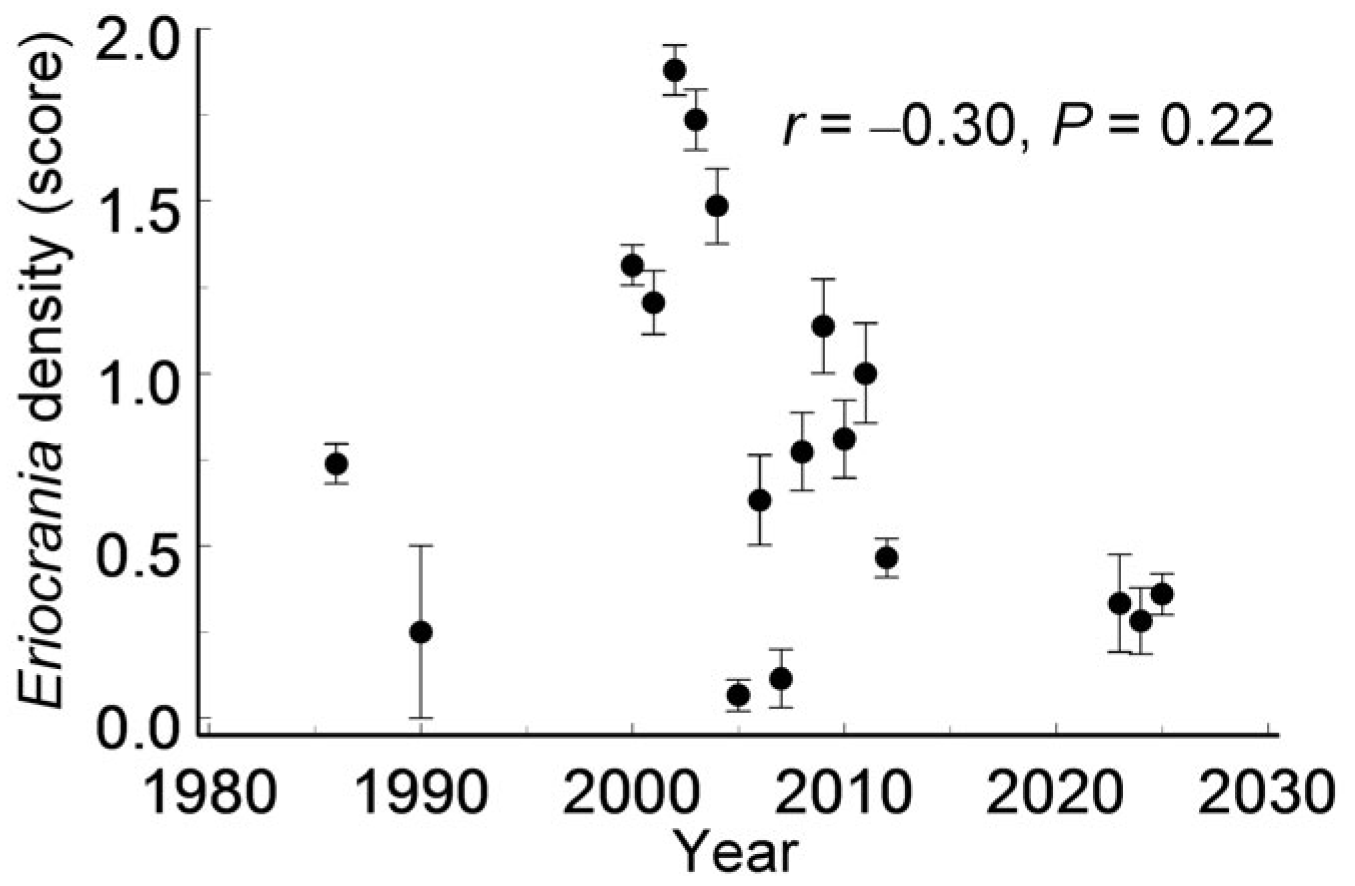
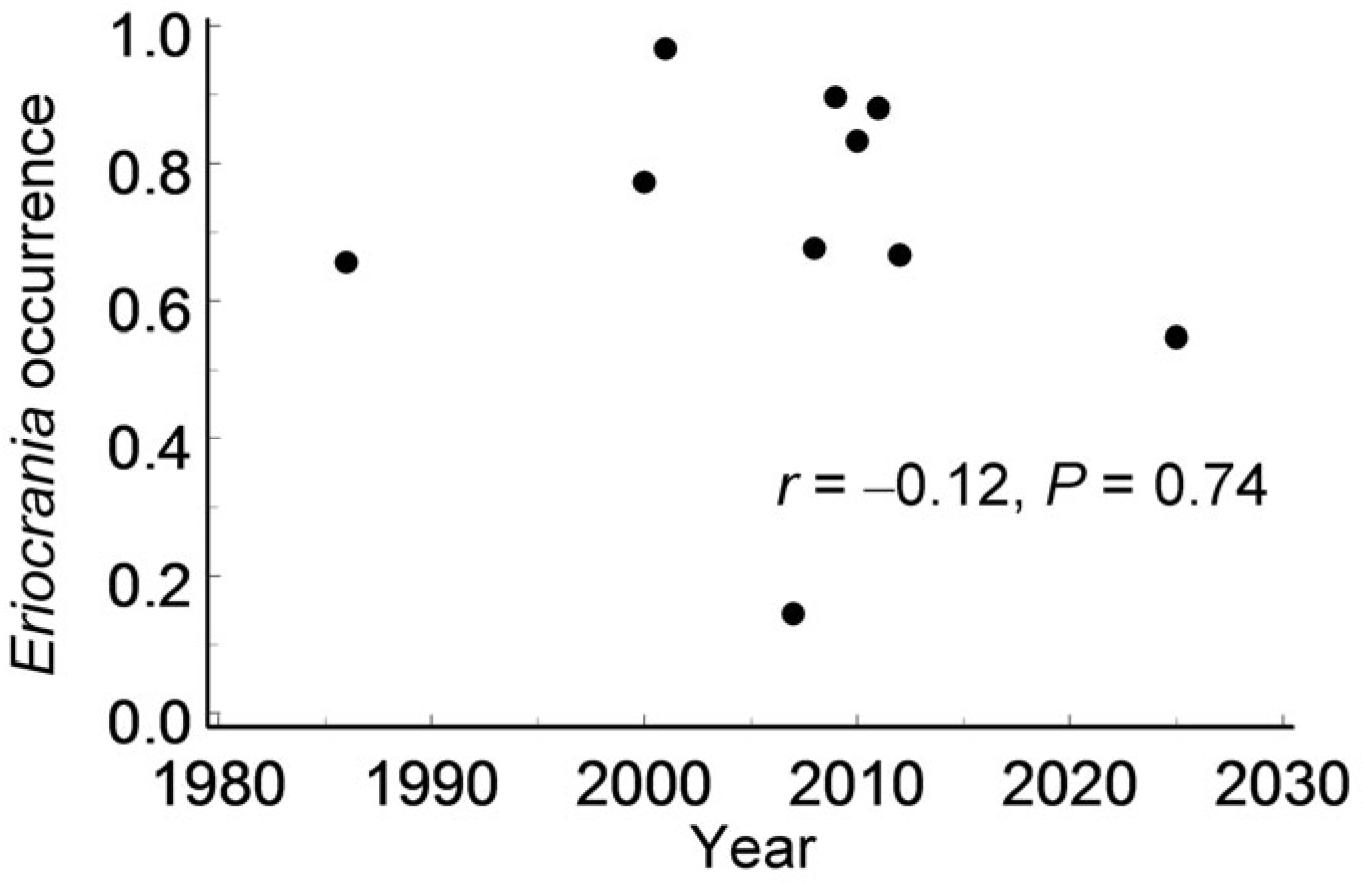
Finally, colonisation and extinction appeared well balanced across
Eriocrania-suitable habitat patches at both spatial and temporal scales. Furthermore, the absence of a detectable directional trend over 40 years disproves Hypothesis 4. This observation is consistent with previous findings from a subarctic region in which
Eriocrania densities showed no directional trend between 1991 and 2016 [
69].
4.5. Limitations and Future Research Directions
Despite the exceptional temporal and spatial coverage of this study, several methodological limitations should be noted. Species-level identification of Eriocrania was not possible using mine morphology alone, limiting the precision of herbivore data. The large spatial extent precluded complete annual censusing, and changes in social access—such as locked gates and fences—hampered visits to some sites from the early 2000s, restricting close examination of certain birches in some years.
An additional methodological limitation is inherent to the survey approach: the time-restricted search for Eriocrania leaf mines in birch foliage may have led to occasional false absences, particularly in large or inaccessible trees. This limitation should be considered when interpreting patch occupancy and extinction risk, and it highlights the need for complementary approaches in future studies.
Future research could build on these findings by incorporating higher-resolution temporal sampling and molecular identification to clarify species-specific responses to urbanisation. Investigating additional environmental drivers—such as microclimate, pollution and urban management—would improve mechanistic understanding of patch dynamics. Comparative studies across multiple plant–herbivore systems and cities would help assess the generality of observed patterns.
The unique insights revealed by this 40-year dataset—insights that could not have been obtained from shorter time series—underscore the value of extended monitoring for understanding ecological processes in heterogeneous urban environments. Such efforts are essential for detecting subtle trends, informing conservation and guiding urban planning.