Simple Summary
Pyrethroid resistance in Anopheles sinensis, a major malaria vector in Asia, severely compromises insecticide-based vector control. This study identified carboxylesterases as important factors in the mechanism of resistance to deltamethrin. Transcriptomic comparison of deltamethrin-resistant and susceptible strains revealed four upregulated carboxylesterase (CCE) genes. Based on prior evidence, we evaluated eight candidate CCEs, confirming significant upregulation of five genes (AsAe9, AsAe10, AsAce1, AsAce2, and AsBe4) in resistant strains via quantitative real-time PCR (qRT-PCR). The gene silencing mediated by RNA interference (RNAi) reduced the knockdown time and increased the mortality rates of resistant mosquitoes when exposed to deltamethrin. This study revealed carboxylesterase genes involved in pyrethroid resistance in An. sinensis, providing critical insights into resistance mechanisms and management strategies in mosquitoes.
Abstract
Carboxylesterases (CCEs) have been demonstrated to be involved in pyrethroid resistance in insect species. This study aims to investigate CCE-mediated resistance mechanisms in Anopheles sinensis, a major malaria vector. Through comparative transcriptomics of a deltamethrin-resistant strain (CQ-LR) versus susceptible strain (WX-LS) of An. sinensis, we identified differentially expressed CCE genes across five developmental stages, five tissues, and three time points post-blood-meal. Four candidate genes (AsAe9, AsAe10, AsAce2, AsUn5) showed significantly upregulated expression. Subsequent qRT-PCR validation across four field-derived resistant strains (WX-LR, AH-LR, YH-LR, CQ-LR) and the susceptible strain confirmed significant upregulation of AsAe9, AsAe10, AsAce1, AsAce2, and AsBe4 in more than two resistant populations. RNAi-based functional validation showed that silencing AsAe10 or AsBe4 in the WX-LR strain significantly decreased knockdown time and raised 24 h mortality upon diagnostic deltamethrin exposure, with AsAe10 silencing having the strongest effect. This study identifies CCE genes involved in deltamethrin resistance in An. sinensis, providing valuable insights into the resistance mechanisms of pyrethroid and a theoretical basis for mosquito resistance management.
1. Introduction
Anopheles sinensis, a primary vector for malaria and filariasis, exhibits extensive distribution across China and other East Asian nations [1,2,3]. The vivax malaria outbreak demonstrates strong epidemiological association with elevated vectorial capacity in An. sinensis for Plasmodium vivax [4]. Current strategies for controlling An. sinensis predominantly rely on pyrethroid-based interventions—insecticide-treated nets and indoor residual spraying—alongside supplemental organophosphate, organochlorine, and carbamate applications [5]. Alarmingly, the development of mosquito resistance to insecticides, particularly to pyrethroids, has compromised disease control efficacy [6,7,8,9]. Field-derived An. sinensis populations from Hainan, China, have developed resistance to DDT, cypermethrin, and malathion [10].
Target-site insensitivity and enhanced metabolic detoxification represent two principal mechanisms underlying insecticide resistance in mosquitoes [3,11]. Carboxylesterases, cytochrome P450s, and glutathione S-transferases in mosquitoes are typical enzymes correlated with pyrethroid resistance [11,12,13,14,15]. In recent years, our research has focused on An. sinensis to elucidate the mechanisms of insecticide resistance. Multiple gene families in mosquitoes have been confirmed to be involved in insecticide resistance mechanisms [16,17,18,19,20,21], including cytochromes P450, glutathione S-transferases, ATP-binding cassette transporters, and carboxylesterases. Carboxylesterases, members of the α/β-hydrolase fold superfamily [22], mediate specific hydrolysis of carboxyl ester bonds in organophosphates and pyrethroids [23,24,25]. For example, carboxylesterase Ha006a facilitates metabolic detoxification of pyrethroids (fenvalerate, λ-cyhalothrin, and deltamethrin) and organophosphates (paraoxon ethyl, profenofos, and chlorpyrifos) in Helicoverpa armigera [26]. Similarly, carboxylesterase RpCarE can hydrolyze isoprocarb and cyhalothrin in Rhopalosiphum padi [27]. Notably, Boest1 contributes to malathion and bifenthrin detoxification in Bradysia odoriphaga [28].
Gene mutation and overexpression represent two primary mechanisms for carboxylesterase-mediated pyrethroid resistance [29]. Specific mutations significantly enhance hydrolysis efficiency: CarE001C (H423I/R322L) in H. armigera elevates fenvalerate hydrolysis, while E3 (W251L/F309L) in Lucilia cuprina increases permethrin degradation [30,31]. In pyrethroid-resistant Musca domestica, MdαE7 mutations are accompanied by elevated expression and gene amplification, enhancing pyrethroid hydrolysis [32,33]. Overexpression of carboxylesterase genes further contributes to pyrethroid resistance. Upregulated expression of esterase genes in fenvalerate-resistant H. armigera midgut/fat body correlates with enhanced activity [34]. Constitutive/inductive PxαE14 overexpression facilitates multi-pyrethroid detoxification in Plutella xylostella [35]. Studies have revealed significantly elevated carboxylesterase expression in pyrethroid-resistant mosquito populations, exemplified by upregulated CPIJ018231, CPIJ018232, and CPIJ018233 in permethrin-resistant Culex quinquefasciatus [36], overexpressed CCEae3a in deltamethrin-resistant Aedes aegypti [37], and increased COEAE1F expression in deltamethrin-resistant Anopheles gambiae [38]. Moreover, increased esterase activity was observed in deltamethrin-resistant Cx. pipiens pallens and Aedes aegypti strains [39,40]. Correlative analysis by Chang et al. identified carboxylesterases as contributors to deltamethrin/permethrin resistance in An. sinensis [3], while Wu et al. found upregulated carboxylesterase genes in deltamethrin-resistant strains [16]. Collectively, these findings establish a correlation between pyrethroid resistance and carboxylesterase overexpression in mosquitoes, suggesting potential involvement of An. sinensis carboxylesterases in pyrethroid resistance. However, identification and functional analyses of specific pyrethroid resistance-associated carboxylesterase genes in An. sinensis remain limited.
In this study, comparative transcriptomics of susceptible and resistant An. sinensis strains were utilized to identify CCE genes exhibiting upregulated expression. Based on these findings and prior evidence of CCE-mediated resistance mechanisms, we identified candidate CCE genes of An. sinensis associated with deltamethrin resistance. Functionally, qRT-PCR was performed to confirm five upregulated genes across different resistant strains, and their causal roles in resistance were further elucidated through RNA interference (RNAi) and bioassays. These results enhance our mechanistic understanding of CCE-facilitated pyrethroid resistance and provide a theoretical basis for optimizing mosquito control strategies.
2. Materials and Methods
2.1. Mosquito Strains
The susceptible strain WX-LS, originally sourced from Wuxi, Jiangsu Province, China, has been maintained at the Institute of Entomology and Molecular Biology, Chongqing Normal University, China, under insecticide-free conditions. The resistant strain WX-LR was derived from WX-LS through deltamethrin selection. The pyrethroid-resistant strains CQ-LR, AH-LR, and YN-LR were established as near-isogenic lines via backcrossing for over ten generations between laboratory-susceptible strains and resistant populations collected from Chongqing, Anhui, and Yunnan, China, respectively. All five An. sinensis strains were reared separately under controlled conditions of 27 ± 1 °C and 70% ± 10% relative humidity. The susceptibility of adult female mosquitoes was evaluated three days post-emergence using the WHO standard bioassay protocol with filter papers impregnated with a diagnostic concentration of 0.05% deltamethrin (CAS: 52918-63-5, Sigma-Aldrich, St. Louis, MO, USA) [5]. The LC50 of deltamethrin for the laboratory-susceptible strain was 0.0067 mg/L. After a one-hour exposure to 0.05% deltamethrin-treated filter papers, the laboratory-resistant strains exhibited mortality rates of 14.29% for WX-LR, 13.33% for YN-LR, 17.14% for CQ-LR, and 11.43% for AH-LR following a 24 h recovery period.
2.2. Identification of Pyrethroid Resistance-Associated CCEs Using RNA-Seq
Transcriptomic analysis was performed on An. sinensis, encompassing both susceptible and resistant strains. Specimens were procured from diverse developmental phases, including fourth-instar larvae, female and male pupae, and adult females and males. Additionally, tissues from adult mosquitoes, namely the antennae, cuticle, fat body, Malpighian tubules, and midgut, were also collected. For blood-fed females, samples were gathered at 1, 3, and 12 h post-feeding. Three biological replicates were used to ensure reliability. The sequencing was conducted by Novogene Co., Ltd. (Beijing, China) using the Illumina HiSeq™ 2000 platform (Illumina, San Diego, CA, USA). Subsequently, following previously described methods [41,42], the data were analyzed by the Institute of Entomology and Molecular Biology, Chongqing Normal University. CCE genes associated with pyrethroid resistance in An. sinensis were identified based on the transcriptional profiles of distinct developmental stages, tissues, and blood-fed females from both deltamethrin-resistant (CQ-LR) and susceptible (WX-LS) strains. The sequencing reads were aligned to the An. sinensis genome using TopHat [43]. The expression levels of the CCE genes were quantified in FPKM (fragments per kilobase of transcript per million mapped reads) by employing Cufflinks [44]. Additionally, Cufflinks was utilized to assess the differential expression of transcripts between the resistant and susceptible strains. A gene was deemed significantly differentially expressed if it satisfied the following criteria: | log2 (fold change in FPKM) | ≥ 1 and p-value ≤ 0.05. These thresholds enabled the identification of potential CCE genes implicated in pyrethroid resistance. The differential expression patterns of the CCE genes were visualized using GraphPad Prism 10.0 software (GraphPad Software Inc., San Diego, CA, USA).
2.3. qRT-PCR Verification of Pyrethroid Resistance-Associated CCE Genes
Previous research has demonstrated that five genes (CarE001G, MdαE7, Pxαe28, and Ldace1) in H. armigera, M. domestica, P. xylostella, and Leptinotarsa decemlineata are involved in insecticide metabolism or are resistance-conferring [45,46,47,48]. Their corresponding homologs in An. sinensis have been identified as AsBe4, AsAe4, AsUn4, and AsAce1. These genes, along with the genes identified through RNA-seq analysis in this study, were selected as candidate target genes for validation via qRT-PCR. Total RNA was extracted using the Trizol Reagent Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) in accordance with the manufacturer’s instructions. The cDNA template was synthesized from the total RNA using the PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa, Biotech Co., Ltd., Dalian, China). The qRT-PCR assays were conducted using an iTaq™ SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA). Each treatment was replicated three times biologically. The ribosomal protein L49 (RPL49) of An. sinensis was employed as the reference gene. The primers for qRT-PCR were designed using Primer Premier 5.0 (Supplementary Files S1). The relative expression levels were determined using the 2−ΔΔCt method [49].
2.4. RNAI-Based Validation of Pyrethroid Resistance-Related CCE Genes
To elucidate the involvement of specific CCE genes in the pyrethroid resistance of An. sinensis, RNA interference (RNAi) was employed in conjunction with deltamethrin bioassays on female mosquitoes from the WX-LR strain. Primers for dsRNA synthesis were designed based on the cDNA sequences of the five CCE genes and enhanced green fluorescent protein (EGFP) using Primer Premier 5.0 (Supplementary Files S1). The dsRNA fragments were synthesized in vitro using the T7 RiboMAX Express RNAi System (Promega (Beijing) Biotech Co., Ltd., Beijing, China), with EGFP dsRNA serving as a negative control. The dsRNA concentrations were measured using Nano-Drop™ 1000 (Thermo Fisher Scientific Inc., Waltham, MA, USA). An 800 ng amount of the dsRNA fragments was injected into the hemocoel at the abdomen region between the second and third segment of the pupa at 10 h post-pupation of An. sinensis. The efficiency of gene silencing by dsRNA was evaluated using qRT-PCR in female adult mosquitoes three hours after emergence. The bioassay procedure adhered to the WHO standard insecticide susceptibility testing protocol [5]. Vigorous female adults were transferred to holding tubes for 1 h pre-exposure. Moribund/dead mosquitoes were discarded. Paper impregnated with 0.05% deltamethrin was secured to the inner surface of the exposure tubes. Following retraction of the sliding mesh partition, mosquitoes were gently blown into exposure tubes. Mosquitoes were exposed for 1 h with knockdown counts recorded at 10 min intervals. Survivors were transferred to holding tubes with 10% sugar water for 24 h recovery prior to mortality assessment. A mosquito was classified as dead or knocked down if it was immobile or unable to stand or take off. The knockdown curves of each treatment were fitted and the half-knockdown time (KT50) was calculated using GraphPad Prism 10.0 software.
2.5. Statistical Analysis
In this study, the disparities in gene expression levels and biological assay data across diverse treatments were analyzed. One-way analysis of variance (ANOVA) was initially conducted to assess the overall differences in expression levels among the distinct populations or developmental stages of An. sinensis, and Tukey’s multiple comparison tests were employed to discern the pairwise differences. The difference in knockdown rates between the control and treatment groups during the 1 h exposure to deltamethrin was analyzed using the Log-rank (Mantel–Cox) test. These analyses were performed using GraphPad Prism version 10.0 software.
3. Results and Discussion
3.1. Identification of Carboxylesterase Genes Associated with Pyrethroid Resistance in An. sinensis
3.1.1. Identification of CCEs via Comparative Transcriptomics
The overexpression of esterase genes in mosquitoes facilitates their insecticide resistance [12,36,37,50,51]. In this study, RNA-seq was employed to profile differential expression of CCE genes in An. sinensis across five developmental stages, five tissue types, and three post-blood meal time points in paired deltamethrin-resistant (CQ-LR) and susceptible (WX-LS) strains. Developmental stage-specific profiling revealed that AsAe10 and AsBe3 exhibited significantly upregulated expression in four stages, excluding the fourth instar of larva (Figure 1a). The majority of overexpressed genes belonged to the Ae or Be classes, including AsAe9. In contrast, the measured CCE genes in the fourth larva exhibited significant downregulation in expression levels (Figure 1a). Tissue-specific profiling identified significantly upregulated CCE genes in An. sinensis: five in both the antennae and cuticle, four in the Malpighian tubules and midgut, and three in the fat body (Figure 1b). AsAe10 exhibited significant overexpression across all five examined tissues, whereas AsBe2 showed significant upregulation in three tissues (Figure 1b). Widespread upregulation was also observed for Ae-class genes, with AsAe9 showing specific overexpression in antennae. The newly identified AsAce2 exhibited upregulated expression in the fat body (Figure 1b). Specific temporal patterns of CCE gene upregulation emerged post-blood meal: four genes at 1 h, twelve genes at 3 h, and three genes at 12 h (Figure 1c). Asle1 and AsAe10 exhibited significant upregulation across two distinct temporal intervals. AsAce2 and AsUn5 had significant overexpression at 3 h after blood meal (Figure 1c). As shown in Figure 1, AsAe9 and AsAe10 exhibited significant upregulation across the three experiments, and AsAce2 was overexpressed in both tissue-specific and post-blood meal assays. Notably, the homolog of AsAe10 in H. armigera, CarE001H, has been functionally validated as a key gene involved in pyrethroid detoxification [52]. Similarly, the ortholog of AsAce2 in P. xylostella (Pxαe18) plays an analogous role in detoxification processes [46]. Therefore, we preliminarily identify AsAe9, AsAe10, AsAce2, and AsUn5 as genes associated with deltamethrin resistance in An. sinensis.

Figure 1.
RNA sequencing analyses of differentially expressed CCE genes in deltamethrin-resistant An. sinensis strain (CQ-LR) versus susceptible strain (WX-LS). (a) Developmental stage-specific expression; (b) tissue-specific expression; (c) temporal dynamics of gene expression post-blood meal. Vertical dotted lines mark the ±1 of log2 (fold changes in FPKM value) values on the x-axis, and horizontal dotted lines denote the p-value = 0.05 of −log10 (p-value) on the y-axis. Criteria for significantly upregulated genes: |log2 (fold change in FPKM)| ≥ 1 and p-value ≤ 0.05.
Developmental stage- and tissue-specific upregulation of carboxylesterase genes is involved in resistance mechanisms in insects. For instance, in a pyrethroid-resistant strain of Cx. quinquefasciatus, two esterase genes exhibit upregulated expression levels in both larval and adult stages relative to the susceptible strain [15]. Conversely, another study revealed that resistant larvae—but not adults—of the same species demonstrate significant overexpression of three distinct carboxylesterase genes [36]. This study proposes that constitutive upregulation of CCE genes in pupal and adult stages of the resistant An. sinensis strain reflects stage-specific selection pressure. Given that insecticide treatments target advanced developmental stages, these alterations in expression profiles could be associated with adaptive genetic responses to insecticide exposure, thereby driving resistance evolution. The midgut and fat body are typically organs for tissue-specific carboxylesterase expression, which is critical for metabolic detoxification [53,54,55,56,57,58]. In P. xylostella, the fipronil resistance-associated carboxylesterase genes Pxae22 and Pxae31 exhibit upregulated expression in fourth-instar larvae and the midgut [56]. Wu et al. documented 1.9-fold elevated esterase activity in a fenvalerate-resistant H. armigera strain (1690-fold resistance), concomitant with 2-to-90-fold upregulated expression of four esterase genes in the midgut and fat body tissues relative to a susceptible strain [34]. Our earlier investigation revealed spatiotemporal overexpression patterns of pyrethroid resistance-associated P450 genes in An. sinensis: elevated in resistant strains specifically within pupae/adults, the adult midgut/cuticle, and at 1- and 3 h post-blood meal [57]. Malpighian tubule-specific gene expression in mosquitoes facilitates dual roles in blood meal digestion and xenobiotic detoxification [58]. Reports indicate that Anopheles with pyrethroid resistance mediated by kdr mutations or P450 overexpression exhibit altered blood-feeding capabilities [59,60]. Conversely, following a single blood meal, pyrethroid-resistant An. funestus exhibits significantly enhanced resistance to permethrin [61]. Researchers consider that insecticide detoxification mechanisms involved in resistance may be activated by a prior blood meal, thereby enhancing the resistance phenotype before insecticide exposure. Moreover, the blood meal sustains DDT and permethrin resistance during mosquito development, likely due to blood meal-induced elevation in glutathione S-transferase activity [62]. We hypothesize that, compared to the susceptible strain, significant blood-induced upregulation of carboxylesterase expression in the resistant mosquito strain may enhance blood meal metabolism. This could boost their fitness and stabilize insecticide resistance inheritance. Consequently, we measured carboxylesterase expression post-blood feeding in both resistant and susceptible mosquitoes in this study. Our integrated transcriptomic approach, validated in An. gambiae, An. sinensis, and M. domestica [38,63,64], consistently identifies carboxylesterase genes as important actors in pyrethroid resistance. Notably, transcriptomic analysis of the deltamethrin-resistant An. sinensis strain established by Zhou et al. did not detect overexpressed CCE genes [64]. This discrepancy with our findings is likely attributable to the strain’s geographic origin (Hubei, China) and differential resistance gene dominance.
3.1.2. Validation of Differential Expression for CCEs via qRT-PCR
Many studies on mosquito resistance mechanisms validate the necessity for qRT-PCR confirmation of expression differences in candidate genes between resistant and susceptible strains [15,16,57]. qRT-PCR has been previously employed to identify pyrethroid resistance-associated P450 and GST genes in An. sinensis [19,57]. The differential expression of eight genes—comprising four previously reported genes (AsAe4, AsAce1, AsBe4, AsUn4) and four genes newly identified in this study (AsAe9, AsAe10, AsAce2, AsUn5)—was quantified via qRT-PCR in deltamethrin-resistant and susceptible An. sinensis strains.
While AsAe4 showed no significant upregulation in four An. sinensis-resistant strains compared with the susceptible WX-LS strain (Figure 2), its homolog MdαE7 in resistant M. domestica is involved in permethrin detoxification [47,65]. Additionally, the P. xylostella homolog PxEst-6 exhibits competence in binding and metabolizing bifenthrin, cyfluthrin, cypermethrin, and λ-cyhalothrin [66]. This functional difference likely reflects species-specific variations in pyrethroid detoxification metabolism mediated by the target gene. Significant upregulation of AsAe9, AsAce1, and AsBe4 was detected in two An. sinensis resistant strains (Figure 2). Ace1 shows upregulated expression in a pyrethroid-treated L. decemlineata strain, which may be implicated in insecticide resistance mechanisms [45]. A homolog of AsBe4 (carboxylesterase CarE001G in H. armigera) exhibits metabolic activity toward β-cypermethrin, λ-cyhalothrin, and fenvalerate in vitro [48]. AsBe4 (corresponding to Bemisia tabaci carboxylesterase BTbe5) demonstrates upregulation following imidacloprid exposure, and RNAi-mediated gene silence functionally validates its role in the insecticidal mechanism [67]. Elevated relative expression of AsAe10 was observed across all four resistant strains, especially in the WX-LR strain (Figure 2), consistent with RNA-seq data from this study. The homolog of AsAe10 in H. armigera (CarE001H) has been functionally validated as a key metabolic detoxification gene involved in pyrethroid catabolism [52]. CCEae3A (homolog of Ae10) exhibits significant upregulation in the Ae. aegypti temephos-resistant strain, and its overexpression in transgenic An. gambiae confers resistance to α-cypermethrin [51]. AsAce2 exhibited significantly upregulated expression in four An. sinensis resistant strains (Figure 2). Its homolog Pxae18 is overexpressed in chlorpyrifos-resistant P. xylostella, with RNAi confirming resistance involvement [46]. AsUn4 and AsUn5 exhibited significant upregulation in the AH-LR and WX-LR strains, respectively (Figure 2). The homolog of AsUn4 in P. xylostella (PxαE28) exhibits significant upregulation in chlorpyrifos-resistant strains, and RNAi-mediated gene silencing restores insecticide susceptibility, confirming its functional role in resistance [46]. Consequently, based on comparative transcriptomics and qRT-PCR validation, AsAe9, AsAe10, AsAce1, AsAce2, and AsBe4 were selected as deltamethrin resistance-associated carboxylesterase genes for subsequent functional analysis.
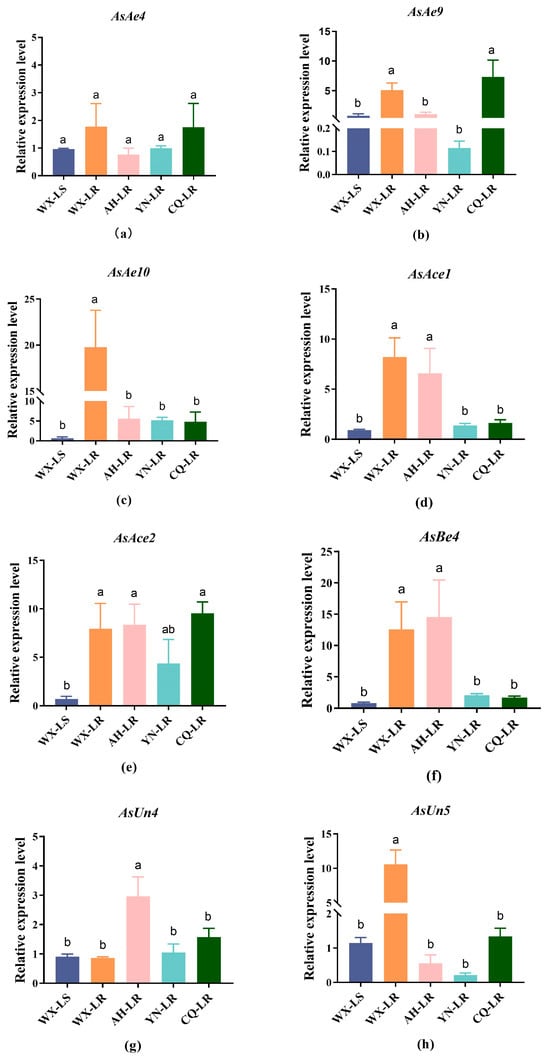
Figure 2.
Validation of expression levels for eight selected carboxylesterase genes in deltamethrin-resistant (WX-LR, AH-LR, YN-LR, CQ-LR) and susceptible (WX-LS) An. sinensis strains. (a) AsAe4; (b) AsAe9; (c) AsAe10; (d) AsAce1; (e) AsAce2; (f) AsBe4; (g) AsUn4; (h) AsUn5. The genes’ relative expression levels are presented as the mean ± SD (standard deviation) from three biological replicates with three technical replicates each. Columns marked with the different lowercase letters indicate significantly differential expression across the strains (p < 0.05).
3.2. Function Analysis of Carboxylesterase Genes in Pyrethroid Resistance of An. sinensis
3.2.1. Determination of Time Points for CCE Silencing
Functional validation was performed through RNAi silencing to confirm the involvement of AsAe9, AsAe10, AsAce1, AsAce2, and AsBe4 in pyrethroid resistance in An. sinensis. To determine the optimal developmental stage for dsRNA injection, the specific expression profiles of these five genes in pupae and adults in both pyrethroid-resistant (WX-LR) and susceptible (WX-LS) strains were acquired using qRT-PCR (Figure 3). All five genes exhibited expression through the pupal and adult stages, but had various expression levels across the different periods. In the WX-LS strain, AsAe9, AsAce1, and AsAce2 exhibited maximal relative expression levels at Plast (terminal pupal stage), whereas AsAe10 and AsBe4 demonstrated divergent peak timing (Figure 3). In the WX-LR strain, three target genes (AsAe10, AsAce2, AsBe4) achieved peak expression at A72 (adults 72 h post-emergence). Compared to the susceptible strain, all five genes had significant upregulation in at least one adult stage of WX-LR (p < 0.05), with three genes concurrently overexpressed during pupal development (Figure 3). The five genes in both strains exhibited the lowest expression levels at the P10 stage (10 h post-pupation), with no significantly differential expression between strains (p > 0.05, Figure 3). This expression convergence established P10 as the optimal developmental stage for dsRNA microinjection in subsequent RNAi-mediated functional validation.
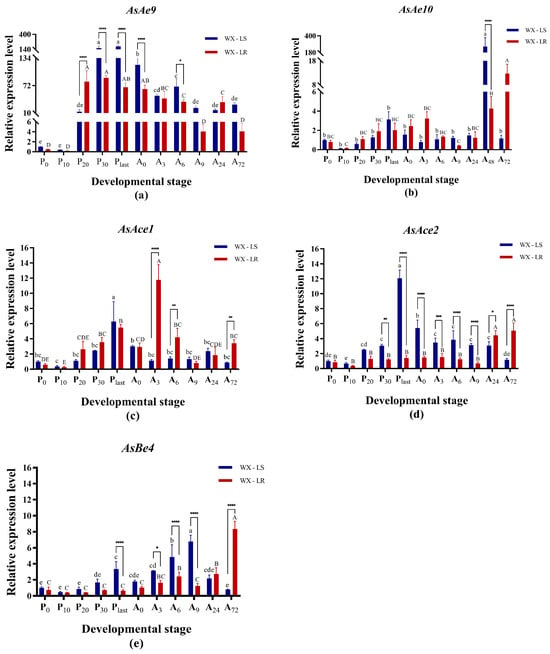
Figure 3.
Developmental stage-specific expression profiling of five carboxylesterase genes in deltamethrin-resistant and susceptible An. sinensis strains. (a): AsAe9; (b) AsAe10; (c) AsBe4; (d) AsAce1; (e) AsAce2. The relative expression levels of genes were normalized to 0 h post-pupation of WX-LS, and are shown as the mean ± SD from three biological replicates in qRT-PCR analysis. Columns marked with homogeneous uppercase (for WX-LS) or lowercase (for WX-LR) letters indicate no significant difference in expression across developmental stages (p > 0.05). Asterisks indicate significant inter-strain differential expression at matched timepoints (*: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001). Pupal phases: P0 (0 h post-pupation), P10 (10 h post-pupation), P20 (20 h post-pupation), P30 (30 h post-pupation), Plast (terminal pupal stage). Adult stages: A0 (0 h post-emergence, newly emerged adults), A3 (3 h post-emergence), A6 (6 h post-emergence), A9 (9 h post-emergence), A24 (24 h post-emergence), A72 (72 h post-emergence).
3.2.2. Functional Analysis of CCE Genes in Pyrethroid Resistance
RNAi experiments targeting the genes AsAe9, AsAe10, AsAce1, AsAce2, and AsBe4 in the deltamethrin-resistant strain WX-LR revealed that the relative expression levels of the first four genes were significantly reduced (p < 0.01; Figure 4A–D), with silencing efficiencies of 79.8%, 51.8%, 55.4%, and 66.3%, respectively. In contrast, silencing of AsBe4 did not result in a significant reduction in expression, showing a 30.4% decrease (Figure 4E). In the subsequent bioassay, silencing of AsAe10, AsAce1, AsAce2, and AsBe4 resulted in significantly shortened knockdown times (p < 0.05; Figure 4b–e) in resistant An. sinensis following 1 h of exposure to 0.05% deltamethrin. The most significant reduction in knockdown time occurred in the AsAe10-silenced group, followed by AsBe4 (Figure 4b–e). Integrated analysis of the knockdown data revealed that silencing each of the five genes resulted in a reduction in KT50 in resistant mosquitoes. Compared to the control groups, gene-silenced An. sinensis exhibited reductions in KT50 values: AsAe9 and AsAe10 decreased from a common control value of 55.12 min (95% fiducial limit [FL]: 53.73–56.66) to 51.09 min (95% FL: 50.36–51.82) and 43.83 min (95% FL: 40.91–46.74), while AsAce1, AsAce2, and AsBe4—sharing a common control value of 57.43 min (95% FL: 56.77–58.15)—declined to 49.09 min (95% FL: 46.27–52.08), 50.08 min (95% FL: 46.16–54.26), and 48.02 min (95% FL: 43.63–52.23), respectively. The non-overlapping 95% fiducial limits between the silenced groups and the control groups indicate a statistically significant reduction in knockdown time. The most significant decrease in KT50 was observed in the AsAe10-silenced group, which is consistent with the findings described above. RNAi-mediated silencing of five carboxylesterase genes in the pyrethroid-resistant strain enhanced deltamethrin susceptibility, resulting in increased mortality rates of 40.3% (AsAe9), 25.3% (AsAe10), 9.5% (AsAce1), 24.9% (AsAce2), and 14.2% (AsBe4) after 1 h exposure to deltamethrin followed by 24 h recovery (Figure 4I–V). Silencing of AsAe9, AsAe10, and AsBe4 significantly compromised the resistance phenotype, with AsAe10 exhibiting the most pronounced effect (p < 0.05, Figure 4I–V). In conclusion, among the five genes investigated, although silencing of several genes led to reduced knockdown time and increased mortality in the resistant strain after deltamethrin exposure, only silencing of AsAe10 and AsBe4 resulted in statistically significant changes in both endpoints. Notably, AsAe10 silencing produced the most substantial phenotypic alteration. Furthermore, the expression of AsAe10 was significantly upregulated in the resistant strain, suggesting that it may be strongly associated with pyrethroid resistance in An. sinensis. Its overexpression could potentially enhance the metabolic detoxification of deltamethrin in mosquitoes. Interestingly, AsBe4 silencing also significantly compromised resistance despite its relatively lower silencing efficiency, suggesting its potentially critical role in the resistance phenotype.
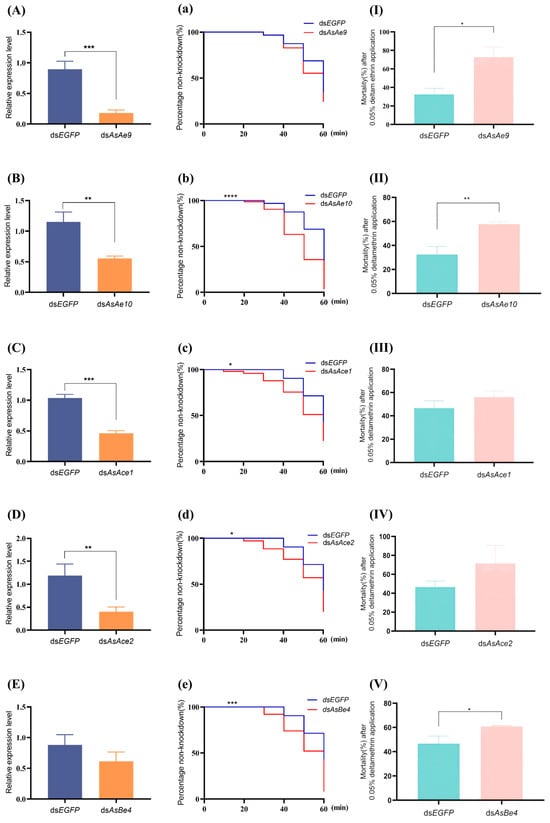
Figure 4.
Functional validation of the carboxylesterase genes AsAe9 (A,a,I), AsAe10 (B,b,II), AsAce1 (C,c,III), AsAce2 (D,d,IV), and AsBe4 (E,e,V) of An. sinensis in its deltamethrin resistance. (A–E) The expression levels of AsAe9, AsAe10, AsAce1, AsAce2, and AsBe4post-dsRNA injection (qRT-PCR; mean ± SD). (a–e) Time–knockdown profiles of An. sinensis during 0.05% deltamethrin exposure. (I–V) Mortality of An. sinensis after 24 h recovery following 0.05% deltamethrin exposure (mean ± SD). The groups injected with dsRNA of enhanced green fluorescent protein (dsEGFP) were negative controls. The increases in knockdown number were recorded at 10 min intervals for 1 h. The asterisks indicate significant differences between groups (*: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001).
Insect esterases can hydrolyze pyrethroids, thereby contributing to detoxification. Enhanced resistance phenotypes correlate with gene mutations that elevate both the expression and hydrolytic activity of these enzymes [68,69]. In An. gambiae, transcriptional upregulation of carboxylesterase genes significantly correlates with deltamethrin resistance [38]. In the deltamethrin-resistant Cx. pipiens pallens, carboxylesterase activity is associated with resistance, with resistance declining concomitantly upon relaxation of deltamethrin selection pressure [39]. In Ae. aegypti, resistance to permethrin and deltamethrin has been linked to elevated carboxylesterase activity [40,70], with CCEae3a overexpression correlating with deltamethrin resistance [37]. Germline transformation of Aedes aegypti with the An. gambiae CCEae3a gene—overexpressed in the donor Ae. Aegypti strain—elevates enzyme activity and significantly increases α-cypermethrin resistance [51]. In the permethrin-resistant strain of Cx. quinquefasciatus, α-esterase and esterase B1 exhibit significant upregulation, respectively, compared to the susceptible stain [15]. Concurrently, three carboxylesterase genes show significant overexpression in the resistant strain, collectively indicating enhanced metabolic detoxification capacity [36]. Carboxylesterase upregulation and enhanced activity represent an important pyrethroid resistance mechanism in mosquitoes. This study confirms that the significantly upregulated carboxylesterase genes in resistant An. sinensis are involved in deltamethrin resistance. Similar roles of carboxylesterases in organophosphorus insecticide resistance have been demonstrated. In Ae. aegypti and Aedes albopictus, temephos resistance is involved in increased carboxylesterase activity, with resistant strains exhibiting significant overexpression of CCEae6a, and concomitant gene amplification [71,72].
RNAi-mediated silencing provides a robust approach for functionally validating the contribution of upregulated carboxylesterase genes to insecticide resistance, a methodology extensively applied in resistance mechanism research [73]. RNAi-based functional validation has elucidated the roles of cytochrome P450 in An. gambiae, the GST in Ae. aegypti, and G-protein-coupled receptor (GPCR) genes in Cx. quinquefasciatus permethrin resistance [74,75,76]. In P. xylostella, the carboxylesterase genes PxαE8, PxαE14, and PxEst-6 exhibit tissue-specific expression in the midgut or Malpighian tubules, and their association with pyrethroid resistance has been validated through RNAi-mediated gene silencing [35,66,77]. Pyrethroid-induced carboxylesterase upregulation (EoCarE592 in Ectropis obliqua; four genes in Grapholita molesta) enhances insect susceptibility upon RNAi-mediated gene silencing [78,79]. Additionally, researchers also have considered carboxylesterase-mediated sequestration of organophosphates as an important mechanism for mosquito resistance [80], and this sequestration is closely associated with its overexpression [81]. Currently, novel RNAi-based insecticides have been commercialized for agricultural pest control [82,83]. Multiple studies have confirmed the feasibility of applying RNAi technology for mosquito management [84], with ongoing research focused on identifying mosquito-specific target genes and optimizing dsRNA delivery methods [85,86]. Prospectively, leveraging the insecticide resistance-associated genes identified in An. sinensis in this study could enable the development of RNAi biopesticides. These agents would reduce carboxylesterase-mediated metabolism of pyrethroids in mosquitoes, thereby delaying resistance evolution. Furthermore, synergistic application with conventional insecticides could extend their effective lifespan.
This study advances our understanding of pyrethroid resistance mechanisms in An. sinensis, and provides a theoretical foundation for optimizing mosquito control strategies. The carboxylesterase genes identified in this research offer molecular tools for resistance management. For AsAe10, future studies will bridge mechanism and application. Bidirectional in vivo validation via transgenic An. sinensis (overexpression/knockout) will delineate carboxylesterase-mediated resistance pathways, while integrated in vitro modeling (molecular docking/eukaryotic expression) will analyze metabolic dynamics. It is also a significant strategy to develop synergistic dsRNA–insecticide combinations for delaying vector resistance.
4. Conclusions
This study integrated transcriptomic screening and functional validation to identify carboxylesterase genes involved in deltamethrin resistance in Anopheles sinensis. Based on comparative transcriptomic analyses between resistant and susceptible strains, AsAe9, AsAe10, AsAce2, and AsUn5 were identified as significantly upregulated carboxylesterase genes exhibiting differential spatiotemporal expression patterns and post-blood meal induction profiles. qRT-PCR confirmed significant overexpression of AsAe10 and AsAce2 in all four resistant strains, with AsAe9, AsAce1, and AsBe4 elevated in two strains. RNA interference targeting the five carboxylesterase genes revealed that silencing of AsAe10 and AsBe4 significantly shortened the knockdown time and increased 24 h mortality in the resistant strain upon exposure to 0.05% deltamethrin, with AsAe10 exhibiting a more pronounced effect. This study identifies these genes as contributors to pyrethroid resistance in An. sinensis, provides further insight into the resistance mechanisms, and offers a theoretical basis for refining resistance management strategies against mosquitoes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects16090938/s1. Supplementary File S1: Primer sequences and coding sequences of genes.
Author Contributions
Conceptualization, B.C., Y.W., F.S. and L.Q.; methodology, B.C., Y.W., F.S. and L.Q.; software, Y.W., X.G. and H.Y.; investigation, X.G. and X.C.; data curation, X.G. and X.C.; writing—original draft preparation, Y.W.; writing—review and editing, B.C. and Y.W.; visualization, Y.W., X.G. and H.Y.; supervision, B.C.; project administration, B.C. and Y.W.; funding acquisition, B.C. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 31672363, 31872262), the National Key Program of Science and Technology Foundation Work of China (grant number 2015FY210300), and the Science and Technology Research Program of Chongqing Municipal Education Commission (grant number KJQN202200516).
Data Availability Statement
The data presented in the study are available in “FigShare” at https://www.doi.org/10.6084/m9.figshare.29975380.
Acknowledgments
The authors would like to express their gratitude to Junfu Chen from Chongqing Normal University for help in the visualization of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rueda, L.M.; Zhao, T.; MA, Y.J.; Gao, Q.; Ding, Z.G.; Khuntirat, B.; Sattabongkot, J.; Wilkerson, R.C. Updated distribution records of the Anopheles (Anopheles) hyrcanus species-group (Diptera: Culicidae) in China. Zootaxa 2007, 1407, 43–55. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef]
- Chang, X.; Zhong, D.; Fang, Q.; Hartsel, J.; Zhou, G.; Shi, L.; Fang, F.; Zhu, C.; Yan, G. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: A major obstacle to mosquito-borne diseases control and elimination in China. PLoS Negl. Trop. Dis. 2014, 8, e2889. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, S.; Zheng, X.; Huang, F.; Wang, D.; Shen, Y.; Su, Y.; Zhou, G.; Liu, F.; Jiang, J. Vector capacity of Anopheles sinensis in malaria outbreak areas of central China. Parasites Vectors 2012, 5, 136. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; pp. 6–35. Available online: https://iris.who.int/handle/10665/250677 (accessed on 30 June 2018).
- Hemingway, J.; Field, L.; Vontas, J. An overview of insecticide resistance. Science 2002, 298, 96–97. [Google Scholar] [CrossRef]
- Zaim, M.; Guillet, P. Alternative insecticides: An urgent need. Trends Parasitol. 2002, 18, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Butler, D. Mosquitoes score in chemical war. Nature 2011, 475, 19. [Google Scholar] [CrossRef]
- Kelvin, A.A. Outbreak of Chikungunya in the Republic of Congo and the global picture. J. Infect. Dev. Ctries 2011, 5, 441–444. [Google Scholar] [CrossRef]
- Sun, D.; Wang, G.; Zeng, L.; Li, S.; He, C.; Hu, X.; Wang, S. Extensive resistance of Anopheles sinensis to insecticides in malaria-endemic areas of Hainan Province, China. Am. J. Trop. Med. Hyg. 2017, 97, 295–298. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Chevillon, C.; Guillemaud, T.; Lenormand, T.; Pasteur, N. An overview of the evolution of overproduced esterases in the mosquito Culex pipiens. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1707–1711. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect cytochrome P450. In Comprehensive Molecular Insect Science; Gilbert, L., Iatrou, K., Gill, S.S., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2005; Volume 4, pp. 1–77. [Google Scholar]
- Marcombe, S.; Mathieu, R.B.; Pocquet, N.; Riaz, M.-A.; Poupardin, R.; Sélior, S.; Darriet, F.; Reynaud, S.; Yébakima, A.; Corbel, V.; et al. Insecticide resistance in the dengue vector Aedes aegypti from Martinique: Distribution, mechanisms and relations with environmental factors. PLoS ONE 2012, 7, e30989. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, G.; Muthusamy, R.; Narayanan, M.; Shivakumar, M.S.; Kweka, E.J. Overexpression of cytochrome P450 and esterase genes involved in permethrin resistance in larvae and adults of Culex quinquefasciatus. Parasitol. Res. 2023, 122, 3205–3212. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Si, F.; Li, J.; Yan, Z.; Yan, Z.; He, X.; Chen, B. Identification of carboxylesterase genes associated with pyrethroid resistance in the malaria vector Anopheles sinensis (Diptera: Culicidae). Pest Manag. Sci. 2018, 74, 159–169. [Google Scholar] [CrossRef]
- Yan, Z.; He, Z.; Yan, Z.; Si, F.; Zhou, Y.; Chen, B. Genome-wide and expression-profiling analyses suggest the main cytochrome P450 genes related to pyrethroid resistance in the malaria vector, Anopheles sinensis (Diptera Culicidae). Pest. Manag. Sci. 2018, 74, 1810–1820. [Google Scholar] [CrossRef]
- He, Q.; Yan, Z.; Si, F.; Zhou, Y.; Fu, W.; Chen, B. ATP-binding cassette (ABC) transporter genes involved in pyrethroid resistance in the malaria vector Anopheles sinensis: Genome-wide identification, characteristics, phylogenetics, and expression profile. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef]
- Tao, F.; Si, F.; Hong, R.; He, X.; Li, X.; Qiao, L.; He, Z.; Yan, Z.; He, S.; Chen, B. Glutathione S-transferase (GST) genes and their function associated with pyrethroid resistance in the malaria vector Anopheles sinensis. Pest Manag. Sci. 2022, 78, 4127–4139. [Google Scholar] [CrossRef]
- Si, F.; Qiao, L.; He, Q.; Zhou, Y.; Yan, Z.; Chen, B. HSP superfamily of genes in the malaria vector Anopheles sinensis: Diversity, phylogenetics and association with pyrethroid resistance. Malar. J. 2019, 18, 132. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, L.; He, Q.; Zhou, Y.; Ren, S.; Chen, B. Genome-wide identification, characterization and evolution of cuticular protein genes in the malaria vector Anopheles sinensis (Diptera: Culicidae). Insect. Sci. 2018, 25, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Cygler, M.; Schrag, J.D.; Sussman, J.L.; Harel, M.; Silman, I.; Gentry, M.K.; Doctor, B.P. Relationship between sequence conservation and three-dimensional structure in a large family of esterases, lipases, and related proteins. Protein Sci. 1993, 2, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qiao, C. Mechanism and application of insect detoxification enzymes in bioremediation of pesticide contamination. J. Agro-Environ. Sci. 2002, 21, 285–287. Available online: https://www.aes.org.cn/nyhjkxxb/ch/reader/view_abstract.aspx?file_no=8574&flag=1 (accessed on 30 June 2002).
- Oakeshott, J.G.; Devonshire, A.L.; Claudianos, C.; Sutherland, T.D.; Horne, I.; Campbell, P.M.; Ollis, D.L.; Russell, R.J. Comparing the organophosphorus and carbamate insecticide resistance mutations in cholin-and carboxyl-esterases. Chem. Biol. Interact. 2005, 157, 269–275. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, Y.; Lin, Z.; Bhatt, P.; Chen, S. New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 2020, 182, 109138. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Rode, S.; Lonare, S.; Demiwal, P.; Narasimhappa, P.; Arun, E.; Kumar, R.; Das, J.; Ramamurthy, P.C.; Sircar, D.; et al. Heterologous expression, biochemical characterization and prospects for insecticide biosensing potential of carboxylesterase Ha006a from Helicoverpa armigera. Pestic. Biochem. Physiol. 2024, 200, 105844. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, Y.; Li, X.; Chen, M. Functional analysis of a carboxylesterase gene associated with isoprocarb and cyhalothrin resistance in Rhopalosiphum padi (L.). Front. Physiol. 2018, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, X.; Sang, Z.; Wang, R.; Ullah, F.; Gao, X.; Song, D. Characterization of the insecticide detoxification carboxylesterase Boest1 from Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae). Pest Manag. Sci. 2022, 78, 591–602. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Han, Y.; Wang, L.; Liu, Z.; Guo, H.; Fang, J. Transcript-level analysis of detoxification gene mutation-mediated chlorpyrifos resistance in Laodelphax striatellus (Hemiptera: Delphacidae). J. Econ. Entomol. 2019, 112, 1285–1291. [Google Scholar] [CrossRef]
- Xu, J.; Chang, Y.; Lu, M.; Tie, Y.; Dong, Y.; Chen, G.; Ma, Z.; Liu, X.; Li, Y. Two single mutations in carboxylesterase 001C improve fenvalerate hydrolase activity in Helicoverpa armigera. Pestic. Biochem. Physiol. 2021, 179, 104969. [Google Scholar] [CrossRef]
- Heidari, R.; Devonshire, A.L.; Campbell, B.E.; Dorrian, S.J.; Oakeshott, J.G.; Russell, R.J. Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis. Insect Biochem. Mol. Biol. 2005, 35, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Feng, X. Functional Characterization of Carboxylesterases in Pyrethroid Resistant House Fly, Musca Domestica. PhD Thesis, Auburn University, Auburn, AL, USA, 2018. Available online: https://www.proquest.com/openview/0d65cf2c4f2c2640ed6c3a2986ef362a/1?cbl=18750&diss=y&pq-origsite=gscholar (accessed on 4 August 2018).
- Zhang, L.; Shi, J.; Shi, X.; Liang, P.; Gao, J.; Gao, X. Quantitative and qualitative changes of the carboxylesterase associated with beta-cypermethrin resistance in the housefly, Musca domestica (Diptera: Muscidae). Comp. Biochem. Physiol. B. 2010, 156, 6–11. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Yuan, G.; Campbell, P.M.; Teese, M.G.; Russell, R.J.; Oakeshott, J.G.; Wu, Y. Overexpressed esterases in a fenvalerate resistant strain of the cotton bollworm. Helicoverpa armigera. Insect Biochem. Mol. Biol. 2011, 41, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, B.; Hu, X.; Shi, X.; Qi, L.; Liang, P.; Gao, X. Overexpression of PxαE14 contributing to detoxification of multiple insecticides in Plutella xylostella (L.). J. Agric. Food Chem. 2022, 70, 5794–5804. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Li, T.; Liu, N. Molecular and functional characterization of three novel carboxylesterases in the detoxification of permethrin in the mosquito, Culex quinquefasciatus. Insect Sci. 2022, 29, 199–214. [Google Scholar] [CrossRef]
- Goindin, D.; Delannay, C.; Gelasse, A.; Ramdini, C.; Gaude, T.; Faucon, F.; David, J.-P.; Gustave, J.; Vega-Rua, A.; Fouque, F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect. Dis. Poverty 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Oumbouke, W.A.; Pignatelli, P.; Barreaux, A.M.G.; Tia, I.Z.; Koffi, A.A.; Alou, L.P.A.; Sternberg, E.D.; Thomas, M.B.; Weetman, D.; ’Guessan, R.N. Fine scale spatial investigation of multiple insecticide resistance and underlying target-site and metabolic mechanisms in Anopheles gambiae in central Côte d’Ivoire. Sci. Rep. 2020, 10, 15066. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hu, H.; Ma, K.; Zhou, D.; Yu, J.; Zhong, D.; Fang, F.; Chang, X.; Hu, S.; Zou, F.; et al. Development of resistance to pyrethroid in Culex pipiens pallens population under different insecticide selection pressures. PLoS Negl. Trop. Dis. 2015, 9, e0003928. [Google Scholar] [CrossRef]
- Aponte, H.A.; Penilla, R.; Dzul-Manzanilla, F.; Che-Mendoza, A.; López, A.D.; Solis, F.; Manrique-Saide, P.; Ranson, H.; Lenhart, A.; McCall, P.J.; et al. The pyrethroid resistance status and mechanisms in Aedes aegypti from the Guerrero state, Mexico. Pestic. Biochem. Physiol. 2013, 107, 226–234. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; He, Z.; Li, W.; Si, F.; Tang, Y.; He, Q.; Qiao, L.; Yan, Z.; Fu, W.; et al. De novo transcriptome sequencing and sequence analysis of the malaria vector Anopheles sinensis (Diptera: Culicidae). Paras. Vector. 2014, 7, 314. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, Y.; Chen, B. ASDB: A comprehensive omics database for Anopheles sinensis. Genomics 2021, 113, 976–982. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; World, B.J.; Pachter, L.P. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Lü, F.G.; Fu, K.Y.; Li, Q.; Guo, W.C.; Ahmat, T.; Li, G.Q. Identification of carboxylesterase genes and their expression profiles in the Colorado potato beetle Leptinotarsa decemlineata treated with fipronil and cyhalothrin. Pestic. Biochem. Physiol. 2015, 122, 86–95. [Google Scholar] [CrossRef]
- Xie, M.; Ren, N.; You, Y.; Chen, W.; Song, Q.; You, M. Molecular characterization of two α-esterase genes involving chlorpyrifos detoxification in the diamondback moth, Plutella xylostella. Pest Manag. Sci. 2017, 73, 1204–1212. [Google Scholar] [CrossRef]
- Feng, X.; Liu, N. Functional characterization of carboxylesterases in insecticide resistant house flies, Musca domestica. J. Vis. Exp. 2018, 138, 58106. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, C.; Xu, J.; Feng, C.; Li, Y.; Dong, Y.; Ma, Z. Identification and biochemical characterization of carboxylesterase 001G associated with insecticide detoxification in Helicoverpa armigera. Pestic. Biochem. Physiol. 2019, 157, 69–79. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Paton, M.G.; Karunaratne, S.H.P.; Giakoumaki, E.; Roberts, N.; Hemingway, J. Quantitative analysis of gene amplification in insecticide-resistant Culex mosquitoes. Biochem. J. 2000, 346, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Poulton, B.C.; Fraser, C.; Amalia, A.; Sattelle, D.B.; Lycett, G.J. Aedes aegypti CCEae3A carboxylase expression confers carbamate, organophosphate and limited pyrethroid resistance in a model transgenic mosquito. PLoS Negl. Trop. Dis. 2024, 18, e0011595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, L.; Zhao, C.; Xu, J.; Sun, Z.; Dong, Y.; Li, D.; Liu, X.; Ma, Z. Functional characterization of two carboxylesterase genes involved in pyrethroid detoxification in Helicoverpa armigera. J. Agric. Food Chem. 2020, 68, 3390–3402. [Google Scholar] [CrossRef]
- Li, H.; Buczkowski, G.; Mittapalli, O.; Xie, J.; Wu, J.; Westerman, R.; Schemerhorn, B.J.; Murdock, L.L.; Pittendrigh, B.R. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol. Biol. 2008, 17, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.; Soulages, J. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Mao, K.; Ren, Z.; Li, W.; Cai, T.; Qin, X.; Wan, H.; Jun, B.; He, S.; Li, J. Carboxylesterase genes in nitenpyram-resistant brown planthoppers, Nilaparvata lugens. Insect Sci. 2021, 28, 1049–1060. [Google Scholar] [CrossRef]
- Ren, N.; Xie, M.; You, Y.; Li, J.; Chen, W.; Cheng, X.; You, M. Fipronil-resistance mediated by carboxylesterases in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol. Sin. 2015, 58, 288–296. [Google Scholar] [CrossRef]
- Guo, Y.; Si, F.; Han, B.; Qiao, L.; Chen, B. Identification and functional validation of P450 genes associated with pyrethroid resistance in the malaria vector Anopheles sinensis (Diptera: Culicidae). Acta Trop. 2024, 260, 107413. [Google Scholar] [CrossRef]
- Piermarini, P.M.; Esquivel, C.J.; Denton, J.S. Malpighian tubules as novel targets for mosquito control. Int. J. Environ. Res. Public Health 2017, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.M.; Chandre, F.; Rossignol, M.; Porciani, A.; Chateau, M.; Moiroux, N.; Pennetier, C. Sub-lethal insecticide exposure affects host biting efficiency of Kdr-resistant Anopheles gambiae. Peer Commun. J. 2021, 1, E28. [Google Scholar] [CrossRef]
- Nouage, L.; Elanga-Ndille, E.; Binyang, A.; Tchouakui, M.; Atsatse, T.; Ndo, C.; Kekeunou, S.; Wondji, C.S. Influence of GST- and P450-based metabolic resistance to pyrethroids on blood feeding in the major African malaria vector Anopheles funestus. PLoS ONE 2020, 15, e0230984. [Google Scholar] [CrossRef]
- Spillings, B.L.; Coetzee, M.; Koekemoer, L.L.; Brooke, B.D. The effect of a single blood meal on the phenotypic expression of insecticide resistance in the major malaria vector Anopheles funestus. Malar. J. 2008, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.V.; Brooke, B.D. The effect of multiple blood-feeding on the longevity and insecticide resistant phenotype in the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Parasites Vectors 2014, 7, 390. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; William, R.R.; Zhang, L.; Scott, J.G.; Gao, X.; Kristensen, M.; Liu, N. A whole transcriptomal linkage analysis of gene co-regulation in insecticide resistant house flies, Musca domestica. BMC Genom. 2013, 14, 803. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, X.; Sun, Y.; Ma, L.; Shen, B.; Zhu, C. Genomic analysis of detoxification supergene families in the mosquito Anopheles sinensis. PLoS ONE 2015, 10, e0143387. [Google Scholar] [CrossRef]
- Feng, X.; Liu, N. Functional analyses of house fly carboxylesterases involved in insecticide resistance. Front. Physiol. 2020, 11, 595009. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Tian, Z.; Li, Y.; Ye, X.; Li, R.; Li, X.; Zheng, S.; Liu, J.; Zhang, Y. Identification of key residues of carboxylesterase PxEst-6 involved in pyrethroid metabolism in Plutella xylostella (L.). J. Hazard. Mater. 2021, 407, 124612. [Google Scholar] [CrossRef]
- Xia, J.; Xu, H.; Yang, Z.; Pan, H.; Yang, X.; Guo, Z.; Yang, F.; Guo, L.; Sun, X.; Wang, S.; et al. Genome-wide analysis of carboxylesterases (COEs) in the whitefly, Bemisia tabaci (Gennadius). Int. J. Mol. Sci. 2019, 20, 4973. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Heidari, R.; Huang, H.Z.; Hammock, B.D.; Russell, R.J.; Oakeshot, J.G. Hydrolysis of individual isomers of fluorogenic pyrethroid analogs by mutant carboxylesterases from Lucilia cuprina. Insect Biochem. Molec. 2007, 37, 891–902. [Google Scholar] [CrossRef]
- Li, Y.; Farnsworth, C.A.; Coppin, C.W.; Teese, M.G.; Liu, J.W.; Scott, C.; Zhang, X.; Russell, R.J.; Oakeshott, J.G. Organophosphate and pyrethroid hydrolase activities of mutant esterases from the cotton bollworm Helicoverpa armigera. PLoS ONE 2013, 8, e77685. [Google Scholar] [CrossRef]
- Marcombe, S.; Fustec, B.; Cattel, J.; Chonephetsarath, S.; Thammavong, P.; Phommavanh, N.; David, J.P.; Corbel, V.; Sutherland, I.W.; Hertz, J.C.; et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl. Trop. Dis. 2019, 13, e0007852. [Google Scholar] [CrossRef]
- Poupardin, R.; Srisukontarat, W.; Yunta, C.; Ranson, H. Identification of carboxylesterase genes implicated in temephos resistance in the dengue vector Aedes aegypti. PLoS Negl. Trop. Dis. 2014, 8, e2743. [Google Scholar] [CrossRef] [PubMed]
- Grigoraki, L.; Lagnel, J.; Kioulos, I.; Kampouraki, A.; Morou, E.; Labbe, P.; Weill, M.; Vontas, J. Transcriptome profiling and genetic study reveal amplified carboxylesterase genes implicated in temephos resistance, in the Asian tiger mosquito Aedes albopictus. PLoS Negl. Trop. Dis. 2015, 9, e0003771. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Lycett, G.J.; McLaughlin, L.A.; Ranson, H.; Hemingway, J.; Kafatos, F.C.; Loukeris, T.G.; Paine, M.J.I. Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol. 2006, 15, 321–327. [Google Scholar] [CrossRef]
- Lumjuan, N.; Rajatileka, S.; Changsom, D.; Wicheer, J.; Leelapat, P.; Prapanthadara, L.A.; Somboon, P.; Lycett, G.; Ranson, H. The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochem. Mol. Biol. 2011, 41, 203–209. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of G-protein-coupled receptor-related genes in insecticide resistance of the mosquito, Culex quinquefasciatus. Sci. Rep. 2014, 4, 6474. [Google Scholar] [CrossRef]
- Li, R.; Sun, X.; Liang, P.; Gao, X. Characterization of carboxylesterase PxαE8 and its role in multi-insecticide resistance in Plutella xylostella (L.). J. Integr. Agric. 2022, 21, 1713–1721. [Google Scholar] [CrossRef]
- Peng, C.; Yin, H.; Liu, Y.; Mao, X.F.; Liu, Z.Y. RNAi mediated gene silencing of detoxification related genes in the Ectropis oblique. Genes 2022, 13, 1141. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Zhang, D.; Li, Z.; Zhang, S.; Liu, X. Molecular identification of carboxylesterase genes and their potential roles in the insecticides susceptibility of Grapholita molesta. Insect Mol. Biol. 2023, 32, 305–315. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Hawkes, N.J.; Hemingway, J. Co-amplification explains linkage disequilibrium of two mosquito esterase genes in insecticide resistant Culex quinquefasciatus. Biochem. J. 1997, 325, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, C.E.; Shan, G.; Ottea, J. Overview of carboxylesterases and their role in the metabolism of insecticides. J. Pest Sci. 2005, 30, 75–83. [Google Scholar] [CrossRef]
- Yan, J.; Nauen, R.; Reitz, S.; Alyokhin, A.; Zhang, J.; Mota-Sanchez, D.; Kim, Y.; Palli, S.R.; Rondon, S.I.; Nault, B.A.; et al. The new kid on the block in insect pest management: Sprayable RNAi goes commercial. Sci. China Life Sci. 2024, 67, 1766–1768. [Google Scholar] [CrossRef]
- Narva, K.; Toprak, U.; Alyokhin, A.; Groves, R.; Jurat-Fuentes, J.L.; Moar, W.; Nauen, R.; Whipple, S.; Head, G. Insecticide resistance management scenarios differ for RNA-based sprays and traits. Insect Mol. Biol. 2025, 34, 518–526. [Google Scholar] [CrossRef]
- Airs, P.M.; Bartholomay, L.C. RNA Interference for Mosquito and Mosquito-Borne Disease Control. Insects 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, R.M.; Duman-Scheel, M. Advances in oral RNAi for disease vector mosquito research and control. Curr. Opin. Insect Sci. 2020, 40, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Berthaud, V.; Martin, E.; Vallon, L.; Rebollo, R.; Vallier, A.; Vigneron, A.; Hay, A.; Moro, C.V.; Minard, G. Evaluation of non-invasive dsRNA delivery methods for the development of RNA interference in the Asian tiger mosquito Aedes albopictus. J. Pest Sci. 2025, 98, 581–596. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).