Lipid Composition Analysis of Cricket Oil from Crickets Fed with Broken Rice-Derived Bran
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
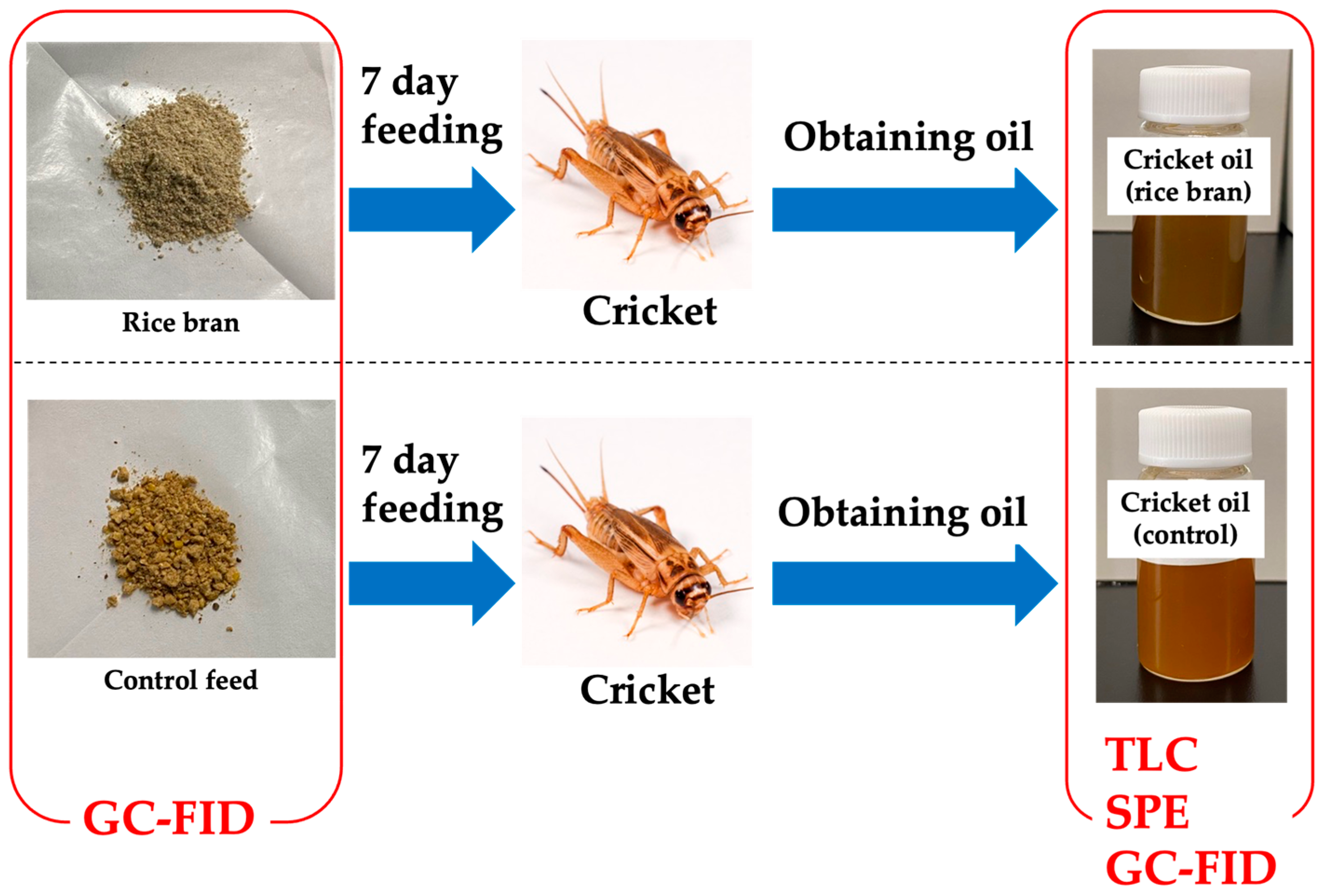
2.1. Materials
2.2. Extraction of Total Lipids from Cricket Feed
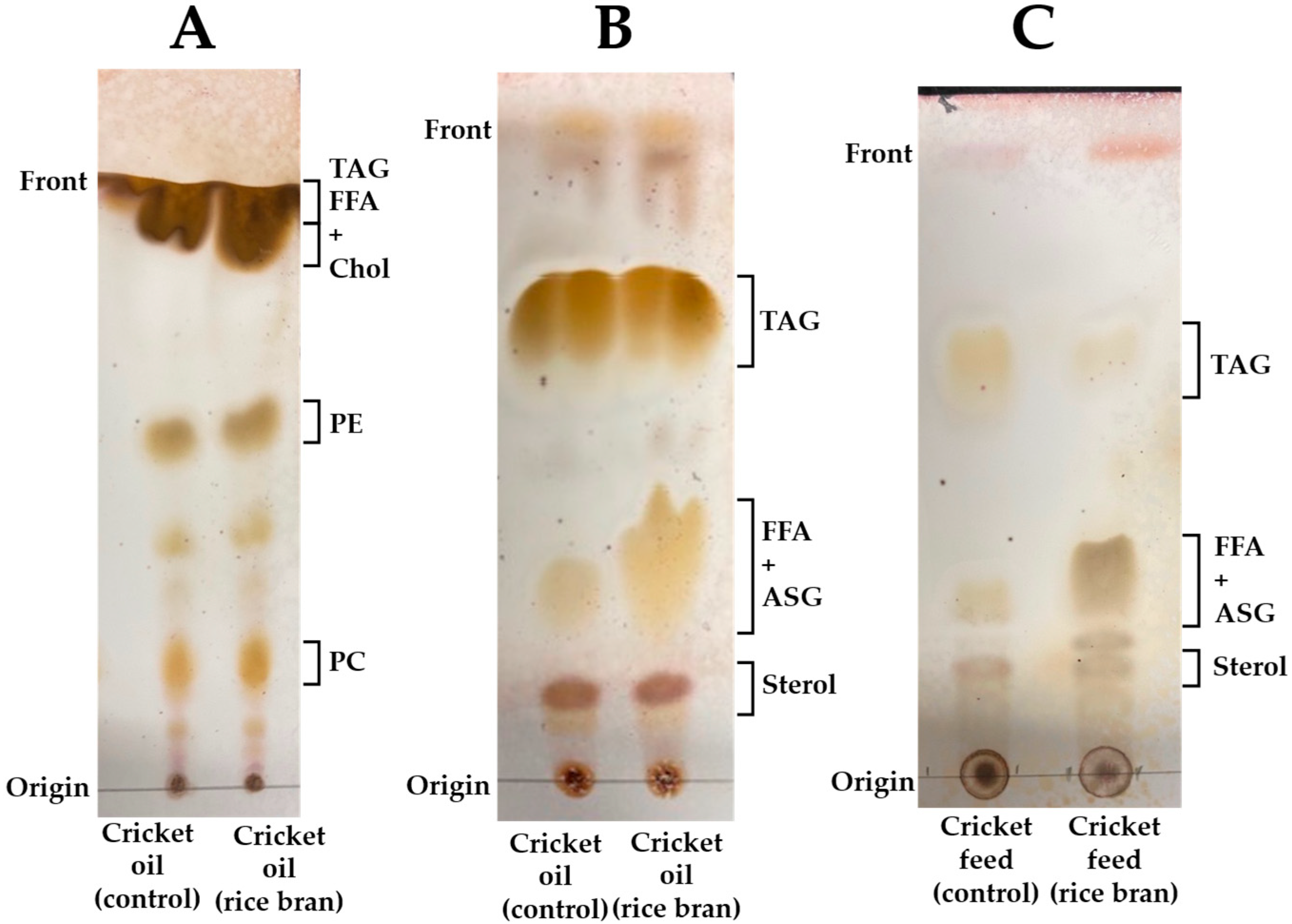
2.3. Identification of Lipid Classes by Thin-Layer Chromatography (TLC)
2.4. Fractionation of Lipid Classes Using Solid-Phase Extraction (SPE)
2.5. Analysis of Fatty Acid Composition by GC-FID
2.6. Statistical Analysis
3. Results
3.1. Identification of Lipid Classes in Cricket Oil and Their Feed
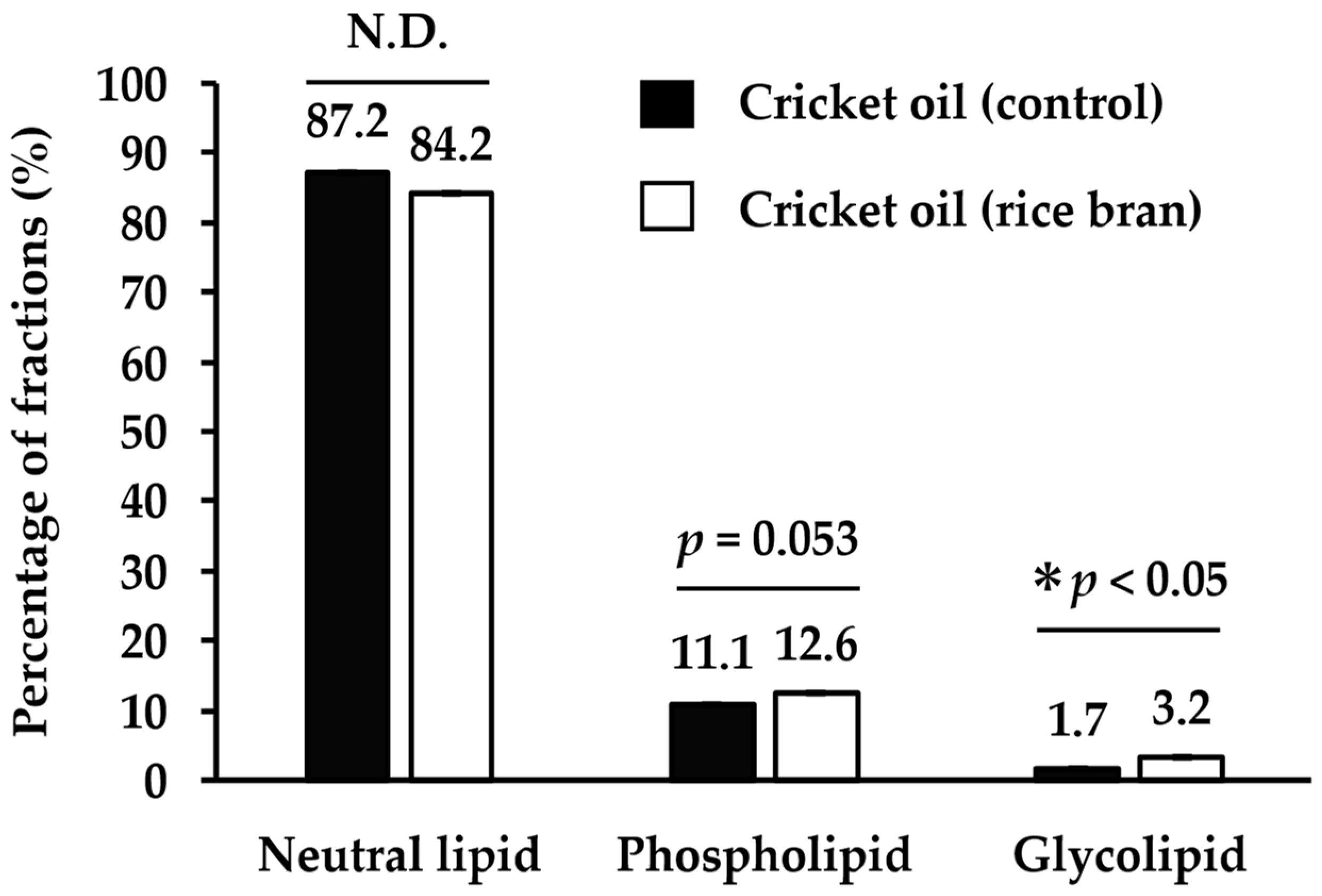
3.2. Lipid Class Composition of Cricket Oil
3.3. Fatty Acid Composition of Control Feed and Rice Bran
3.4. Fatty Acid Composition of Cricket Oil
3.5. Fatty Acid Composition of Neutral Lipid Fractions Extracted from Cricket Oils
3.6. Fatty Acid Composition of Phospholipid Fraction Extracted from Cricket Oils
3.7. Fatty Acid Composition of Glycolipid Fraction Extracted from Cricket Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. World Population Prospects 2022: Summary of Results. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 23 January 2025).
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects Future Prospects for Food Feed Security; FAO: Rome, Italy, 2013; Available online: https://www.fao.org/4/i3253e/i3253e.pdf (accessed on 23 January 2025).
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional Composition of Five Commercial Edible Insects in South Korea. J. Asia Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Mark, D.F. Complete Nutrient Composition of Commercially Raised Invertebrates Used as Food for Insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Kolobe, S.D.; Manyelo, T.G.; Malematja, E.; Sebola, N.A.; Mabelebele, M. Fats and Major Fatty Acids Present in Edible Insects Utilised as Food and Livestock Feed. Vet. Anim. Sci. 2023, 22, 100312. [Google Scholar] [CrossRef]
- Ityotagher, P.A.; Casimir, C.A. A Sustainable Approach to Functional Lipids. INFORM 2024, 35, 20–23. [Google Scholar]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Nyangena, D.N.; Osuga, I.M.; Cheseto, X.; Subramanian, S.; Ekesi, S.; Tanga, C.M. Physicochemical Properties of Edible Cricket Oils: Implications for Use in Pharmaceutical and Food Industries. Future Foods 2024, 9, 100316. [Google Scholar] [CrossRef] [PubMed]
- Kourimska, L.; Adámkova, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Zhou, S.; Duan, H.; Guo, J.; Yan, W. Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review. Foods 2022, 11, 3961. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santaescolastica, C.; de Pril, I.; van de Voorde, I.; Fraeye, I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods 2023, 12, 4090. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- Mafu, A.; Ketnawa, S.; Phongthai, S.; Schonlechner, R.; Rawdkuen, S. Whole Wheat Bread Enriched with Cricket Powder as an Alternative Protein. Foods 2022, 11, 2142. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed. Edible Insects on the European Market; International Platform of Insects for Food and Feed (IPIFF): Brussels, Belgium, 2020; Available online: https://ipiff.org/wp-content/uploads/2020/06/10-06-2020-IPIFF-edible-insects-market-factsheet.pdf (accessed on 28 January 2025).
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta Domesticus) and Field Cricket (Gryllus Bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Fresch, M.; Scherber, C.; Brockmeyer, J. The Lipidome of an Omnivorous Insect Responds to Diet Composition and Social Environment. Ecol. Evol. 2022, 12, e9497. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Marnila, P.; Mooney, P.; Mäki, M.; Siljander-Rasi, H.; et al. The Plant-Based by-Product Diets for the Mass-Rearing of Acheta Domesticus and Gryllus Bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Solofondranohatra, C.L.; Hugel, S.; Fisher, B.L. Weeds and Agro By-Products for Sustainable Farming of Edible Field Cricket, Gryllus Madagascarensis (Orthoptera: Gryllidae). PLoS ONE 2025, 20, e0313083. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of Diet on the Growth Performance, Feed Conversion, and Nutrient Content of the House Cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Starcevic, K.; Gavrilovic, A.; Gottstein, Z.; Masek, T. Influence of Substitution of Sunflower Oil by Different Oils on the Growth, Survival Rate and Fatty Acid Composition of Jamaican Field Cricket (Gryllus Assimilis). Anim. Feed Sci. Technol. 2017, 228, 66–71. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary Enrichment of Edible Insects with Omega 3 Fatty Acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Umebara, I.; Akutsu, K.; Kubo, M.; Iijima, A.; Sakurai, R.; Masutomi, H.; Ishihara, K. Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta Domesticus) Reared on Apple By-Products. Foods 2024, 13, 1668. [Google Scholar] [CrossRef]
- Japan Ministry of Agriculture, Forestry and Fisheries. Major Data, Etc. on the Draft Basic Guidelines for Rice. 2025. Available online: https://www.maff.go.jp/j/council/seisaku/syokuryo/250131/attach/pdf/250131-7.pdf (accessed on 26 January 2025).
- Lim, J.S.; Abdul Manan, Z.; Hashim, H.; Wan Alwi, S.R. Towards an Integrated, Resource-Efficient Rice Mill Complex. Resour. Conserv. Recycl. 2013, 75, 41–51. [Google Scholar] [CrossRef]
- Nahemiah, D.; Nkama, I.; Paul Yahaya, I.; Halidu Badau, M.; Umar, A. Advances in Rice Postharvest Loss Reduction Strategies in Africa through Low Grade Broken Rice Fractions and Husk Value Addition. In Recent Advances in Rice Research; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Pastell, H.; Mellberg, S.; Ritvanen, T.; Raatikainen, M.; Mykkänen, S.; Niemi, J.; Latomäki, I.; Wirtanen, G. How Does Locally Produced Feed Affect the Chemical Composition of Reared House Crickets (Acheta Domesticus)? ACS Food Sci. Technol. 2021, 1, 625–635. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method For The Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Miyazawa, T.; Higuchi, O.; Sogame, R.; Miyazawa, T. Determination of Plasmalogen Molecular Species in Hen Eggs. Molecules 2024, 29, 4795. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Miyazawa, T.; Kinoshita, M.; Miyazawa, T. Shishitsu Sankashishitsu Bunsekihou Nyuumon; Saiwai Shobo: Tokyo, Japan, 2023. [Google Scholar]
- Chen, G.; Jiang, Y.; Chen, F. Fatty Acid and Lipid Class Composition of the Eicosapentaenoic Acid-Producing Microalga, Nitzschia Laevis. Food Chem. 2007, 104, 1580–1585. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P.P. Determination of Sterol and Fatty Acid Compositions, Oxidative Stability, and Nutritional Value of Six Walnut (Juglans Regia L.) Cultivars Grown in Portugal. J. Agric Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef]
- Fukasawa, R.; Miyazawa, T.; Abe, C.; Bhaswant, M.; Toda, M. Quantification and Comparison of Nutritional Components in Oni Walnut (Juglans Ailanthifolia Carr.), Hime Walnut (Juglans Subcordiformis Dode.), and Cultivars. Horticulturae 2023, 9, 1221. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Dewettink, K.; Provijin, P.; Brouwers, J.F.; de Meulenaer, B.; Oonincx, D.G.A.B. Lipidome of cricket species used as food. Food Chem. 2021, 349, 129077. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.H.; Juliano, B.O. Lipids in Developing and Mature Rice Grain. Phytochemistry 1980, 19, 1063–1069. [Google Scholar] [CrossRef]
- Zhou, Z.; Blanchard, C.; Helliwell, S.; Robards, K. Fatty Acid Composition of Three Rice Varieties Following Storage. J. Cereal Sci. 2003, 37, 327–335. [Google Scholar] [CrossRef]
- Hirayama, O.; Matsuda, H. Lipid Components and Distribution in Brown Rice. J. Agric. Chem. Soc. Jpn. 1973, 47, 371–377. [Google Scholar] [CrossRef]
- Hemavathy, J.; Prabhakar, J.V. Lipid composition of rice (Oryza sativa L.) bran. J. Am. Oil Chem. Soc. 1987, 64, 1016–1019. [Google Scholar] [CrossRef]
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric. 2014, 94, 929–934. [Google Scholar] [CrossRef]
- Blomoquist, G.J.; Borgeson, C.E.; Vundla, M. Polyunsaturated fatty acids and eicosanoids in insects. Insect Biochem. 1991, 21, 99–106. [Google Scholar] [CrossRef]
- Lambremont, E.N.; Dial, P.F. Fatty Acid Composition of Major Phospholipids from the Fat Body, Flight Muscle and Testis of the House Cricket, and Positional Distribution of Fatty Acids in Testicular Phospholipids. Comp. Biochem. Physiol. Part B Comp. Biochem. 1980, 66, 327–330. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Loher, W.; Blomquist, G.J. Biosynthesis of Polyunsaturated Fatty Acids by The Australian Field Cricket, Teleogryllus commodus. Insect Biochem. 1986, 16, 387–393. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Fernando, I. Acheta Domesticus (House Cricket) as Human Foods—An Approval of the European Commission—A Systematic Review. Food Front. 2024, 5, 435–473. [Google Scholar] [CrossRef]
- Bonanome, A.; Grundy, S.M. Effect of Dietary Stearic Acid on Plasma Cholesterol and Lipoprotein Levels. N. Engl. J. Med. 1988, 318, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
| Cricket Feed (Control) | Cricket Feed (Rice Bran) | |
|---|---|---|
| C12:0 (Lauric acid) | 0.04 ± 0.01 | N.D. |
| C14:0 (Myristic acid) | 1.69 ± 0.12 | 0.35 ± 0.01 |
| C15:0 (Pentadecanoic acid) | 0.22 ± 0.01 | 0.07 ± 0.00 |
| C16:0 (Palmitic acid) | 20.39 ± 0.14 | 16.88 ± 0.08 |
| C16:1 (cis-9 Palmitoleic acid | 2.67 ± 0.09 | 0.18 ± 0.01 |
| C18:0 (Stearic acid) | 6.62 ± 0.05 | 1.96 ± 0.00 |
| C18:1 (trans-9 Elaidic acid) | 0.63 ± 0.02 | N.D. |
| C18:1 (cis-9 Oleic acid) | 31.63 ± 0.32 | 39.41 ± 0.05 |
| C18:2 (cis-9,12 Linoleic acid) | 22.87 ± 0.52 | 37.42 ± 0.05 |
| C18:3 (cis-9,12,15 α-Linolenic acid) | 1.36 ± 0.02 | 1.40 ± 0.00 |
| C20:0 (Arachidic acid) | 0.35 ± 0.00 | 0.58 ± 0.00 |
| C20:1 (cis-11 Eicosenoic acid) | 1.90 ± 0.24 | 0.47 ± 0.01 |
| C20:2 (cis-11,14 Eicosadienoic acid) | 0.15 ± 0.01 | 0.02 ± 0.00 |
| C20:4 (cis-5,8,11,14 Arachidonic acid) | 0.46 ± 0.01 | N.D. |
| C20:5 (cis-5,8,11,14,17 Eicosapentaenoic acid) | 3.25 ± 0.11 | N.D. |
| C22:0 (Behenic acid) | 0.30 ± 0.15 | 0.39 ± 0.00 |
| C22:1 (cis-13 Erucic acid) | 0.31 ± 0.02 | 0.02 ± 0.01 |
| C22:6 (cis-4,7,10,13,16,19 Docosahexaenoic acid) | 4.34 ± 0.16 | N.D. |
| C23:0 (Tricosanoic acid) | 0.03 ± 0.00 | 0.06 ± 0.01 |
| C24:0 (Lignoceric acid) | 0.28 ± 0.00 | 0.76 ± 0.00 |
| C24:1 (Nervonic acid) | 0.29 ± 0.05 | 0.01 ± 0.00 |
| SFA ratio | 29.93 ± 0.33 | 21.05 ± 0.08 |
| MUFA ratio | 37.49 ± 0.09 | 40.10 ± 0.04 |
| PUFA ratio | 32.58 ± 0.29 | 38.85 ± 0.05 |
| ω-6 ratio | 23.58 ± 0.51 | 37.45 ± 0.05 |
| ω-3 ratio | 9.00 ± 0.25 | 1.40 ± 0.00 |
| ω-6/ω-3 ratio | 2.63 ± 0.13 | 26.69 ± 0.04 |
| SFA/UFA ratio | 0.43 ± 0.01 | 0.27 ± 0.00 |
| PUFA/MUFA ratio | 0.87 ± 0.01 | 0.97 ± 0.00 |
| Total Lipid | Neutral Lipid Fraction | Phospholipid Fraction | Glycolipid Fraction | |||||
|---|---|---|---|---|---|---|---|---|
| Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | Cricket oil (Control Feed) | Cricket oil (Rice Bran) | Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | |
| C12:0 (Lauric acid) | 0.1 ± 0 | 0.1 ± 0 ** | 0 ± 0 | 0 ± 0 | ND | ND | 0 ± 0 | 0 ± 0 |
| C14:0 (Myristic acid) | 0.9 ± 0 | 0.8 ± 0 ** | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.1 ± 0 | 0.1 ± 0 | 0.6 ± 0 | 0.5 ± 0 * |
| C14:1 (cis-9 Myristoleic acid) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | N.D. | N.D. | N.D. | N.D. |
| C15:0 (Pentadecanoic acid) | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.2 ± 0 | 0.1 ± 0 * |
| C16:0 (Palmitic acid) | 28 ± 0.1 | 27 ± 0 ** | 28 ± 0.2 | 27 ± 0.2 | 12 ± 0.1 | 13 ± 0.2 | 23 ± 0.2 | 26 ± 0.2 ** |
| C16:1 (cis-9 Palmitoleic acid) | 1.1 ± 0 | 0.9 ± 0 ** | 1.1 ± 0 | 0.8 ± 0 ** | 0.5 ± 0 | 0.4 ± 0 | 1.1 ± 0 | 0.8 ± 0 ** |
| C18:0 (Stearic acid) | 7.7 ± 0 | 7.4 ± 0 ** | 7.3 ± 0 | 6.9 ± 0 ** | 14 ± 0.1 | 15 ± 0.1 ** | 9.6 ± 0.1 | 7.9 ± 0 ** |
| C18:1 (trans-9 Elaidic acid) | 0.3 ± 0 | 0.3 ± 0 | 0.5 ± 0 | 0.3 ± 0 * | 0.6 ± 0 | 0.6 ± 0 | 0.5 ± 0 | 0.3 ± 0 * |
| C18:1 (cis-9 Oleic acid) | 28 ± 0 | 30 ± 0 ** | 29 ± 0.1 | 32 ± 0.1 ** | 16 ± 0.1 | 15 ± 0 | 27 ± 0.2 | 27 ± 0.1 |
| C18:2 (trans-9,12 Linolelaidic acid) | N.D. | N.D. | N.D. | N.D. | 0 ± 0 | 0 ± 0 | 0.1 ± 0 | 0 ± 0 |
| C18:2 (cis-9,12 Linoleic acid) | 32 ± 0 | 32 ± 0 ** | 31 ± 0.2 | 30 ± 0.1 * | 54 ± 0.2 | 54 ± 0.1 | 35 ± 0.3 | 36 ± 0.2 * |
| C18:3 (cis-9,12,15 Linolenic acid) | 0.7 ± 0 | 0.5 ± 0 ** | 0.7 ± 0 | 0.5 ± 0 ** | 0.4 ± 0 | 0.3 ± 0 ** | 0.7 ± 0 | 0.4 ± 0 ** |
| C20:0 (Arachidic acid) | 0.3 ± 0 | 0.3 ± 0 | 0.3 ± 0 | 0.3 ± 0 | 0.3 ± 0 | 0.4 ± 0 * | 0.5 ± 0 | 0.4 ± 0 |
| C20:1 (cis-11 Eicosenoic acid) | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 * | 0.1 ± 0 | 0 ± 0 * | 0.3 ± 0 | 0.1 ± 0 ** |
| C20:2 (cis-11,14 Eicosadienoic acid) | 0 ± 0 | 0 ± 0 * | 0 ± 0 | 0 ± 0 | 0.1 ± 0 | 0.1 ± 0 * | 0.1 ± 0 | 0 ± 0 ** |
| C20:3 (cis-8,11,14 Eicosatrienoic acid) | N.D. | N.D. | N.D. | N.D. | 0 ± 0 | 0 ± 0 | N.D. | N.D. |
| C20:4 (cis-5,8,11,14 Arachidonic acid) | 0.2 ± 0 | 0.1 ± 0 ** | 0.1 ± 0 | 0.1 ± 0 * | 1 ± 0 | 0.7 ± 0 ** | 0.3 ± 0 | 0.2 ± 0 ** |
| C20:5 (cis-5,8,11,14,17 Eicosapentaenoic acid) | 0.4 ± 0 | 0.3 ± 0 ** | 0.3 ± 0 | 0.2 ± 0 ** | 1.3 ± 0.1 | 1 ± 0.1 * | 0.6 ± 0 | 0.3 ± 0 ** |
| C22:0 (Behenic acid) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | N.D. | N.D. | 0.2 ± 0 | 0.1 ± 0 |
| C22:1 (cis-13 Erucic acid) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.1 ± 0 | 0 ± 0 ** |
| C22:6 (cis-4,7,10,13,16,19 Docosahexaenoic acid) | 0.1 ± 0 | 0 ± 0 ** | 0.1 ± 0 | 0 ± 0 ** | 0.1 ± 0 | 0 ± 0 ** | 0.2 ± 0 | 0 ± 0 ** |
| C23:0 (Tricosanoic acid) | 0.2 ± 0 | 0.3 ± 0 ** | 0.2 ± 0 | 0.3 ± 0 ** | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0.1 |
| C24:0 (Lignoceric acid) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.3 ± 0 | 0.1 ± 0 * |
| Total Lipid | Neutral Lipid Fraction | Phospholipid Fraction | Glycolipid Fraction | |||||
|---|---|---|---|---|---|---|---|---|
| Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | Cricket Oil (Control Feed) | Cricket Oil (Rice Bran) | |
| SFA | 37 ± 0.1 | 36 ± 0 ** | 37 ± 0.3 | 35 ± 0.2 * | 27 ± 0.1 | 28 ± 0.1 ** | 35 ± 0.2 | 35 ± 0.1 * |
| MUFA | 29 ± 0.1 | 31 ± 0 ** | 31 ± 0.1 | 33 ± 0.1 ** | 17 ± 0.1 | 16 ± 0 * | 29 ± 0.1 | 28 ± 0.1 * |
| PUFA | 34 ± 0 | 33 ± 0 ** | 32 ± 0.2 | 31 ± 0.1 ** | 57 ± 0.3 | 56 ± 0.1 | 37 ± 0.3 | 37 ± 0.2 |
| ω-6 | 33 ± 0 | 32 ± 0 ** | 31 ± 0.2 | 31 ± 0.1 * | 55 ± 0.3 | 55 ± 0.1 | 35 ± 0.3 | 36 ± 0.2 * |
| ω-3 | 1.1 ± 0 | 0.8 ± 0 ** | 1.1 ± 0 | 0.7 ± 0 ** | 1.7 ± 0.1 | 1.3 ± 0.1 * | 1.6 ± 0 | 0.8 ± 0 ** |
| ω-6/ω-3 | 29 ± 0.1 | 41 ± 0.1 ** | 29 ± 0 | 41 ± 0.6 ** | 32 ± 1.1 | 43 ± 2.7 * | 22 ± 0.2 | 46 ± 0.8 ** |
| SFA/UFA | 0.6 ± 0 | 0.6 ± 0 ** | 0.6 ± 0 | 0.6 ± 0 * | 0.4 ± 0 | 0.4 ± 0 ** | 0.5 ± 0 | 0.5 ± 0 |
| PUFA/MUFA | 1.2 ± 0 | 1.1 ± 0 ** | 1 ± 0 | 0.9 ± 0 ** | 3.4 ± 0 | 3.5 ± 0 | 1.3 ± 0 | 1.3 ± 0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogame, R.; Miyazawa, T.; Toda, M.; Iijima, A.; Bhaswant, M.; Miyazawa, T. Lipid Composition Analysis of Cricket Oil from Crickets Fed with Broken Rice-Derived Bran. Insects 2025, 16, 951. https://doi.org/10.3390/insects16090951
Sogame R, Miyazawa T, Toda M, Iijima A, Bhaswant M, Miyazawa T. Lipid Composition Analysis of Cricket Oil from Crickets Fed with Broken Rice-Derived Bran. Insects. 2025; 16(9):951. https://doi.org/10.3390/insects16090951
Chicago/Turabian StyleSogame, Ryosuke, Taiki Miyazawa, Masako Toda, Akihiro Iijima, Maharshi Bhaswant, and Teruo Miyazawa. 2025. "Lipid Composition Analysis of Cricket Oil from Crickets Fed with Broken Rice-Derived Bran" Insects 16, no. 9: 951. https://doi.org/10.3390/insects16090951
APA StyleSogame, R., Miyazawa, T., Toda, M., Iijima, A., Bhaswant, M., & Miyazawa, T. (2025). Lipid Composition Analysis of Cricket Oil from Crickets Fed with Broken Rice-Derived Bran. Insects, 16(9), 951. https://doi.org/10.3390/insects16090951






