Recurrent Duplication, Testis-Biased Expression, and Functional Diversification of Esf2/ABT1 Family Genes in Drosophila
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Homolog Search and Identification
2.2. Phylogenetic Analysis
2.3. Z-Test of Neutrality
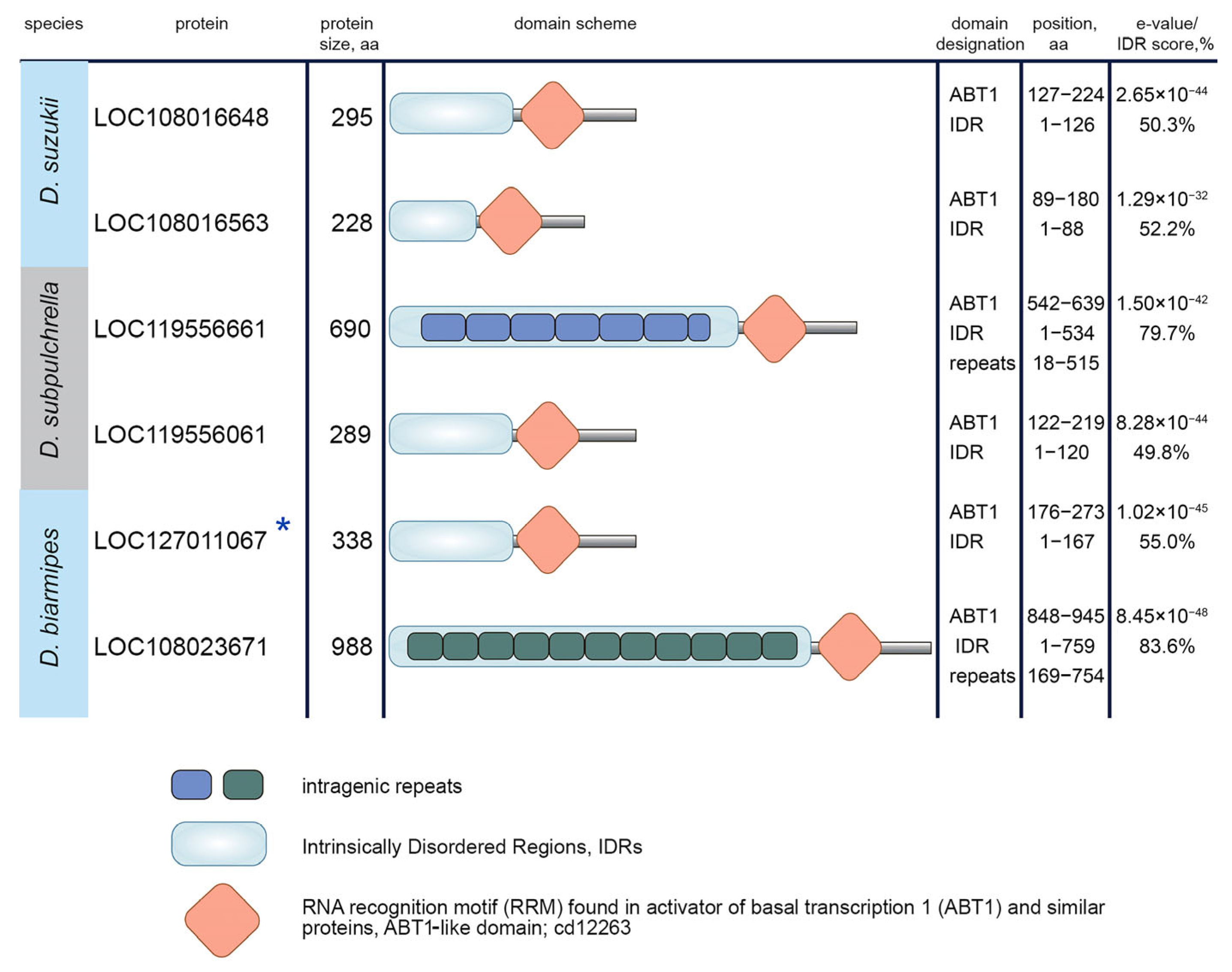
2.4. Search for Protein Domains
2.5. Fly Stocks
2.6. RT-qPCR Analysis
2.7. Copy Number Estimation of Woodpecker Genes
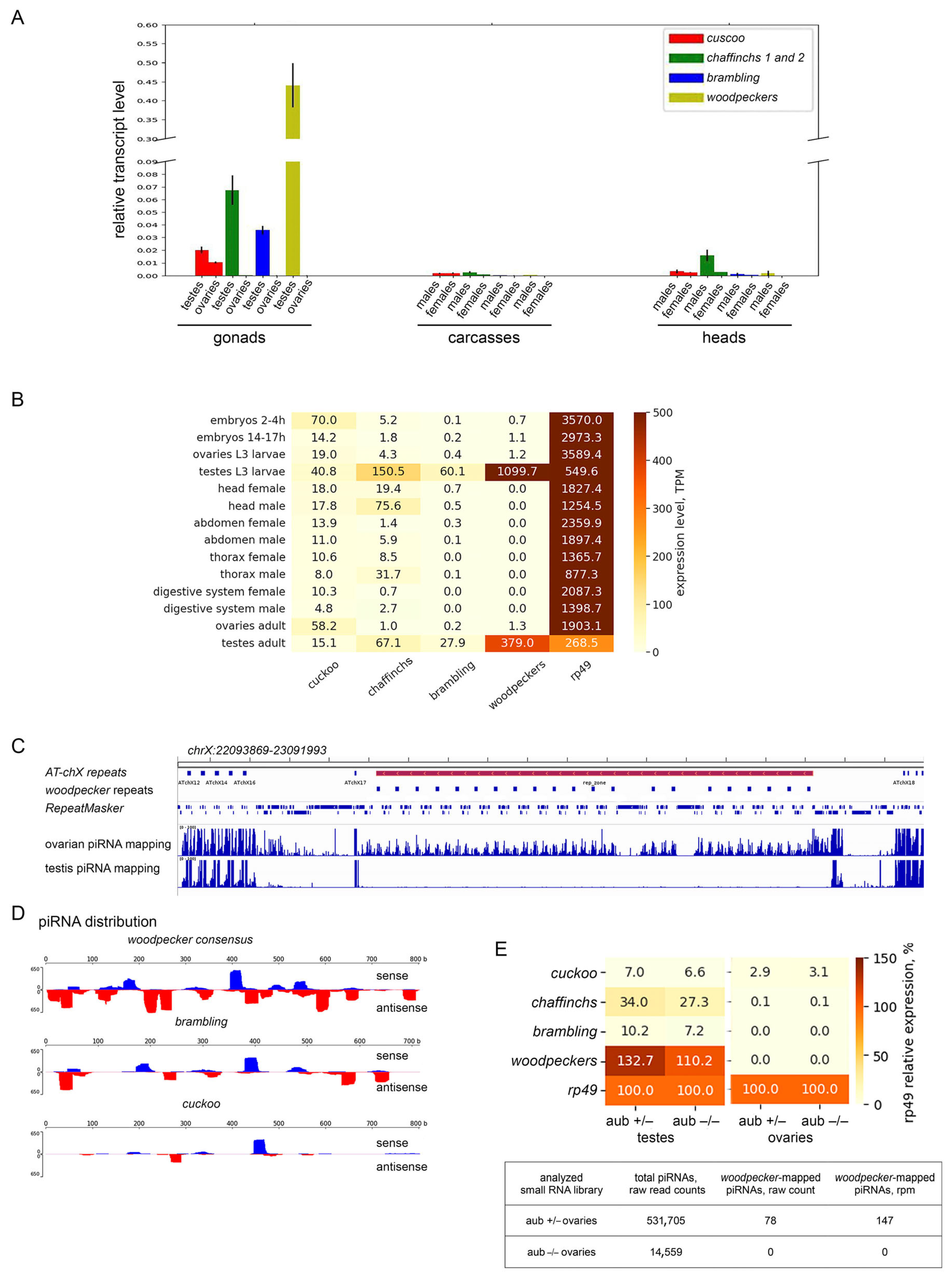
2.8. Transcriptomic Analyses of Esf2/ABP1 Family Genes Using Deep Sequencing Library Data
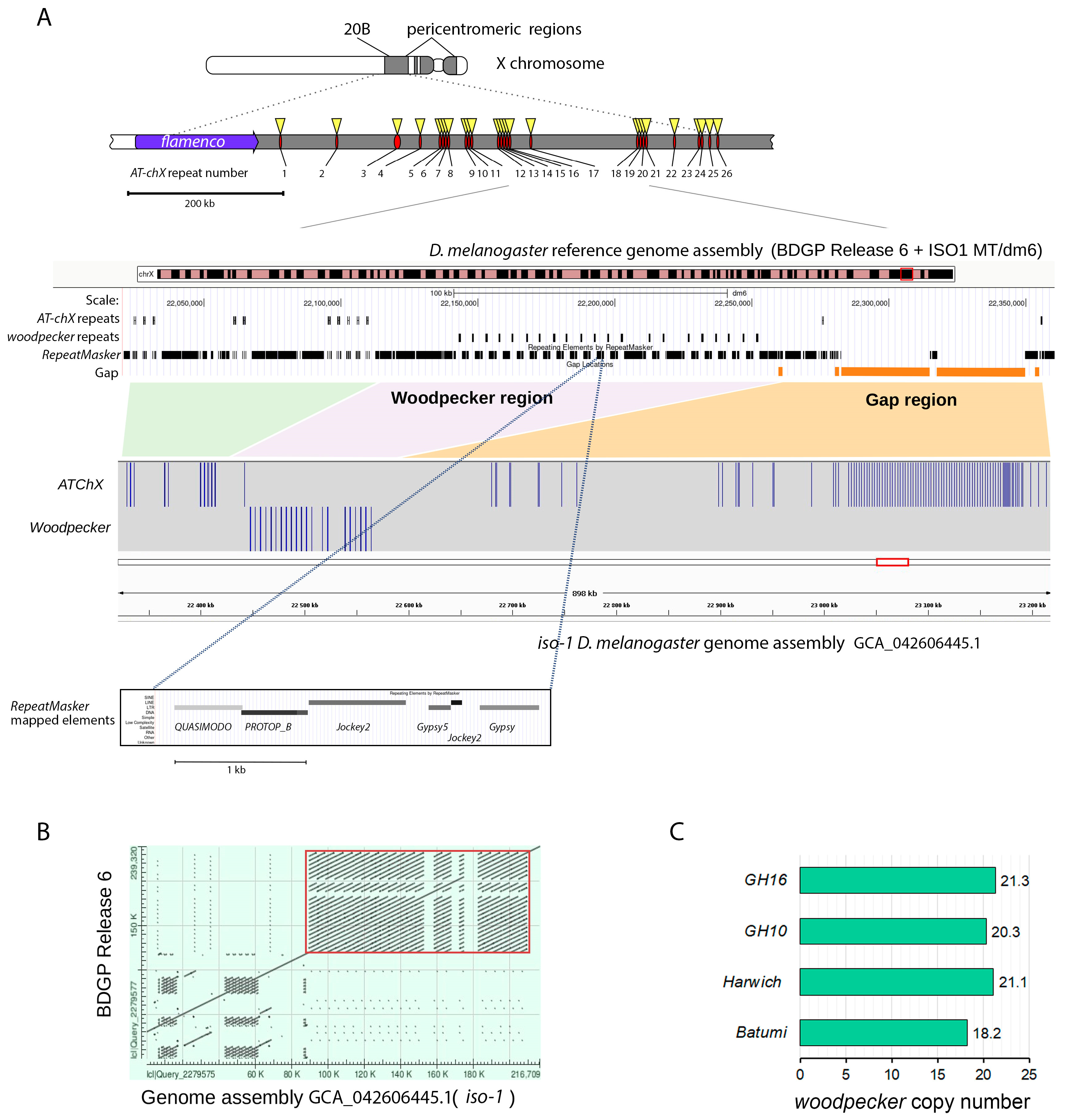
2.9. Bioinformatics Analysis of piRNAs
3. Results
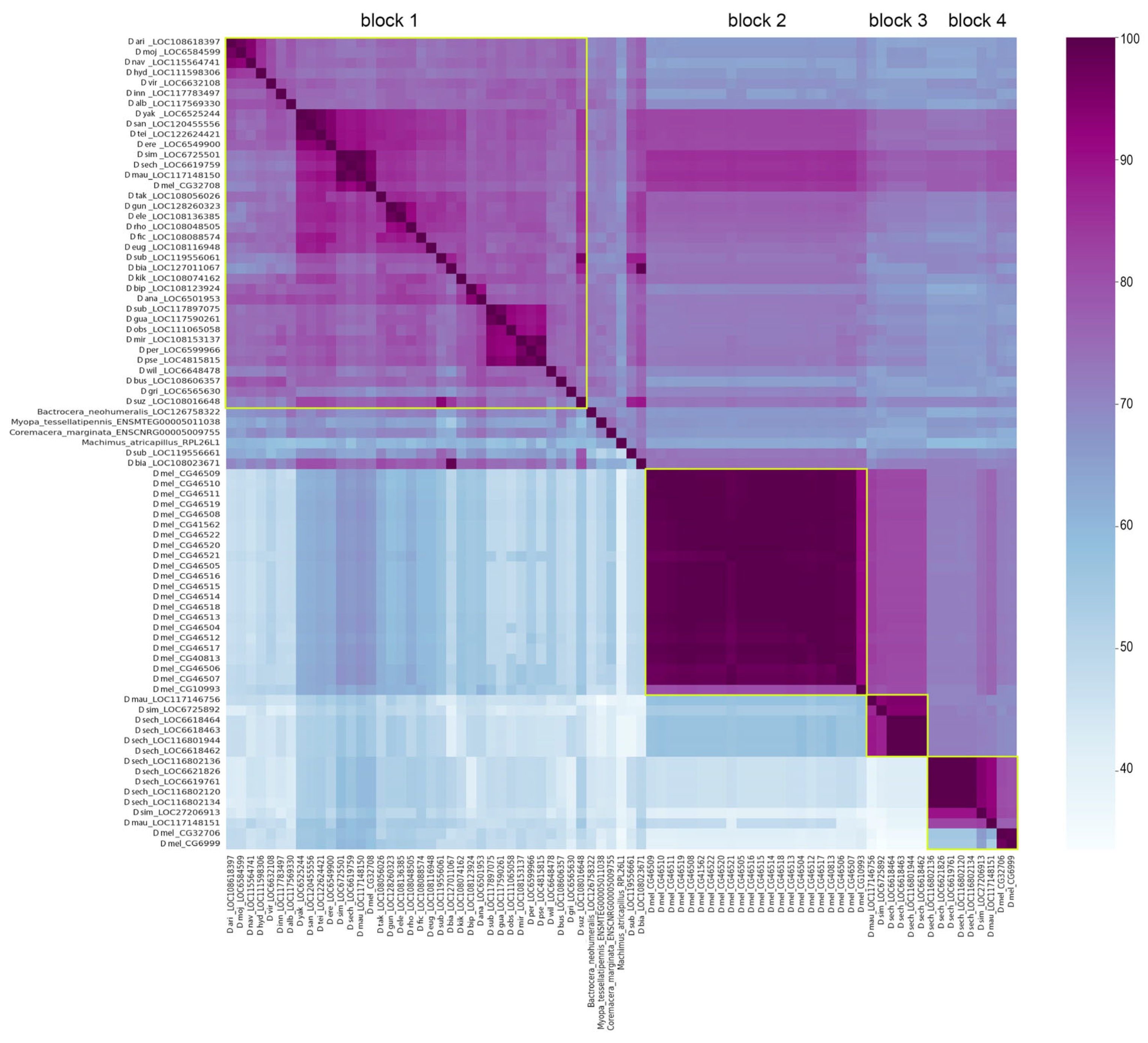
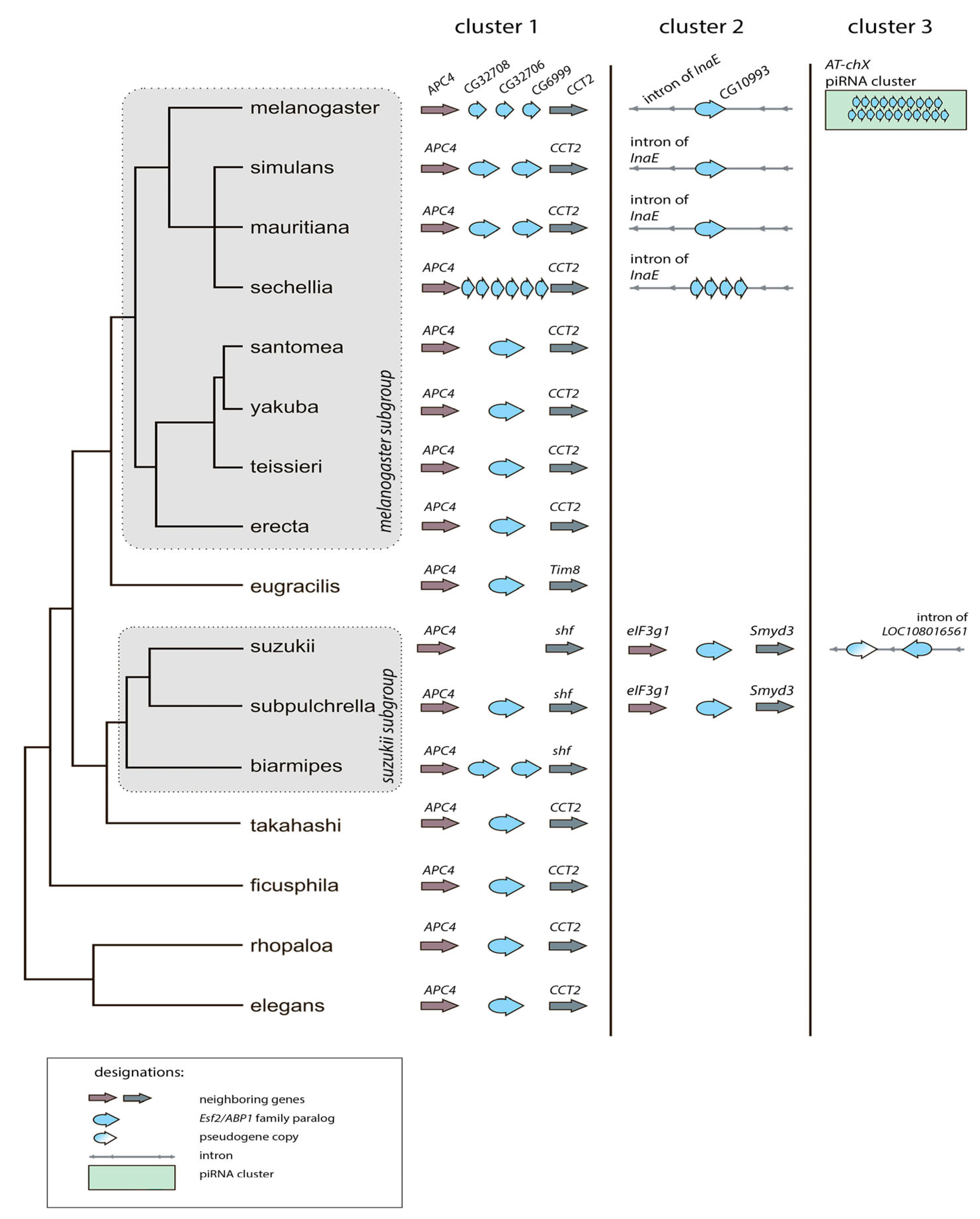
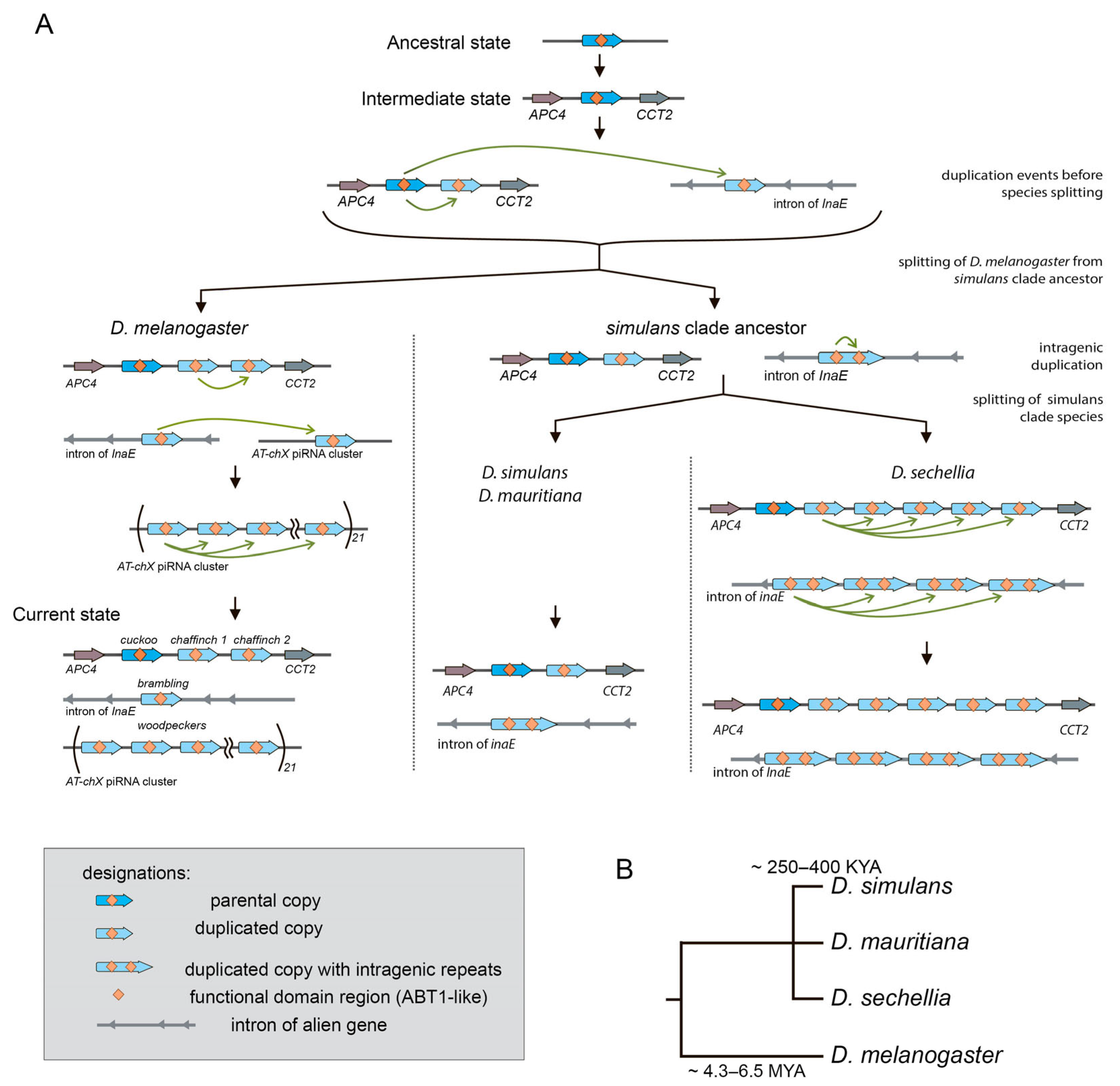
3.1. Survey of Recurrent Duplications of Esf2/ABP1 Family Genes in Drosophila
3.2. Phylogenetic Analysis for Homologous Genes of Melanogaster Subgroup
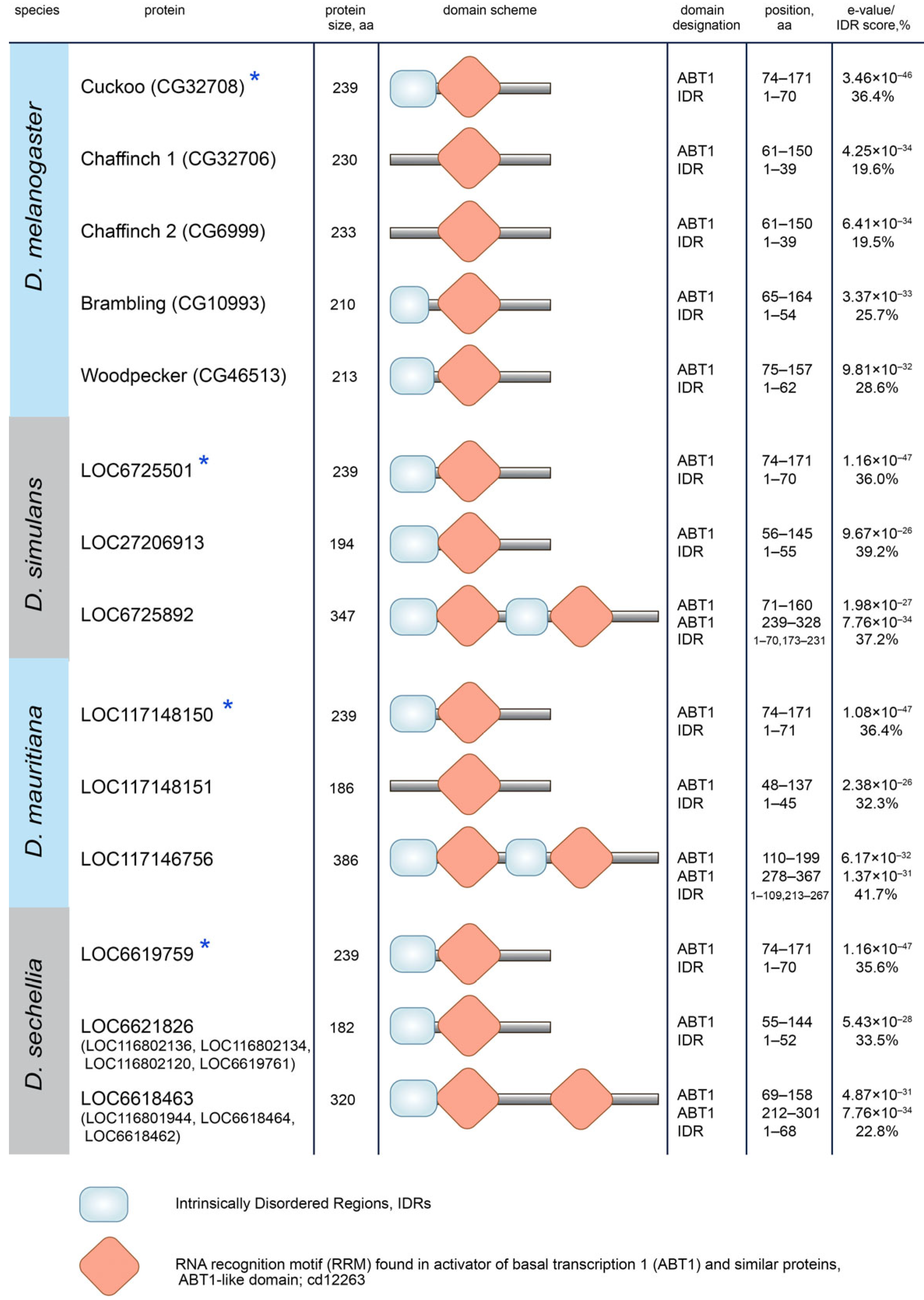
3.3. Protein Domain Search in Esf2/ABP1 Proteins
3.4. Diversification of the Expression Pattern of Esf2/ABP1 Paralogs in D. melanogaster and D. sechellia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, M.; Betrán, E.; Thornton, K.; Wang, W. The origin of new genes: Glimpses from the young and old. Nat. Rev. Genet. 2003, 4, 865–875. [Google Scholar] [CrossRef]
- Conant, G.C.; Wolfe, K.H. Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef]
- Ohno, S. Evolution by Gene Duplication; Springer: New York, NY, USA, 1970; pp. 1–160. [Google Scholar]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef]
- Assis, R.; Bachtrog, D. Neofunctionalization of young duplicate genes in Drosophila. Proc. Natl. Acad. Sci. USA 2013, 110, 17409–17414. [Google Scholar] [CrossRef] [PubMed]
- Innan, H.; Kondrashov, F. The evolution of gene duplications: Classifying and distinguishing between models. Nat. Rev. Genet. 2010, 11, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Kayukawa, K.; Hagiwara, H.; Yudate, H.T.; Masuho, Y.; Murakami, Y.; Tamura, T.A.; Muramatsu, M.A. A novel TATA-binding protein-binding protein, ABT1, activates basal transcription and has a yeast homolog that is essential for growth. Mol. Cell. Biol. 2000, 20, 1407–1418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoang, T.; Peng, W.T.; Vanrobays, E.; Krogan, N.; Hiley, S.; Beyer, A.L.; Osheim, Y.N.; Greenblatt, J.; Hughes, T.R.; Lafontaine, D.L. Esf2p, a U3-associated factor required for small-subunit processome assembly and compaction. Mol. Cell. Biol. 2005, 25, 5523–5534. [Google Scholar] [CrossRef]
- Mummery-Widmer, J.L.; Yamazaki, M.; Stoeger, T.; Novatchkova, M.; Bhalerao, S.; Chen, D.; Dietzl, G.; Dickson, B.J.; Knoblich, J.A. Genome-wide analysis of Notch signaling in Drosophila by transgenic RNAi. Nature 2009, 458, 987–992. [Google Scholar] [CrossRef]
- Schnorrer, F.; Schönbauer, C.; Langer, C.C.; Dietzl, G.; Novatchkova, M.; Schernhuber, K.; Fellner, M.; Azaryan, A.; Radolf, M.; Stark, A.; et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 2010, 464, 287–291. [Google Scholar] [CrossRef]
- Neumüller, R.A.; Richter, C.; Fischer, A.; Novatchkova, M.; Neumüller, K.G.; Knoblich, J.A. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell 2011, 8, 580–593. [Google Scholar] [CrossRef]
- Viswanatha, R.; Li, Z.; Hu, Y.; Perrimon, N. Pooled genome-wide CRISPR screening for basal and context-specific fitness gene essentiality in Drosophila cells. eLife 2018, 7, e36333. [Google Scholar] [CrossRef]
- Clifton, B.D.; Jimenez, J.; Kimura, A.; Chahine, Z.; Librado, P.; Sánchez-Gracia, A.; Abbassi, M.; Carranza, F.; Chan, C.; Marchetti, M.; et al. Understanding the early evolutionary stages of a tandem Drosophila melanogaster-specific gene family: A structural and functional population study. Mol. Biol. Evol. 2020, 37, 2584–2600. [Google Scholar] [CrossRef]
- Chang, C.H.; Mejia Natividad, I.; Malik, H.S. Expansion and loss of sperm nuclear basic protein genes in Drosophila correspond with genetic conflicts between sex chromosomes. eLife 2023, 12, e85249. [Google Scholar] [CrossRef] [PubMed]
- Kuvaeva, E.E.; Cherezov, R.O.; Kulikova, D.A.; Mertsalov, I.B. The Drosophila toothrin gene related to the d4 family genes: An evolutionary view on origin and function. Int. J. Mol. Sci. 2024, 25, 13394. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.L.; Oliver, G.T.; Farkas, I.Z.; Buszczak, M.; Levine, M.T. Recurrent duplication and diversification of a vital DNA repair gene family across Drosophila. Mol. Biol. Evol. 2024, 41, msae113. [Google Scholar] [CrossRef] [PubMed]
- Zakerzade, R.; Chang, C.H.; Chatla, K.; Krishnapura, A.; Appiah, S.P.; Zhang, J.; Unckless, R.L.; Blumenstiel, J.P.; Bachtrog, D.; Wei, K.H. Diversification and recurrent adaptation of the synaptonemal complex in Drosophila. PLoS Genet. 2025, 21, e1011549. [Google Scholar] [CrossRef]
- Chakraborty, M.; Fry, J.D. Parallel functional changes in independent testis-specific duplicates of Aldehyde dehydrogenase in Drosophila. Mol. Biol. Evol. 2015, 32, 1029–1038. [Google Scholar] [CrossRef]
- Clark, A.G.; Eisen, M.B.; Smith, D.R.; Bergman, C.M.; Oliver, B.; Markow, T.A.; Kaufman, T.C.; Kellis, M.; Gelbart, W.; Iyer, V.N. Drosophila 12 Genomes Consortium. Evolution of genes and genomes on the Drosophila phylogeny. Nature 2007, 450, 203–218. [Google Scholar]
- Miller, D.E.; Staber, C.; Zeitlinger, J.; Hawley, R.S. Highly contiguous genome assemblies of 15 Drosophila species generated using nanopore sequencing. G3 Genes|Genomes|Genetics 2018, 8, 3131–3141. [Google Scholar] [CrossRef]
- Kim, B.Y.; Wang, J.R.; Miller, D.E.; Barmina, O.; Delaney, E.; Thompson, A.; Comeault, A.A.; Peede, D.; D’Agostino, E.R.R.; Pelaez, J.; et al. Highly contiguous assemblies of 101 drosophilid genomes. eLife 2021, 10, e66405. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Conte, A.D.; Mehdiabadi, M.; Bouhraoua, A.; Miguel Monzon, A.; Tosatto, S.C.E.; Piovesan, D. Critical assessment of protein intrinsic disorder prediction (CAID)—Results of round 2. Proteins 2023, 91, 1925–1934. [Google Scholar] [CrossRef]
- Bergman, C.M.; Haddrill, P.R. Strain-specific and pooled genome sequences for populations of Drosophila melanogaster from three continents. F1000Res. 2015, 4, 31. [Google Scholar] [CrossRef][Green Version]
- Shukla, H.G.; Chakraborty, M.; Emerson, J.J. Genetic variation in recalcitrant repetitive regions of the Drosophila melanogaster genome. Genome Res. 2025, 35, 2023–2040. [Google Scholar] [CrossRef]
- Teixeira, F.K.; Okuniewska, M.; Malone, C.D.; Coux, R.X.; Rio, D.C.; Lehmann, R. piRNA-mediated regulation of transposon alternative splicing in the soma and germ line. Nature 2017, 552, 268–272. [Google Scholar] [CrossRef]
- Yang, H.; Jaime, M.; Polihronakis, M.; Kanegawa, K.; Markow, T.; Kaneshiro, K.; Oliver, B. Re-annotation of eight Drosophila genomes. Life Sci. Alliance 2018, 1, e201800156. [Google Scholar] [CrossRef]
- Maksimov, D.A.; Laktionov, P.P.; Posukh, O.V.; Belyakin, S.N.; Koryakov, D.E. Genome-wide analysis of SU(VAR)3-9 distribution in chromosomes of Drosophila melanogaster. Chromosoma 2018, 127, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Kotov, A.A.; Godneeva, B.K.; Bazylev, S.S.; Olenina, L.V.; Aravin, A.A. piRNA-mediated gene regulation and adaptation to sex-specific transposon expression in D. melanogaster male germline. Genes Dev. 2021, 35, 914–935. [Google Scholar] [CrossRef]
- Ramalingam, V.; Natarajan, M.; Johnston, J.; Zeitlinger, J. TATA and paused promoters active in differentiated tissues have distinct expression characteristics. Mol. Syst. Biol. 2021, 17, e9866. [Google Scholar] [CrossRef] [PubMed]
- Mahadevaraju, S.; Pal, S.; Bhaskar, P.; McDonald, B.D.; Benner, L.; Denti, L.; Cozzi, D.; Bonizzoni, P.; Przytycka, T.M.; Oliver, B. Diverse somatic Transformer and sex chromosome karyotype pathways regulate gene expression in Drosophila gonad development. bioRxiv 2024. bioRxiv:2024.08.12.607556. [Google Scholar] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Srivastav, S.P.; Feschotte, C.; Clark, A.G. Rapid evolution of piRNA clusters in the Drosophila melanogaster ovary. Genome Res. 2024, 34, 711–724. [Google Scholar] [CrossRef]
- Kotov, A.A.; Adashev, V.E.; Godneeva, B.K.; Ninova, M.; Shatskikh, A.S.; Bazylev, S.S.; Aravin, A.A.; Olenina, L.V. piRNA silencing contributes to interspecies hybrid sterility and reproductive isolation in Drosophila melanogaster. Nucleic Acids Res. 2019, 47, 4255–4271. [Google Scholar] [CrossRef]
- Malone, C.D.; Brennecke, J.; Dus, M.; Stark, A.; McCombie, W.R.; Sachidanandam, R.; Hannon, G.J. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 2009, 137, 522–535. [Google Scholar] [CrossRef]
- Antoniewski, C. Computing siRNA and piRNA overlap signatures. Methods Mol. Biol. 2014, 1173, 135–146. [Google Scholar]
- Fan, C.; Chen, Y.; Long, M. Recurrent tandem gene duplication gave rise to functionally divergent genes in Drosophila. Mol. Biol. Evol. 2008, 25, 1451–1458. [Google Scholar] [CrossRef]
- Lachaise, D.; Cariou, M.L.; David, J.R.; Lemeunier, F.; Tsacas, L.; Ashburner, M. Historical biogeography of the Drosophila melanogaster species subgroup. Evol. Biol. 1988, 22, 159–225. [Google Scholar]
- Russo, C.A.; Takezaki, N.; Nei, M. Molecular phylogeny and divergence times of drosophilid species. Mol. Biol. Evol. 1995, 12, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Subramanian, S.; Kumar, S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol. 2004, 21, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Garrigan, D.; Kingan, S.B.; Geneva, A.J.; Andolfatto, P.; Clark, A.G.; Thornton, K.R.; Presgraves, D.C. Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res. 2012, 22, 1499–1511. [Google Scholar] [CrossRef]
- Suvorov, A.; Kim, B.Y.; Wang, J.; Armstrong, E.E.; Peede, D.; D’Agostino, E.R.R.; Price, D.K.; Waddell, P.; Lang, M.; Courtier-Orgogozo, V.; et al. Widespread introgression across a phylogeny of 155 Drosophila genomes. Curr. Biol. 2022, 32, 111–123.e5. [Google Scholar] [CrossRef]
- Konstantinidou, P.; Loubalova, Z.; Ahrend, F.; Friman, A.; Almeida, M.V.; Poulet, A.; Horvat, F.; Wang, Y.; Losert, W.; Lorenzi, H.; et al. A comparative roadmap of PIWI-interacting RNAs across seven species reveals insights into de novo piRNA-precursor formation in mammals. Cell Rep. 2024, 43, 114777. [Google Scholar] [CrossRef]
- Chang, C.H.; Gregory, L.E.; Gordon, K.E.; Meiklejohn, C.D.; Larracuente, A.M. Unique structure and positive selection promote the rapid divergence of Drosophila Y chromosomes. eLife 2022, 11, e75795. [Google Scholar] [CrossRef]
- Montanari, F.; Shields, D.C.; Khaldi, N. Differences in the number of intrinsically disordered regions between yeast duplicated proteins, and their relationship with functional divergence. PLoS ONE 2011, 6, e24989. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Aravin, A.A.; Naumova, N.M.; Tulin, A.V.; Vagin, V.V.; Rozovsky, Y.M.; Gvozdev, V.A. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 2001, 11, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef]
- Kotov, A.A.; Bazylev, S.S.; Adashev, V.E.; Shatskikh, A.S.; Olenina, L.V. Drosophila as a model system for studying of the evolution and functional specialization of the Y chromosome. Int. J. Mol. Sci. 2022, 23, 4184. [Google Scholar] [CrossRef] [PubMed]
- Prestel, M.; Feller, C.; Straub, T.; Mitlöhner, H.; Becker, P.B. The activation potential of MOF is constrained for dosage compensation. Mol. Cell 2010, 38, 815–826. [Google Scholar] [CrossRef]
- Lucchesi, J.C.; Kuroda, M.I. Dosage compensation in Drosophila. Cold Spring Harb. Perspect. Biol. 2015, 7, a019398. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, L.; Kuroda, M.I. An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech. Dev. 1998, 71, 107–117. [Google Scholar] [CrossRef]
- Meiklejohn, C.D.; Landeen, E.L.; Cook, J.M.; Kingan, S.B.; Presgraves, D.C. Sex chromosome-specific regulation in the Drosophila male germline but little evidence for chromosomal dosage compensation or meiotic inactivation. PLoS Biol. 2011, 9, e1001126. [Google Scholar] [CrossRef]
- Mahadevaraju, S.; Fear, J.M.; Akeju, M.; Galletta, B.J.; Pinheiro, M.M.L.S.; Avelino, C.C.; Cabral-de-Mello, D.C.; Conlon, K.; Dell’Orso, S.; Demere, Z.; et al. Dynamic sex chromosome expression in Drosophila male germ cells. Nat. Commun. 2021, 12, 892. [Google Scholar] [CrossRef]
- Ariura, M.; Solberg, T.; Ishizu, H.; Takahashi, H.; Carninci, P.; Siomi, H.; Iwasaki, Y.W. Drosophila Piwi distinguishes transposons from mRNAs by piRNA complementarity and abundance. Cell Rep. 2024, 43, 115020. [Google Scholar] [CrossRef]
- Chen, P.; Aravin, A.A. Genetic control of a sex-specific piRNA program. Curr. Biol. 2023, 33, 1825–1835.e3. [Google Scholar] [CrossRef]
- Chang, C.H.; Larracuente, A.M. Heterochromatin-enriched assemblies reveal the sequence and organization of the Drosophila melanogaster Y chromosome. Genetics 2019, 211, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Adashev, V.E.; Kotov, A.A.; Bazylev, S.S.; Shatskikh, A.S.; Aravin, A.A.; Olenina, L.V. Stellate genes and the piRNA pathway in speciation and reproductive isolation of Drosophila melanogaster. Front. Genet. 2021, 11, 610665. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2024, 25, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Intrinsically unstructured proteins evolve by repeat expansion. Bioessays 2003, 25, 847–855. [Google Scholar] [CrossRef]

| Gene Number | New Gene Designation |
|---|---|
| CG32708 | cuckoo |
| CG32706 | chaffinch 1 |
| CG6999 | chaffinch 2 |
| CG10993 | brambling |
| CG40813 | woodpecker 1 |
| CG46504 | woodpecker 2 |
| CG46505 | woodpecker 3 |
| CG46506 | woodpecker 4 |
| CG46507 | woodpecker 5 |
| CG46508 | woodpecker 6 |
| CG46509 | woodpecker 7 |
| CG46510 | woodpecker 8 |
| CG46511 | woodpecker 9 |
| CG46512 | woodpecker 10 |
| CG46513 | woodpecker 11 |
| CG46514 | woodpecker 12 |
| CG46515 | woodpecker 13 |
| CG46516 | woodpecker 14 |
| CG46517 | woodpecker 15 |
| CG46518 | woodpecker 16 |
| CG46519 | woodpecker 17 |
| CG46520 | woodpecker 18 |
| CG46521 | woodpecker 19 |
| CG46522 | woodpecker 20 |
| CG41562 | woodpecker 21 |
| Gene | woodpeckers | brambling | cuckoo | chaffinch1 | chaffinch2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| piRNA Read Type | Sense piRNAs | Antisense piRNAs | Sense piRNAs | Antisense piRNAs | Sense piRNAs | Antisense piRNAs | Sense piRNAs | Antisense piRNAs | Sense piRNAs | Antisense piRNAs |
| Mapped reads (0–2 mm), rpm | 201.6 | 568.6 | 115.8 | 203.8 | 68.8 | 47.2 | 0.6 | 0.2 | 0 | 0.2 |
| Mapped reads (0 mm), rpm | 150.3 | 422.7 | 1.1 | 55.2 | 15.9 | 0.9 | 0 | 0 | 0 | 0 |
| Mapped reads (1 mm), rpm | 40.5 | 129.5 | 25.2 | 51.4 | 10.1 | 3.2 | 0 | 0 | 0 | 0 |
| Mapped reads (2 mm), rpm | 10.7 | 16.4 | 89.5 | 97.1 | 45.7 | 43.2 | 0.6 | 0.2 | 0 | 0.2 |
| Overlap_10, pairs | 365 | 155 | 23 | 0 | 0 | |||||
| z10 score | 2.86 | 3.08 | 1.01 | NA | NA | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davydova, E.D.; Kotov, A.A.; Chernizova, A.V.; Yakovleva, E.Y.; Olenina, L.V. Recurrent Duplication, Testis-Biased Expression, and Functional Diversification of Esf2/ABT1 Family Genes in Drosophila. Insects 2025, 16, 956. https://doi.org/10.3390/insects16090956
Davydova ED, Kotov AA, Chernizova AV, Yakovleva EY, Olenina LV. Recurrent Duplication, Testis-Biased Expression, and Functional Diversification of Esf2/ABT1 Family Genes in Drosophila. Insects. 2025; 16(9):956. https://doi.org/10.3390/insects16090956
Chicago/Turabian StyleDavydova, Elizaveta D., Alexei A. Kotov, Alina V. Chernizova, Ekaterina Yu. Yakovleva, and Ludmila V. Olenina. 2025. "Recurrent Duplication, Testis-Biased Expression, and Functional Diversification of Esf2/ABT1 Family Genes in Drosophila" Insects 16, no. 9: 956. https://doi.org/10.3390/insects16090956
APA StyleDavydova, E. D., Kotov, A. A., Chernizova, A. V., Yakovleva, E. Y., & Olenina, L. V. (2025). Recurrent Duplication, Testis-Biased Expression, and Functional Diversification of Esf2/ABT1 Family Genes in Drosophila. Insects, 16(9), 956. https://doi.org/10.3390/insects16090956







