Predation Pressure on Invertebrate Sentinel Prey Depends on Distance to Forest Edge and Seasonality in Kenyan Tea (Camellia sinensis) Plantations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Gatamaiyu
2.1.2. Kericho
2.1.3. Kakamega
2.2. Predation Pressure on Artificial Caterpillars
2.3. Data Analysis
3. Results
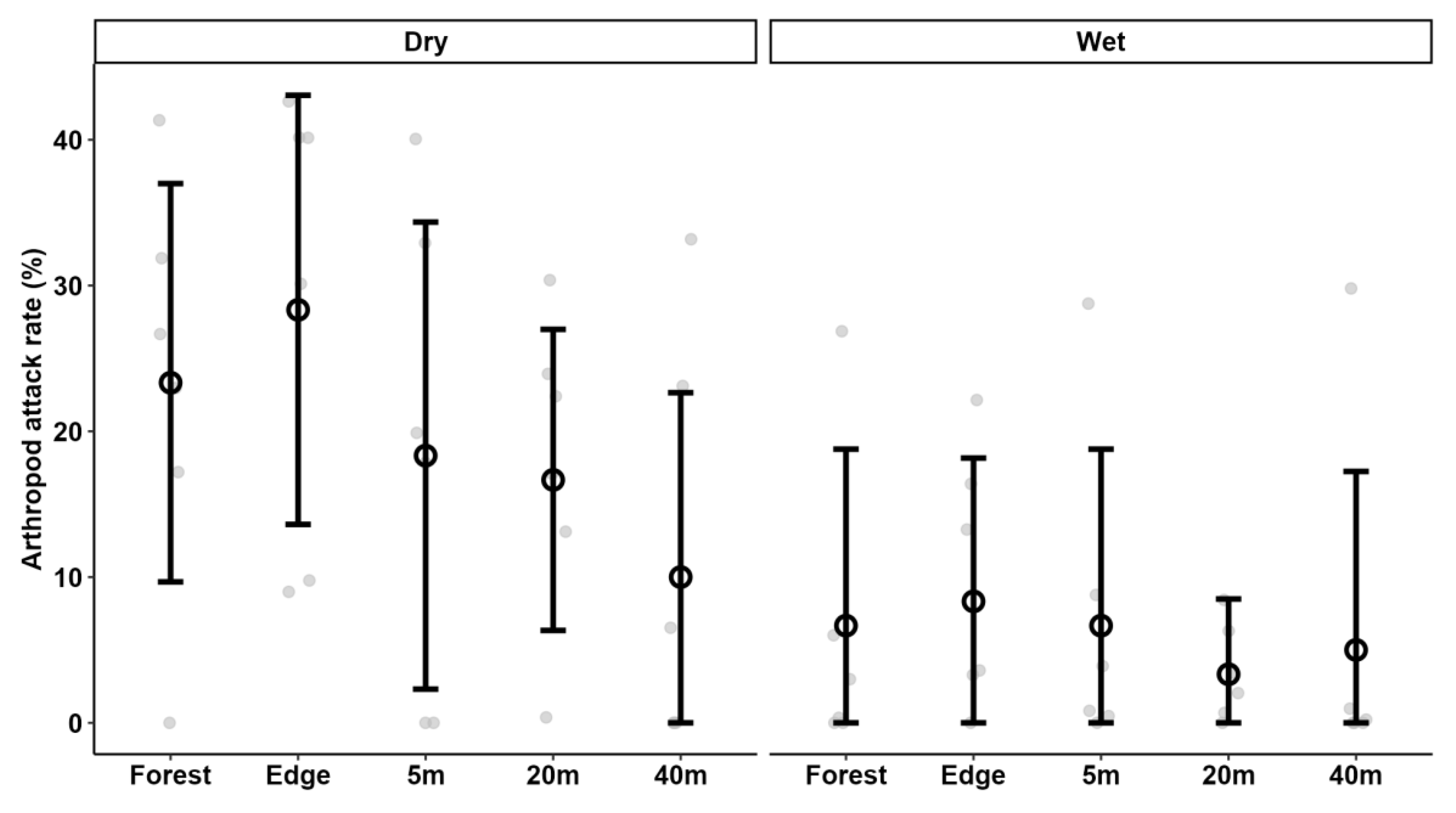
3.1. General Predation Pressure
3.2. Seasonal Variation
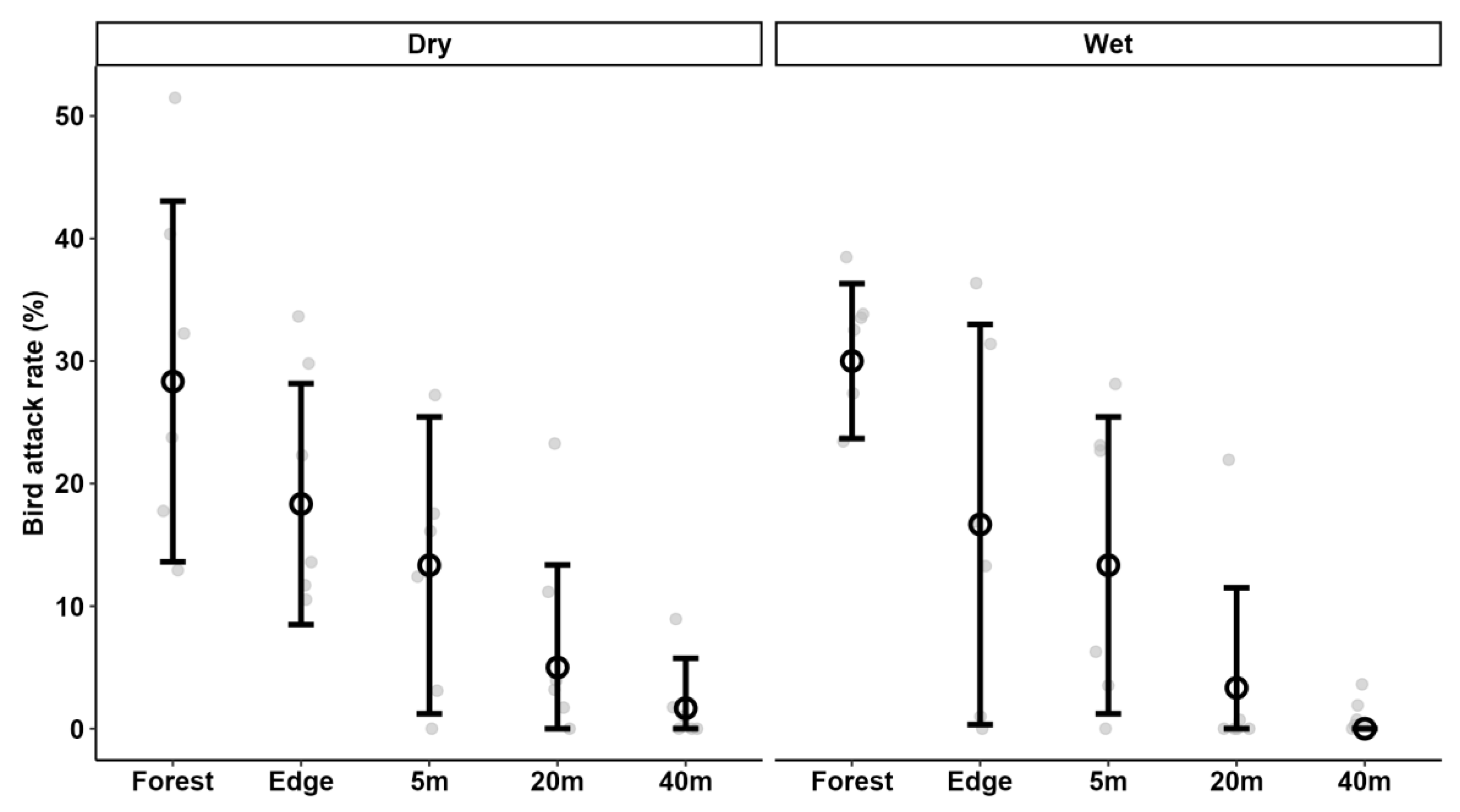
3.3. Predation Pressure in Forest vs. Tea Plantation
3.4. Bird vs. Arthropod Predation Rates
4. Discussion
4.1. Method Limitations
4.2. Comparison with Other Studies in Tea Plantations
4.3. Predation Pressure on Artificial Caterpillars in Africa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Sekercioglu, C.H. Ecosystem functions and services. In Conservation Biology for All; Sodhi, N.S., Erlich, P.R., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 45–72. [Google Scholar]
- MEA (Millennium Ecosystem Assessment). Ecosystems and Human Wellbeing: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M.S. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Mills, N.J. Biological Control. Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Medeiros, H.R.; Grandinete, Y.C.; Manning, P.; Harper, K.A.; Cutler, G.C.; Tyedmers, P.; Righi, C.A.; Ribeiro, M.C. Forest cover enhances natural enemy diversity and biological control services in Brazilian sun coffee plantations. Agron. Sustain. Dev. 2019, 39, 50. [Google Scholar] [CrossRef]
- Babin, R. Pest management in organic cacao. In Handbook of Pest Management in Organic Farming; Vacante, V., Kreiter, S., Eds.; CAB International: Wallingford, UK, 2018; pp. 502–518. [Google Scholar]
- Benn, J.A. Tea in China: A Religious and Cultural History; University of Hawai‘i Press: Honolulu, HI, USA, 2015; p. 288. [Google Scholar]
- Kawai, A. Prospect for Integrated Pest Management in Tea Cultivation in Japan. Japan Agric. Res. Quart. 1997, 31, 213–217. [Google Scholar]
- Kamunya, S.; Ochanda, S.; Cheramgoi, E.; Chalo, R.; Sitienei, K.; Muku, O.; Kirui, W.; Bore, J.K. Tea Growers Guide; Kenya Agricultural & Livestock Research Organization: Nairobi, Kenya, 2019; p. 233. [Google Scholar]
- Mertz, O.; Ravnborg, H.M.; Lövei, G.L.; Nielsen, I.; Konijnendijk, C.C. Ecosystem services and biodiversity in developing countries. Biodivers. Conserv. 2007, 16, 2729–2737. [Google Scholar] [CrossRef]
- Karp, D.S.; Mendenhall, C.D.; Sandí, R.F.; Chaumont, N.; Ehrlich, P.R.; Hadly, E.A.; Daily, G.C. Forest bolsters bird abundance, pest control and coffee yield. Ecol. Lett. 2013, 16, 1339–1347. [Google Scholar] [CrossRef]
- Howe, A.; Lövei, G.L.; Nachmann, G. Dummy caterpillars as a simple method to assess predation rates of invertebrates in tropical agroecosystem. Entomol. Exp. Appl. 2009, 131, 325–329. [Google Scholar] [CrossRef]
- Ferrante, M.; Howe, A.G.; Lövei, G.L. Experimental considerations support the use of artificial sentinel prey—A comment on Rodriguez-Campbell et al. J. Biogeogr. 2024, 51, 2152–2155. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Odum, E.P. Fundamentals of Ecology, 3rd ed.; Saunders: Philadelphia, PA, USA, 1971; p. 302. [Google Scholar]
- D’Angelo, S.A.; Andrade, A.C.S.; Laurance, S.G.; Laurance, W.F.; Mesquita, R.C.G. Inferred causes of tree mortality in fragmented and intact Amazonian forests. J. Trop. Ecol. 2004, 20, 243–246. [Google Scholar] [CrossRef]
- Tabarelli, M.; Gascon, C. Lessons from fragmentation research: Improving management and policy guidelines for biodiversity conservation. Conserv. Biol. 2005, 19, 734–739. [Google Scholar] [CrossRef]
- Imboma, T.S.; Gao, D.-P.; You, M.-S.; You, S.-J.; Lövei, G.L. Predation pressure in tea (Camellia sinensis) plantations in southeastern China measured by the sentinel prey method. Insects 2020, 11, 212. [Google Scholar] [CrossRef]
- Wolda, H. Seasonality in tropical insects. J. Anim. Ecol. 1980, 49, 277–290. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 15 December 2023).
- Barton, K. Package ‘mumin’, Version 1.48. 2025. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 31 March 2025).
- Wickham, H. Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Bolker, B.M. Linear and generalized linear mixed models. In Ecological Statistics: Contemporary Theory and Application; Fox, A., Negrete-Yankelevich, S., Sosa, V.J., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 309–333. [Google Scholar]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–13. [Google Scholar] [CrossRef]
- Symonds, M.R.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Lövei, G.L.; Ferrante, M. A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Sci. 2017, 24, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. Tropical agroecosystems. Science 1973, 182, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Keast, A. (Ed.) Biogeography and Ecology of Forest Bird Communities; SPB Academic Publishing: The Hague, The Netherlands, 1990. [Google Scholar]
- Borghesio, L.; Laiolo, P. Seasonal foraging ecology in a forest avifauna of northern Kenya. J. Trop. Ecol. 2004, 20, 145–155. [Google Scholar] [CrossRef]
- Brown, L.; Britton, P.L. The Breeding Seasons of East African Birds; East Africa Natural History Society: Nairobi, Kenya, 1980. [Google Scholar]
- Myers, J.H.; Cory, J.S. Ecology and evolution of pathogens in natural populations of Lepidoptera. Evol. Appl. 2016, 9, 231–247. [Google Scholar] [CrossRef]
- Howe, A.; Nachman, G.; Lövei, G.L. Predation pressure in Ugandan cotton fields measured by a sentinel prey method. Entomol. Exp. Appl. 2015, 154, 161–170. [Google Scholar] [CrossRef]
- Schwab, D.; Wurz, A.; Grass, I.; Rakotomalala, A.A.N.A.; Osen, K.; Soazafy, M.R.; Martin, D.A.; Tscharntke, T. Decreasing predation rates and shifting predator compositions along a land-use gradient in Madagascar’s vanilla landscapes. J. Appl. Ecol. 2021, 58, 360–371. [Google Scholar] [CrossRef]
- Tadesse, Z.; Nemomissa, S.; Lemessa, D. Insect pest predation by arthropods and birds in different land use types with varying woody vegetation composition in agroecosystems of central Oromia, Ethiopia. Acta Oecologica 2023, 121, 103954. [Google Scholar] [CrossRef]
- Dassou, A.G.; Yemadje, P.L.; Atchadé, M.N.; Gohouede, L.C.; Aboua, C.D.; Boulakia, S.; Balarabe, O.; Sekloka, E.; Tittonell, P. Conservation agriculture compared to conventional tillage improves the trade-off between ground-dwelling arthropod trophic groups for natural pest regulation in cotton cropping systems. Glob. Ecol. Conserv. 2024, 55, e03223. [Google Scholar] [CrossRef]
- Kajl, P.J. Effects of Alien Plant Species Removal on Biodiversity and Species Interactions in Two Native Forest Areas on Mauritius. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2015; 61p. [Google Scholar]
| Location/Season/Habitat | Attack Rates (%d−1) by | ||
|---|---|---|---|
| All Predators | Arthropods | Birds | |
| Gatamaiyu | 25.0 ± 16.7 (20) | 12.5 ± 15.5 (20) | 12.5 ± 11.6 (20) |
| Kericho/Mau | 31.5 ± 27.4 (20) | 14.0 ± 14.3 (20) | 15.0 ± 14.7 (20) |
| Kakamega | 21.5 ± 19.0 (20) | 11.5 ± 12.3 (20) | 11.5 ± 15.7 (20) |
| Wet season | 16.7 ± 15.4 (30) | 6.0 ± 10.0 (30) | 12.7 ± 14.4 (30) |
| Dry season | 35.3 ± 23.0 (30) | 19.3 ± 14.1 (30) | 13.3 ± 13.7 (30) |
| Forest | 41.7 ± 14.0 (12) | 15.0 ± 15.1 (12) | 29.2 ± 10.8 (12) |
| Forest edge | 36.7 ± 25.7 (12) | 18.3 ± 15.9 (12) | 17.5 ± 12.9 (12) |
| Tea plantation, 5 m | 26.7 ± 21.9 (12) | 12.5 ± 14.8 (12) | 13.3 ± 11.5 (12) |
| Tea plantation, 20 m | 16.7 ± 13.0 (12) | 10.0 ± 10.4 (12) | 4.2 ± 7.9 (12) |
| Tea plantation, 40 m | 8.3 ± 12.7 (12) | 7.5 ± 12.2 (12) | 0.8 ± 2.9 (12) |
| Predictors | Responses | ||
|---|---|---|---|
| Total Predation | Insect Predation | Bird Predation | |
| (Intercept) | → | ↘ | ↘ |
| Edge | → | → | ↘ |
| Near edge | ↘ | → | ↘ |
| Outer center | ↘ | → | ↘ |
| Center | ↘ | → | ↘ |
| Kakamega | → | – | – |
| Mau/Kericho | ↗ | – | – |
| Wet season | ↘ | ↘ | – |
| Kakamega—Wet season | → | – | – |
| Mau/Kericho—Wet season | ↘ | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imboma, T.S.; Venturo, A.; Lövei, G.L. Predation Pressure on Invertebrate Sentinel Prey Depends on Distance to Forest Edge and Seasonality in Kenyan Tea (Camellia sinensis) Plantations. Insects 2025, 16, 988. https://doi.org/10.3390/insects16090988
Imboma TS, Venturo A, Lövei GL. Predation Pressure on Invertebrate Sentinel Prey Depends on Distance to Forest Edge and Seasonality in Kenyan Tea (Camellia sinensis) Plantations. Insects. 2025; 16(9):988. https://doi.org/10.3390/insects16090988
Chicago/Turabian StyleImboma, Titus S., Alfredo Venturo, and Gábor L. Lövei. 2025. "Predation Pressure on Invertebrate Sentinel Prey Depends on Distance to Forest Edge and Seasonality in Kenyan Tea (Camellia sinensis) Plantations" Insects 16, no. 9: 988. https://doi.org/10.3390/insects16090988
APA StyleImboma, T. S., Venturo, A., & Lövei, G. L. (2025). Predation Pressure on Invertebrate Sentinel Prey Depends on Distance to Forest Edge and Seasonality in Kenyan Tea (Camellia sinensis) Plantations. Insects, 16(9), 988. https://doi.org/10.3390/insects16090988








