Effect of Diet Compositions on Colony Strength Parameters, and the Enzymatic Activity of Apis mellifera L. During Floral Scarcity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Apiary Setup and Experiment Layout
2.2. Diet Preparation
2.3. Diet Consumption
2.4. Brood Area Measurement
2.5. Adult Bee Population
2.6. Worker Bee Longevity
2.7. Honey Production
2.8. Enzymatic Analysis
2.8.1. Amylase Assay
2.8.2. Lipase Assay
2.8.3. Proteinase Assay
2.8.4. α-Glucosidase Assay
2.9. Data Analysis
3. Results
3.1. Diet Consumption
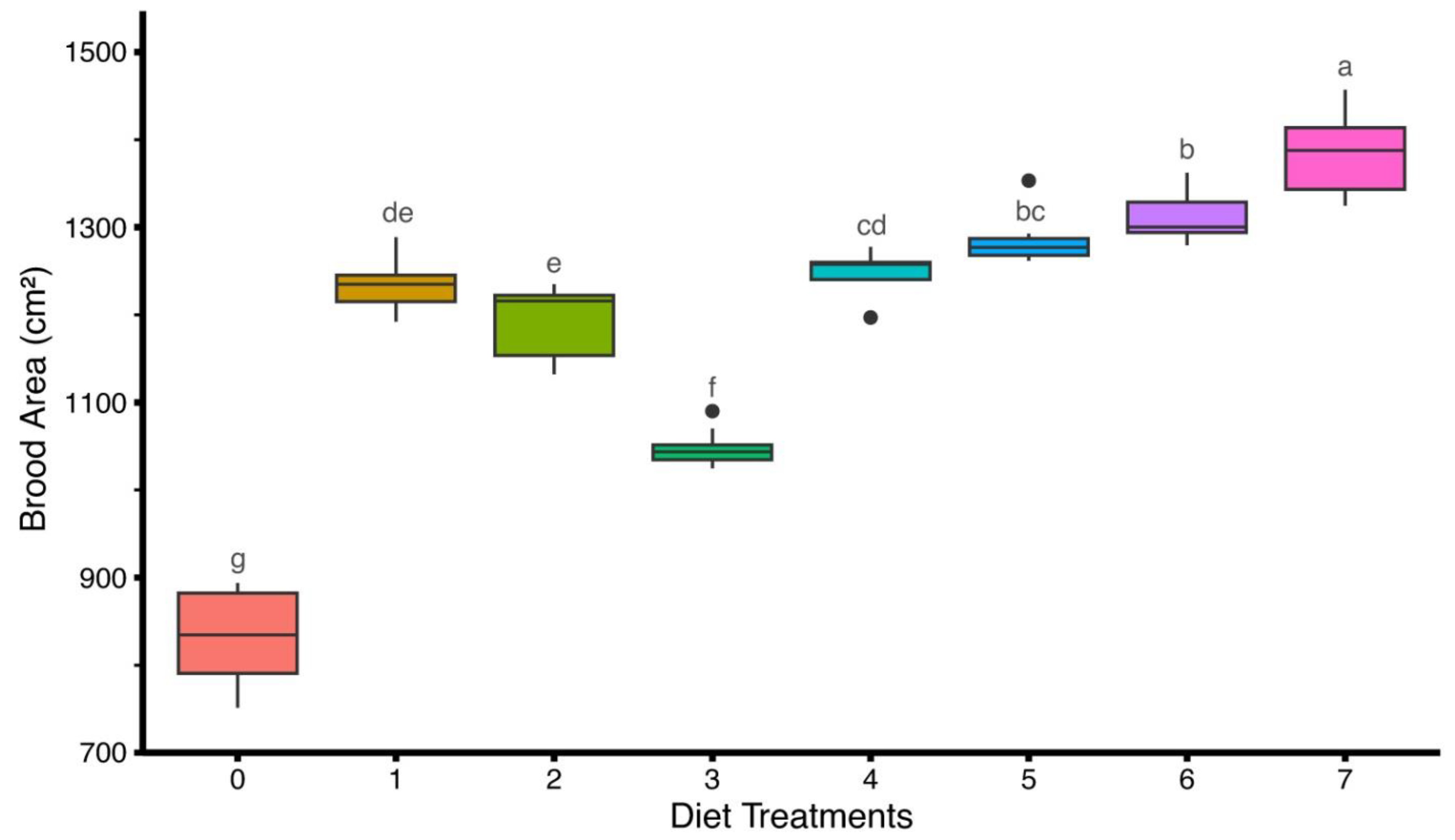
3.2. Brood Area Measurement
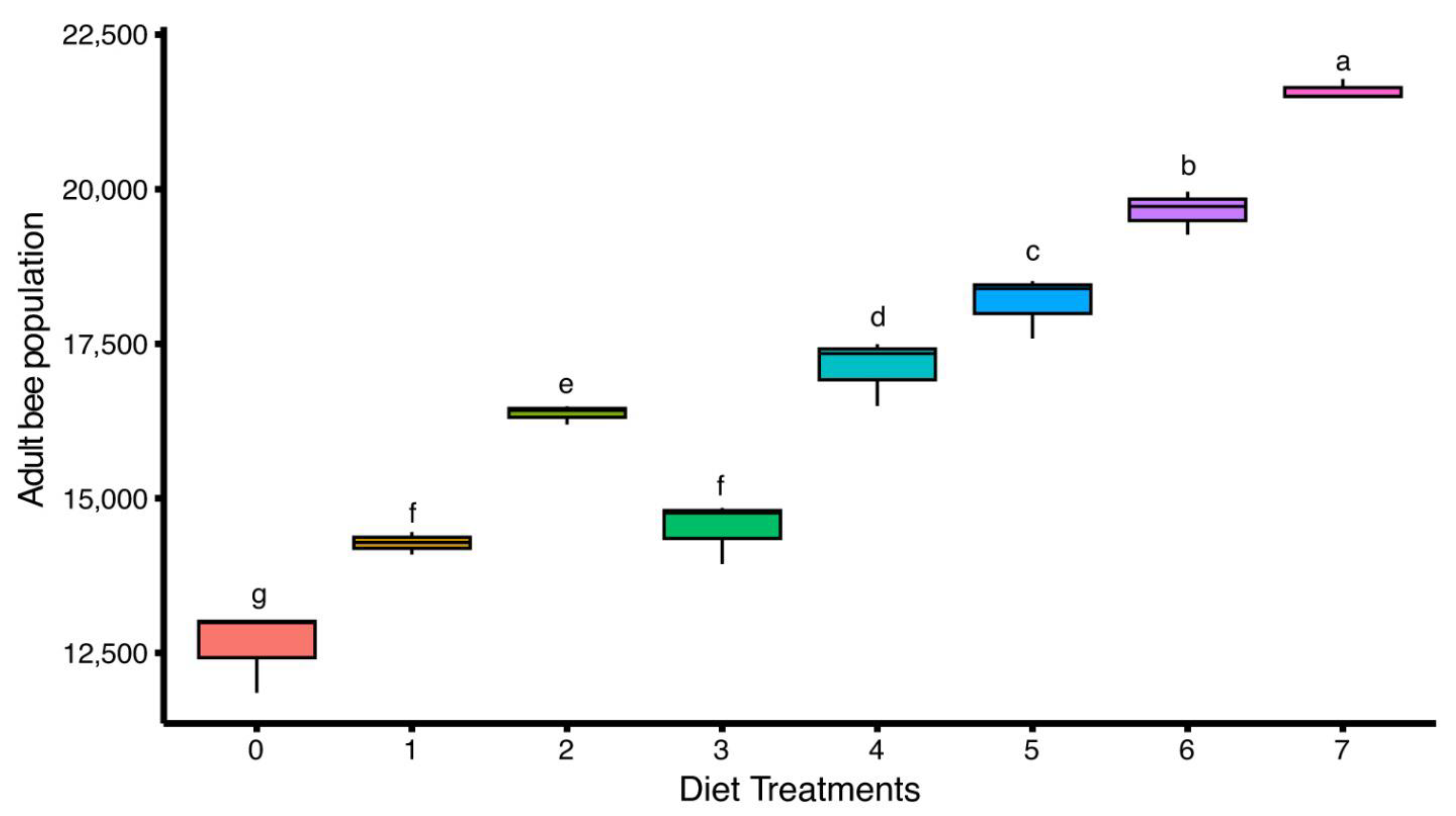
3.3. Adult Bee Population
3.4. Life Span of Worker Bees
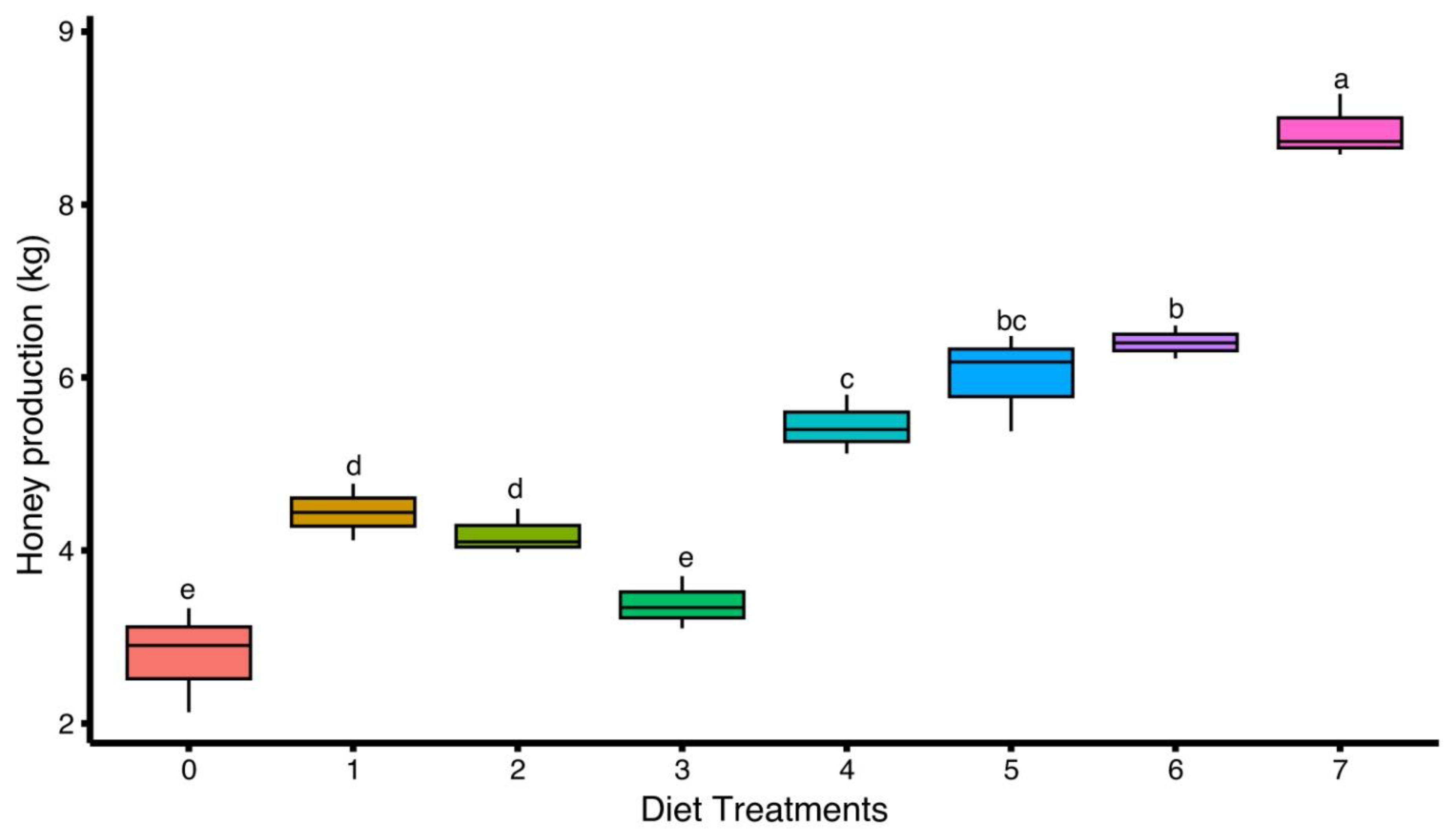
3.5. Honey Production
3.6. Pearson’s Correlation Matrix
3.7. Enzymatic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen-Wardell, G.; Bernhardt, P.; Bitner, R.; Burquez, A.; Buchmann, S.; Cane, J.; Cox, P.A.; Dalton, V.; Feinsinger, P.; Ingram, M. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv. Biol. 1998, 12, 8–17. [Google Scholar] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F. The foraging behaviour of honey bees, Apis mellifera: A review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.; Abdel-Daim, M.M. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A. The honey bee Apis mellifera: An insect at the interface between human and ecosystem health. Biology 2022, 11, 233. [Google Scholar] [CrossRef]
- Waykar, B.; Alqadhi, Y.A. Beekeeping and bee products; boon for human health and wealth. Indian J. Pharm. Biol. Res. 2016, 4, 20. [Google Scholar] [CrossRef]
- Aleixo, K.P.; Menezes, C.; Imperatriz Fonseca, V.L.; da Silva, C.I. Seasonal availability of floral resources and ambient temperature shape stingless bee foraging behavior (Scaptotrigona aff. depilis). Apidologie 2017, 48, 117–127. [Google Scholar] [CrossRef]
- Kumari, B.; Dhankhar, S.S.; Bangarwa, K. Commercial Agriculture; Students’ Counseling & Placement Cell, Directorate of Students’ Welfare, CCS Haryana Agricultural University: Hisar, India, 2015; pp. 1–4. [Google Scholar]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.M.; Vejnović, B.; Stevanović, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet.-Beogr. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Paray, B.A.; Kumari, I.; Hajam, Y.A.; Sharma, B.; Kumar, R.; Albeshr, M.F.; Farah, M.A.; Khan, J.M. Honeybee nutrition and pollen substitutes: A review. Saudi J. Biol. Sci. 2021, 28, 1167–1176. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Williams, S.T.; Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 2022, 18, 52. [Google Scholar] [CrossRef]
- Mazeed, A.; Zidan, E.; Abd El-latif, A. Role of pollinators on Egyptian clover pollination with special reference to honeybee at sohag governorate, Egypt. Arab. Univ. J. Agric. Sci. 2019, 27, 853–860. [Google Scholar] [CrossRef]
- Knoll, S.; Pinna, W.; Varcasia, A.; Scala, A.; Cappai, M.G. The honey bee (Apis mellifera L., 1758) and the seasonal adaptation of productions. Highlights on summer to winter transition and back to summer metabolic activity. A review. Livest. Sci. 2020, 235, 104011. [Google Scholar] [CrossRef]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Meng, Q.; Ye, T.; Wang, J.; Zhao, W.; Tian, Y.; Dong, K. Impact of Comb Cell Diameter on Nectar Evaporation Efficiency in Honey Bees. Insects 2025, 16, 71. [Google Scholar] [CrossRef]
- Mitchell, D. Nectar, humidity, honey bees (Apis mellifera) and varroa in summer: A theoretical thermofluid analysis of the fate of water vapour from honey ripening and its implications on the control of Varroa destructor. J. R. Soc. Interface 2019, 16, 20190048. [Google Scholar] [CrossRef] [PubMed]
- Kartik, A.R.; Singh, G. Artificial Diet Supplementation: A Review for Sustainable Approach to Boost Honeybee Health. J. Sci. Ind. Res. 2024, 83, 914–933. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Bertazzini, M.; Forlani, G. Intraspecific variability of floral nectar volume and composition in rapeseed (Brassica napus L. var. oleifera). Front. Plant Sci. 2016, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Sihag, R.C.; Gupta, M. Testing the Effects of Some Pollen Substitute Diets on Colony Build up and Economics of Beekeeping with Apis mellifera L. J. Entomol. 2013, 10, 120–135. [Google Scholar] [CrossRef]
- Gupta, R.K.; Reybroeck, W.; De Waele, M.; Bouters, A. Bee products: Production and processing. In Beekeeping for Poverty Alleviation and Livelihood Security; Springer: Dordrecht, The Netherlands, 2014; pp. 599–636. [Google Scholar]
- Pande, R.; Thakur, N.; Ngachan, S.; Rajkhowa, D. First record of wax beetle, Platybolium alvearium Blair (Coleoptera: Tenebrionidae), in Eastern Himalaya: A new threat to Indian honey bee (Apis cerana Fabricius) colonies. J. Entomol. Res. 2015, 39, 269–273. [Google Scholar] [CrossRef]
- Islam, S.U.; Aqueel, M.A.; Yousuf, M.U.; Abbasi, A.; Yasin, M.; Iqbal, R.; Raza, M.F.; Parvaiz, A.; Rebouh, N.Y. Evaluating the Influence of Different Artificial Diets on Apis mellifera L. Using Health Biomarkers and Performance Metrics. Insects 2024, 15, 905. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, O.; Mureșan, C.I.; Dobritzsch, D.; Fuszard, M.; Erler, S. The effect of diet on the composition and stability of proteins secreted by honey bees in honey. Insects 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.M.; Erdoğan, Y. Nutrient Needs and Food Gathering Activities of Honeybees. In Bee and Beekeeping; Iksad Publishing House: Ankara, Türkiye, 2023; pp. 1–18. [Google Scholar]
- Farooq, S.; Ngaini, Z. The Enzymatic Role in Honey from Honey Bees and Stingless Bees. Curr. Org. Chem. 2023, 27, 1215–1229. [Google Scholar] [CrossRef]
- Correa-Mosquera, A.R.; Quicazán, M.C.; Zuluaga-Domínguez, C.M. Shelf-life prediction of pot-honey subjected to thermal treatments based on quality attributes at accelerated storage conditions. Food Control 2022, 142, 109237. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Kim, Y.H.; Kim, J.M.; Kim, B.Y.; Lee, K.S.; Jin, B.R. Molecular cloning and characterization of a lipase from the honeybee Apis mellifera. J. Asia-Pac. Entomol. 2022, 25, 101921. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Hamilton, J.A.; Moser, A.; Bengtsson-Olivecrona, G.; Butbul, E.; Carpentier, Y.A.; Gutman, A.; Olivecrona, T. Medium-chain vs long-chain triacylglycerol emulsion hydrolysis by lipoprotein lipase and hepatic lipase: Implications for the mechanisms of lipase action. Biochemistry 1990, 29, 1136–1142. [Google Scholar] [CrossRef]
- Son, S.-Y.; Hur, H.; Hyung, W.J.; Park, Y.-K.; Lee, H.-J.; An, J.Y.; Kim, W.; Kim, H.-I.; Kim, H.-H.; Ryu, S.W. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: 5-year outcomes of the KLASS-02 randomized clinical trial. JAMA Surg. 2022, 157, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Das, D.C.; Sunna, T.C.; Beyene, J.; Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: A systematic review and meta-analysis. EClinicalMedicine 2023, 57, 101860. [Google Scholar] [CrossRef]
- Bryś, M.S.; Strachecka, A. The key role of amino acids in pollen quality and honey bee physiology—A review. Molecules 2024, 29, 2605. [Google Scholar] [CrossRef]
- Sulaiman, I.; Chung, M.; Angel, L.; Tsay, J.-C.J.; Wu, B.G.; Yeung, S.T.; Krolikowski, K.; Li, Y.; Duerr, R.; Schluger, R. Microbial signatures in the lower airways of mechanically ventilated COVID-19 patients associated with poor clinical outcome. Nat. Microbiol. 2021, 6, 1245–1258. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Hawash, M.B.; Sanz-Remón, J.; Grenier, J.-C.; Kohn, J.; Yotova, V.; Johnson, Z.; Lanford, R.E.; Brinkworth, J.F.; Barreiro, L.B. Primate innate immune responses to bacterial and viral pathogens reveals an evolutionary trade-off between strength and specificity. Proc. Natl. Acad. Sci. USA 2021, 118, e2015855118. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Rani, J.; Kamboj, H.; Dhull, S.B.; Rose, P.K.; Bou-Mitri, C.; Goksen, G.; Faliarizao, N. Effect of Different Processing Techniques and Storage Conditions on Honey Properties. In Honey in Food Science and Physiology; Springer: Dordrecht, The Netherlands, 2024; pp. 439–469. [Google Scholar]
- Paquet, P.; Nikkels, A.; Arrese, J.E.; Vanderkelen, A.; Piérard, G.E. Macrophages and tumor necrosis factor a in toxic epidermal necrolysis. Arch. Dermatol. 1994, 130, 605–608. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative estimation of protein in sprouts of Vigna radiate (Mung beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by kjeldahl and lowry methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, C.O.; Soetan, K.O. A review of the health benefits of fenugreek (Trigonella foenum-graecum L.): Nutritional, Biochemical and pharmaceutical perspectives. Am. J. Soc. Issues Humanit. 2014, 4, 3–12. [Google Scholar]
- Gupta, S.; Chhajed, M.; Arora, S.; Thakur, G.; Gupta, R. Medicinal Value of Apricot: A Review. Indian J. Pharm. Sci. 2018, 80, 790–794. [Google Scholar] [CrossRef]
- Kaur, J.; Katyal, P. Baker’s yeast: Industrial applications and health benefits. Appl. Biol. Res. 2019, 21, 105–113. [Google Scholar] [CrossRef]
- Chandel, N.S. Carbohydrate metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040568. [Google Scholar] [CrossRef]
- Phuah, E.-T.; Yap, J.W.-L.; Lau, C.-W.; Lee, Y.-Y.; Tang, T.-K. Vegetable oils and animal fats: Sources, properties and recovery. Recent Adv. Edible Fats Oils Technol. Process. Health Implic. Econ. Environ. Impact 2022, 1–26. [Google Scholar] [CrossRef]
- Amro, A.; Omar, M.; Al-Ghamdi, A. Influence of different proteinaceous diets on consumption, brood rearing, and honey bee quality parameters under isolation conditions. Turk. J. Vet. Anim. Sci. 2016, 40, 468–475. [Google Scholar] [CrossRef]
- Manzoor, A.; Aqueel, M.A.; Islam, S.U.; Dessoky, E.S.; Ahsan, M.H.; Ahmad, B.; Yousuf, M.U.; Saqib, M.; Raza, M.F.; Iqbal, R. Assessment of consumption and digestibility of artificial diets and their effects on few life study parameters of Apis mellifera L. Asian J. Agric. Biol. 2025, 2025, 1–13. [Google Scholar] [CrossRef]
- Islam, N.; Mahmood, R.; Sarwar, G.; Ahmad, S.; Abid, S. Development of pollen substitute diets for Apis mellifera ligustica colonies and their impact on brood development and honey production. Pak. J. Agric. Res. 2020, 33, 192–421. [Google Scholar] [CrossRef]
- Amir, O.; Peveling, R. Effect of triflumuron on brood development and colony survival of free-flying honeybee, Apis mellifera L. J. Appl. Entomol. 2004, 128, 242–249. [Google Scholar] [CrossRef]
- Ismail, A.E.-h.M.; Ghoniemy, H.A.; Owayss, A.A. Combatting mite, Varroa destructor Anderson &Trueman, in honeybee, Apis mellifera Lin., colonies by soft chemicals and/or an integrated pest management. In Proceedings of the 2nd Conference of Farm Integrated Pest Management, 2006; pp. 16–18. Available online: https://saudibi.com/files/image/pdf/conf4/36.pdf (accessed on 11 September 2025).
- Tarpy, D.R.; Lengerich, E.J.; Pettis, J.S. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 2013, 108, 225–233. [Google Scholar] [CrossRef]
- Pettis, J.S.; Rice, N.; Joselow, K.; van Engelsdorp, D.; Chaimanee, V. Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS ONE 2016, 11, e0147220. [Google Scholar] [CrossRef]
- Lee, K.V.; Goblirsch, M.; McDermott, E.; Tarpy, D.R.; Spivak, M. Is the brood pattern within a honey bee colony a reliable indicator of queen quality? Insects 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Morfin, N.; Dainat, B.; Williams, G.R.; van der Steen, J.; Correa-Benítez, A.; Delaplane, K.S. Standard methods to estimate strength parameters, flight activity, comb construction, and fitness of Apis mellifera colonies 2.0. J. Apic. Res. 2025, 64, 533–554. [Google Scholar] [CrossRef]
- Shurjeel, H.K.; Aqueel, M.A.; Ashraf, E.; Ali, A.; Rubab, A. Effect of insecticides on the longevity of Apis mellifera L. (Hymenoptera: Apidae). Sarhad J. Agric. 2020, 36, 768–776. [Google Scholar] [CrossRef]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.-L.; Jaffé, R. Miscellaneous standard methods for Apis mellifera research. J. Apic. Res. 2013, 52, 1–53. [Google Scholar] [CrossRef]
- Aziz, M.A.; Azeem, M.; Ahmed, M.S.; Siddique, F.; Jamal, M. Control of Varroa destructor Anderson and Trueman (Acari: Varroidae) on Apis mellifera linguistica by using thymol and formic acid in Pothwar region of Punjab, Pakistan. Asian J. Agric. Biol. 2015, 3, 150–154. [Google Scholar]
- Murtaza, M.; Abdullah, S.; Hassan, W.; Abbas, K.; Naz, H.; Zia, M.A. Studies on amylase and lipase activity in fishes fed with diet containing different feed ingredients. Punjab Univ. J. Zool. 2016, 31, 165–169. [Google Scholar]
- Lehoczki, G.; Kandra, L.; Gyémánt, G. The use of starch azure for measurement of alpha-amylase activity. Carbohydr. Polym. 2018, 183, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Ismat, N.; Ashraf, M.; Naeem, M.; ur Rehman, M.H. Effect of different feed ingredients on growth and level of intestinal enzyme secretions in juvenile Labeo rohita, Catla catla, Cirrhinus mrigala and Hypophthalmicthys molitrix. Int. J. Aquac. 2013, 3, 85–91. [Google Scholar] [CrossRef]
- Konkit, M.; Kim, W. Activities of amylase, proteinase, and lipase enzymes from Lactococcus chungangensis and its application in dairy products. J. Dairy Sci. 2016, 99, 4999–5007. [Google Scholar] [CrossRef]
- McDonald, C.; Chen, L.L. The Lowry modification of the Folin reagent for determination of proteinase activity. Anal. Biochem. 1965, 10, 175–177. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; Al-Kahtani, S. Macro-and trace elements content in honeybee pollen loads in relation to the harvest season. Saudi J. Biol. Sci. 2020, 27, 1797–1800. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Novel insights into dietary phytosterol utilization and its fate in honey bees (Apis mellifera L.). Molecules 2020, 25, 571. [Google Scholar] [CrossRef]
- Gawali, A.R.; Waykar, B.B. Nutritional requirements and effect of nectar and pollen substitute diets on Apis mellifera L. colonies: A review. J. Apic. Res. 2025, 1–15. [Google Scholar] [CrossRef]
- Noordyke, E.R.; Ellis, J.D. Reviewing the efficacy of pollen substitutes as a management tool for improving the health and productivity of western honey bee (Apis mellifera) colonies. Front. Sustain. Food Syst. 2021, 5, 772897. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Wardell, G.; Ahumada-Segura, F.; Rinderer, T.; Danka, R.; Pettis, J. Comparisons of pollen substitute diets for honey bees: Consumption rates by colonies and effects on brood and adult populations. J. Apic. Res. 2008, 47, 265–270. [Google Scholar] [CrossRef]
- Nabors, L.A. Evaluation in school-based health centers. Psychol. Sch. 2003, 40, 309–320. [Google Scholar] [CrossRef]
- Mattila, H.; Otis, G. Influence of pollen diet in spring on development of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 2006, 99, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; Wiedmer, S.; Retschnig, G.; Neumann, P. Feeding with plant powders increases longevity and body weight of Western honeybee workers (Apis mellifera). Apidologie 2024, 55, 54. [Google Scholar] [CrossRef]
- Amro, A.; Younis, M.; Ghania, A. Physiological effects of some pollen substitutes diets on caged honey bee workers (Apis mellifera L.). Int. J. Environ. 2020, 9, 87–99. [Google Scholar] [CrossRef]
- Standifer, L.; McCaughey, W.; Todd, F.; Kemmerer, A. Relative availability of various proteins to the honey bee. Ann. Entomol. Soc. Am. 1960, 53, 618–625. [Google Scholar] [CrossRef]
- Saffari, A.; Kevan, P.G.; Atkinson, J.L. Palatability and consumption of patty-formulated pollen and pollen substitutes and their effects on honeybee colony performance. J. Apic. Sci. 2010, 54, 63–71. [Google Scholar]
- Pankiw, T.; Page Jr, R.E.; Kim Fondrk, M. Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera). Behav. Ecol. Sociobiol. 1998, 44, 193–198. [Google Scholar] [CrossRef]
- Abou-Shaara, H. Effects of various sugar feeding choices on survival and tolerance of honey bee workers to low temperatures. J. Entomol. Acarol. Res. 2017, 49, 6–12. [Google Scholar] [CrossRef][Green Version]
- El-Kazafy, A.; Ali, M. Determination of heavy metals content in cotton honey in Kafr El-Shiekh province, Egypt. J. Plant Prot. Pathol. 2012, 3, 1211–1219. [Google Scholar] [CrossRef]
- Cremonz, T.M.; De Jong, D.; Bitondi, M.M. Quantification of hemolymph proteins as a fast method for testing protein diets for honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 1998, 91, 1284–1289. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.; Strauss, K.; Pirk, C.; Dietemann, V. Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata). J. Insect Physiol. 2007, 53, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. The foraging abilities of a colony. In The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MA, USA; London, UK, 1995; pp. 46–68. [Google Scholar] [CrossRef]
- Kubota, M.; Tsuji, M.; Nishimoto, M.; Wongchawalit, J.; Okuyama, M.; Mori, H.; Matsui, H.; Surarit, R.; Svasti, J.; Kimura, A. Localization of α-glucosidases I, II, and III in organs of European honeybees, Apis mellifera L., and the origin of α-glucosidase in honey. Biosci. Biotechnol. Biochem. 2004, 68, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.E.; Lau, P.; Rangel, J.; Arnott, R.; De Jong, T.; Moran, N.A. The microbiome and gene expression of honey bee workers are affected by a diet containing pollen substitutes. PLoS ONE 2023, 18, e0286070. [Google Scholar] [CrossRef]
- Kešnerová, L.; Mars, R.A.; Ellegaard, K.M.; Troilo, M.; Sauer, U.; Engel, P. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 2017, 15, e2003467. [Google Scholar] [CrossRef]
- Engel, P.; Martinson, V.G.; Moran, N.A. Disentangling metabolic functions of bacteria in the honey bee gut. ISME J. 2020, 14, 701–714. [Google Scholar]
- Brar, G.; Ngor, L.; McFrederick, Q.S. High abundance of lactobacilli in the gut microbiome of honey bees during winter. Sci. Rep. 2025, 15, 7409. [Google Scholar] [CrossRef] [PubMed]
- Maes, P.W.; Floyd, A.S.; Mott, B.M.; Anderson, K.E. Overwintering honey bee colonies: Effect of worker age and climate on the hindgut microbiota. Insects 2021, 12, 224. [Google Scholar] [CrossRef] [PubMed]

| Diet Treatments | Diet Composition | |||||
|---|---|---|---|---|---|---|
| Diet-1 | 60 g lupin flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-2 | 60 g mung bean flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-3 | 60 g chickpea flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-4 | 30 g lupin flour + 30 g mung bean flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-5 | 30 g mung bean flour + 30 g chickpea flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-6 | 30 g lupin flour + 30 g chickpea flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-7 | 20 g lupin flour + 20 g mung bean flour + 20 g chickpea flour | 2 g fenugreek powder | 10 g yeast | 40 g powder sugar | 10 g dry apricot powder | 10 mL vegetable oil |
| Diet-0 | 1 L of 50% sugar solution | |||||
| Diets | Week 1 (g) | Week 2 (g) | Week 3 (g) | Week 4 (g) | Week 5 (g) | Week 6 (g) | Week 7 (g) | Week 8 (g) |
|---|---|---|---|---|---|---|---|---|
| Diet-1 | 37.42 ± 1.60 f | 36.21 ± 1.71 f | 35.30 ± 1.08 f | 36.41 ± 0.94 f | 43.86 ± 1.92 f | 39.97 ± 1.30 e | 43.48 ± 1.60 e | 43.29 ± 1.64 e |
| Diet-2 | 44.66 ± 1.67 e | 41.83 ± 1.17 e | 41.20 ± 0.91 e | 40.6 ± 1.57 ef | 47.75 ± 2.21 ef | 42.28 ± 1.13 e | 46.36 ± 1.64 e | 46.30 ± 1.25 e |
| Diet-3 | 47.76 ± 1.62 de | 45.46 ± 0.93 de | 42.86 ± 1.40 de | 44.63 ± 0.93 de | 49.84 ± 1.32 de | 47.74 ± 0.98 d | 52.25 ± 0.82 d | 51.59 ± 0.86 d |
| Diet-4 | 51.14 ± 1.61 d | 47.84 ± 1.21 d | 46.89 ± 1.33 d | 48.81 ± 0.79 d | 54.39 ± 1.39 d | 50.61 ± 1.06 d | 55.38 ± 1.59 d | 54.51 ± 1.88 d |
| Diet-5 | 58.22 ± 1.45 c | 56.12 ± 1.34 c | 54.15 ± 1.27 c | 56.12 ± 1.81 c | 62.51 ± 2.30 c | 58.76 ± 1.06 c | 62.32 ± 1.69 c | 62.00 ± 1.22 c |
| Diet-6 | 65.50 ± 2.10 b | 62.96 ± 1.56 b | 62.49 ± 1.55 b | 63.59 ± 1.60 b | 70.12 ± 0.92 b | 65.45 ± 0.95 b | 70.82 ± 1.81 b | 70.40 ± 0.80 b |
| Diet-7 | 79.90 ± 2.19 a | 78.37 ± 2.01 a | 77.58 ± 1.74 a | 76.04 ± 1.94 a | 83.41 ± 2.07 a | 78.73 ± 1.48 a | 84.29 ± 1.61 a | 83.30 ± 2.27 a |
| F-value | 65.2 | 96.8 | 116 | 94.3 | 61.5 | 145 | 84.70 | 89.5 |
| p-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Variables | Brood Area | Adult Bee Population | Honey Production |
|---|---|---|---|
| Adult bee population | r = 0.856 (p < 0.001) | ||
| Honey production | r = 0.856 (p < 0.001) | r = 0.957 (p < 0.001) | |
| Worker bee longevity | r = 0.804 (p < 0.001) | r = 0.920 (p < 0.001) | r = 0.975 (p < 0.001) |
| Diets | Amylase (U/mg) | Lipase (U/mg) | Proteinase (U/mg) | α-Glucosidase (U/mg) |
|---|---|---|---|---|
| Diet-1 | 46.09 ± 0.46 bc | 13.04 ± 0.28 c | 20.20 ± 0.17 e | 35.50 ± 0.17 d |
| Diet-2 | 45.28 ± 0.43 c | 14.00 ± 0.29 c | 21.00 ± 0.14 d | 36.31 ± 0.18 d |
| Diet-3 | 42.01 ± 0.29 d | 13.50 ± 0.29 c | 19.53 ± 0.26 e | 31.26 ± 0.21 e |
| Diet-4 | 46.11 ± 0.35 bc | 15.50 ± 0.14 b | 22.31 ± 0.18 c | 37.51 ± 0.29 c |
| Diet-5 | 47.50 ± 0.29 ab | 16.00 ± 0.14 b | 24.11 ± 0.12 b | 38.76 ± 0.15 ab |
| Diet-6 | 47.00 ± 0.52 ab | 16.25 ± 0.14 ab | 23.10 ± 0.17 c | 38.21 ± 0.12 bc |
| Diet-7 | 48.62 ± 0.23 a | 16.85 ± 0.20 a | 25.21 ± 0.18 a | 39.21 ± 0.21 a |
| Diet-0 | 40.66 ± 0.61 d | 11.93 ± 0.35 d | 17.60 ± 0.26 f | 29.26 ± 0.21 f |
| F-value | 43.2 | 52.5 | 171 | 333 |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.U.; Liaquat, J.; Aqueel, M.A.; Abbasi, A.; Arshad, M.; Rizwan, M.S.; Saqib, M.; Masood, N.; Kavhiza, N.J.; Zafar, S.; et al. Effect of Diet Compositions on Colony Strength Parameters, and the Enzymatic Activity of Apis mellifera L. During Floral Scarcity. Insects 2025, 16, 967. https://doi.org/10.3390/insects16090967
Islam SU, Liaquat J, Aqueel MA, Abbasi A, Arshad M, Rizwan MS, Saqib M, Masood N, Kavhiza NJ, Zafar S, et al. Effect of Diet Compositions on Colony Strength Parameters, and the Enzymatic Activity of Apis mellifera L. During Floral Scarcity. Insects. 2025; 16(9):967. https://doi.org/10.3390/insects16090967
Chicago/Turabian StyleIslam, Shams Ul, Javeria Liaquat, Muhammad Anjum Aqueel, Asim Abbasi, Muhammad Arshad, Muhammad Shahid Rizwan, Muhammad Saqib, Nasir Masood, Nyasha J. Kavhiza, Saba Zafar, and et al. 2025. "Effect of Diet Compositions on Colony Strength Parameters, and the Enzymatic Activity of Apis mellifera L. During Floral Scarcity" Insects 16, no. 9: 967. https://doi.org/10.3390/insects16090967
APA StyleIslam, S. U., Liaquat, J., Aqueel, M. A., Abbasi, A., Arshad, M., Rizwan, M. S., Saqib, M., Masood, N., Kavhiza, N. J., Zafar, S., Avila-Quezada, G. D., Abd_Allah, E. F., Alharbi, D. S., & Hashem, A. (2025). Effect of Diet Compositions on Colony Strength Parameters, and the Enzymatic Activity of Apis mellifera L. During Floral Scarcity. Insects, 16(9), 967. https://doi.org/10.3390/insects16090967








