Changes in Gut Microbiota, Midgut Structure, and Gene Expression of Spodoptera frugiperda Infected by Serratia marcescens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Toxic Effects of S. marcescens on FAW
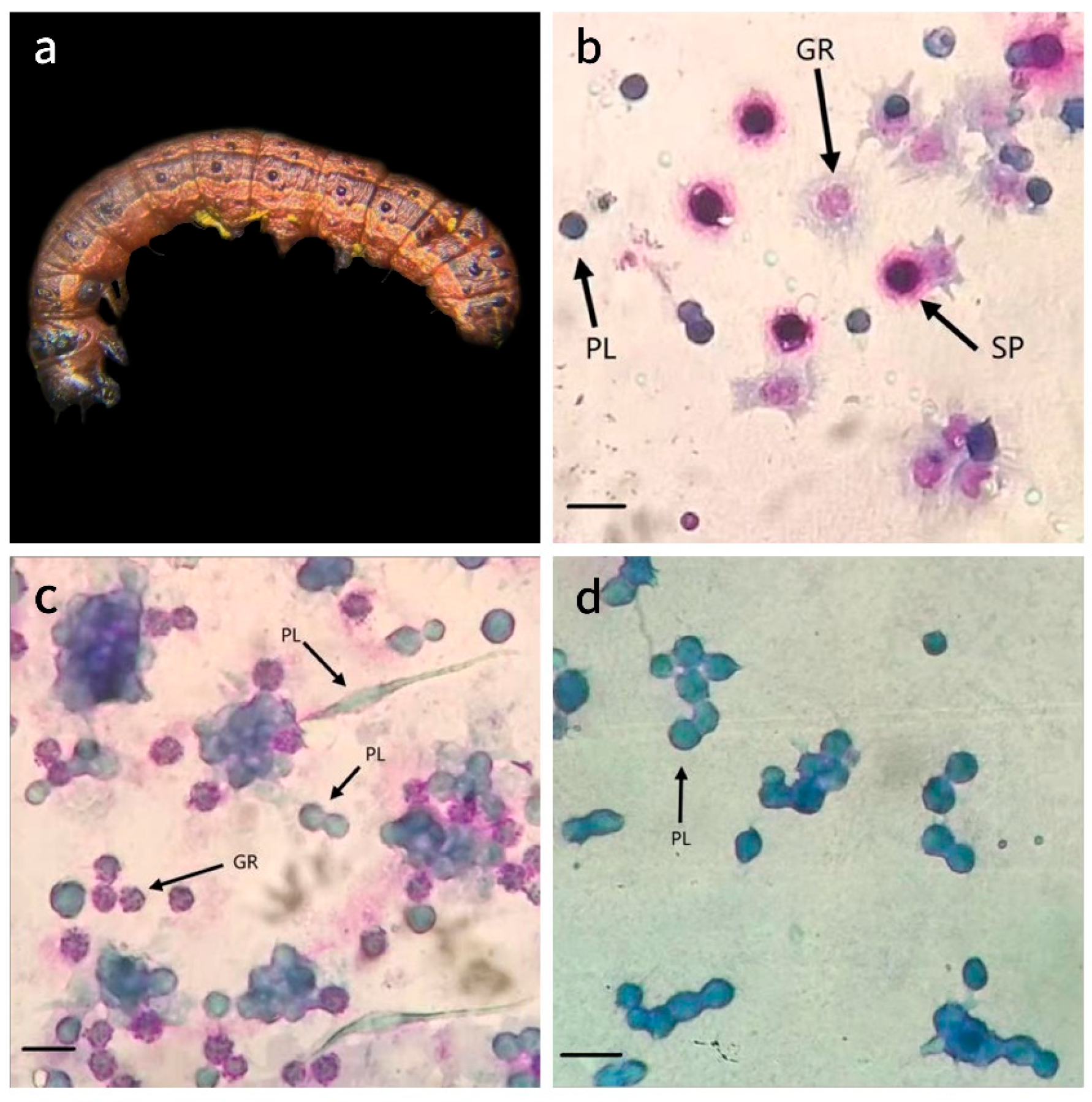
2.3. Effect of S. marcescens on the Hemolymph of FAW Larvae
2.4. Determination of Changes in the Midgut Microbial Community in FAW
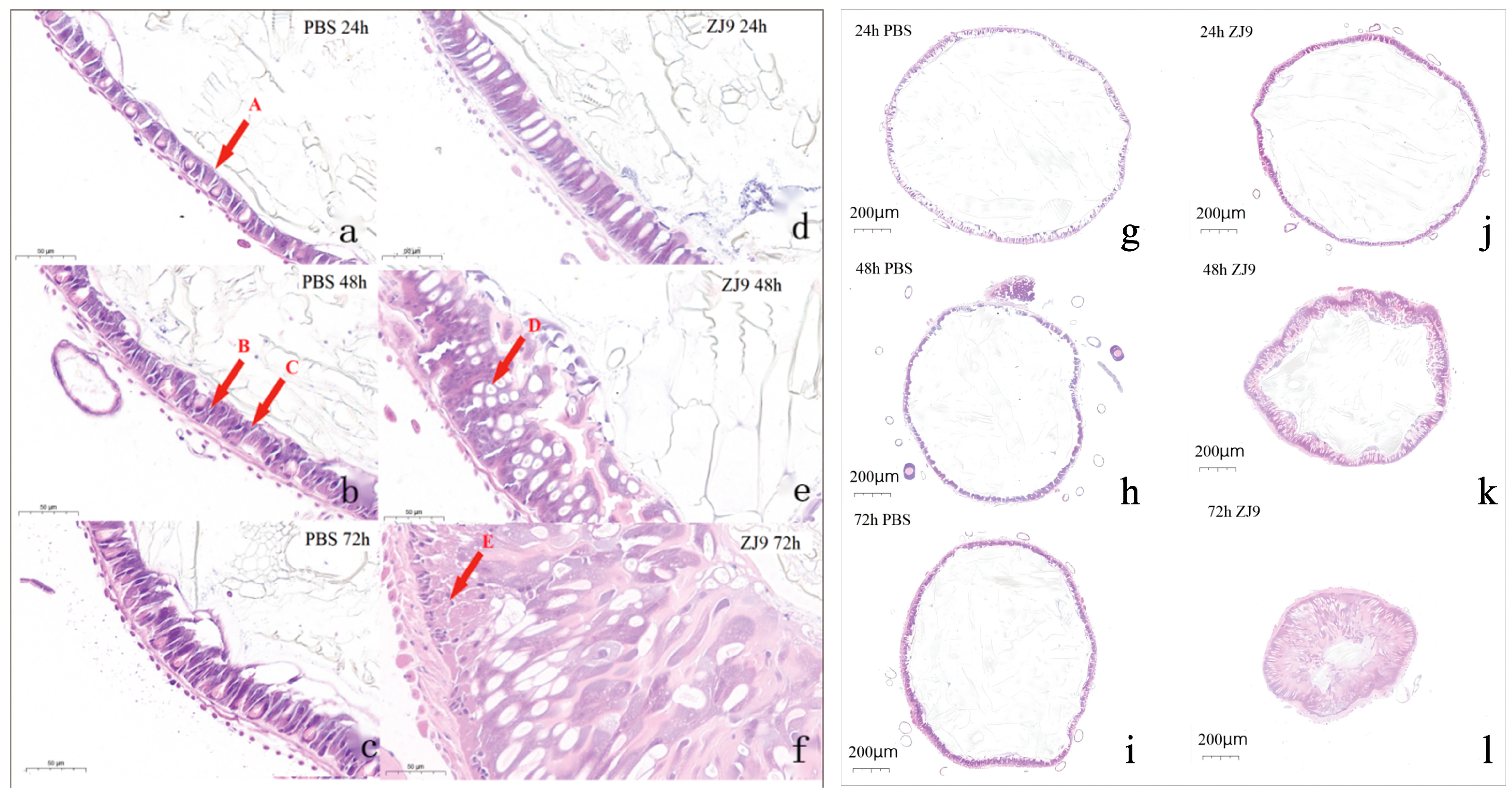
2.5. Midgut Tissue Sectioning and Observation
2.6. Transcriptomic Sequencing of FAW Infected with S. marcescens ZJ9
2.7. The qRT-PCR
3. Results
3.1. Toxic Effects of S. marcescens on FAW
3.2. Effect of S. marcescens on the Hemolymph of FAW Larvae
3.3. The Influence of S. marcescens Infestation on the Midgut of FAW
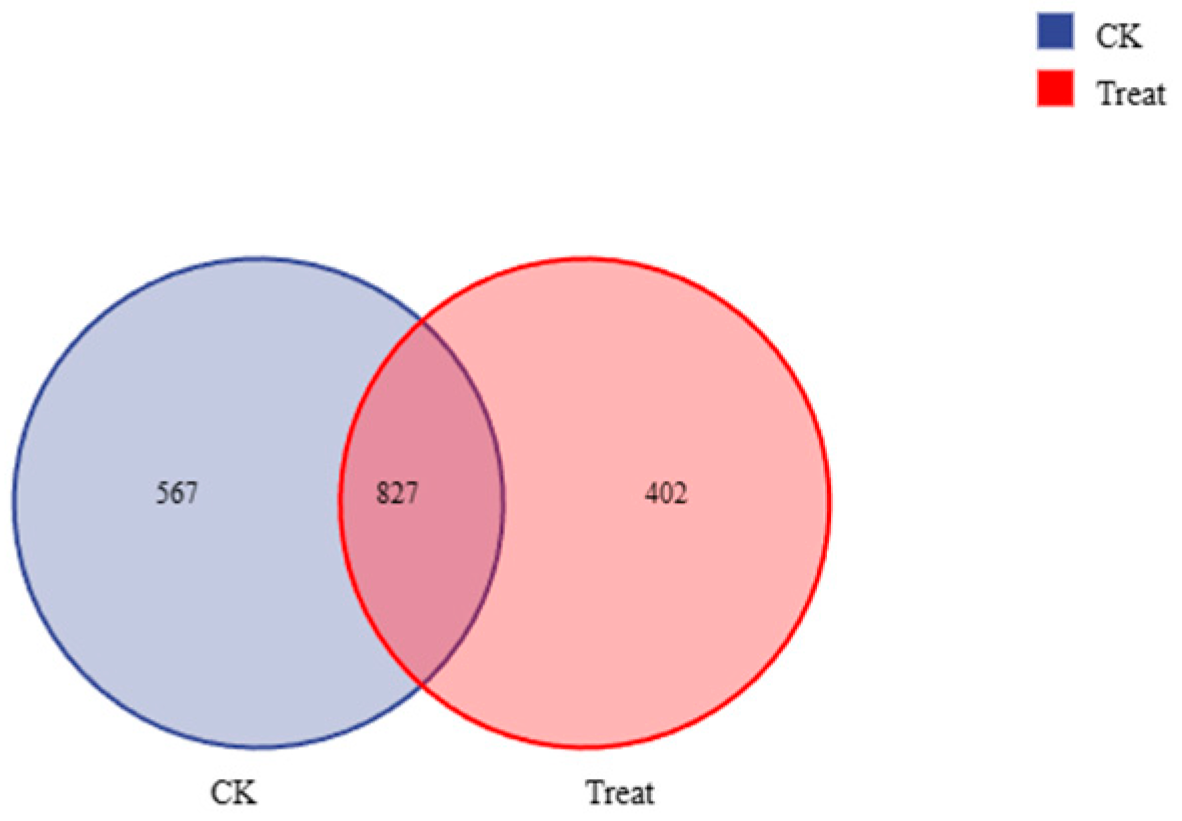
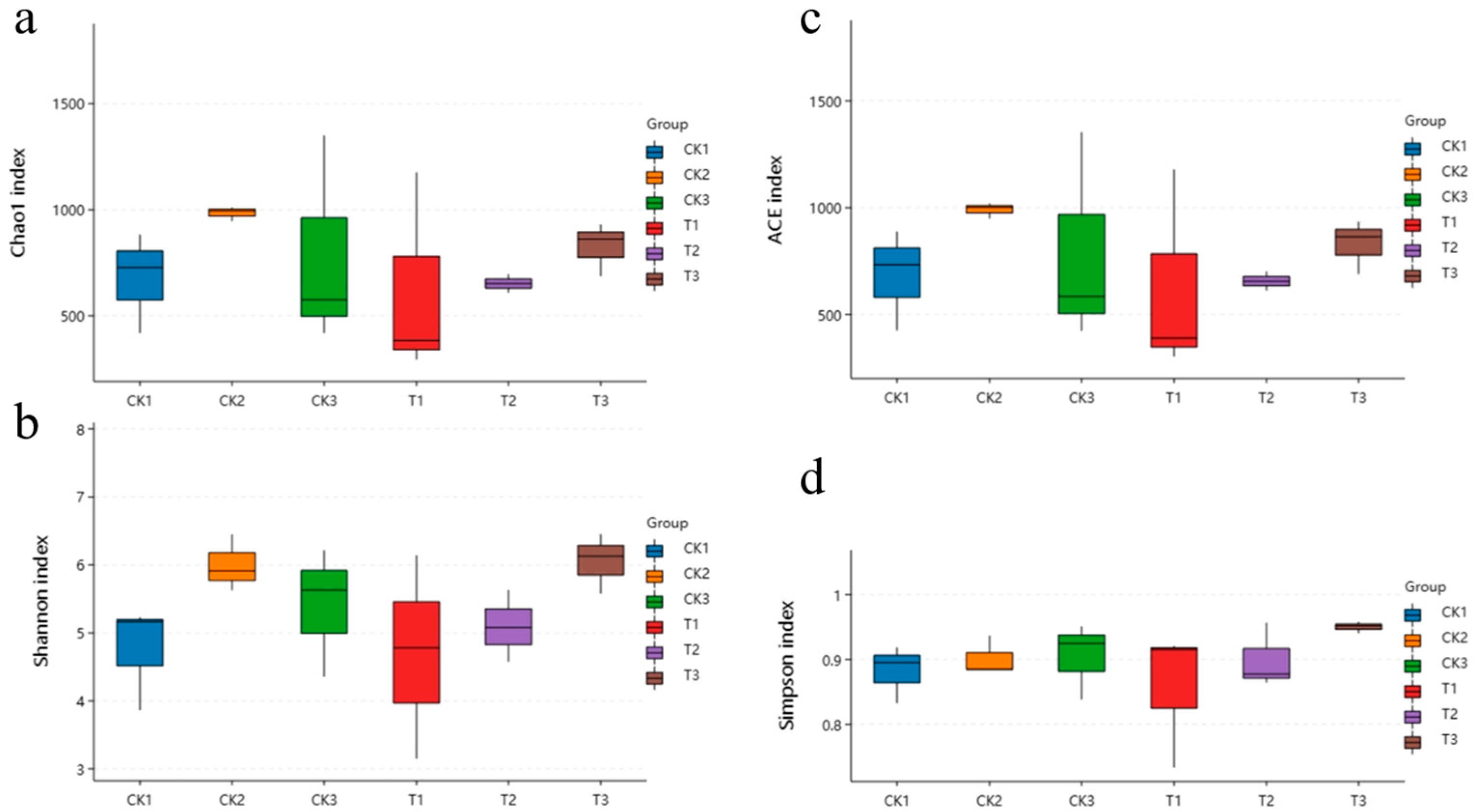
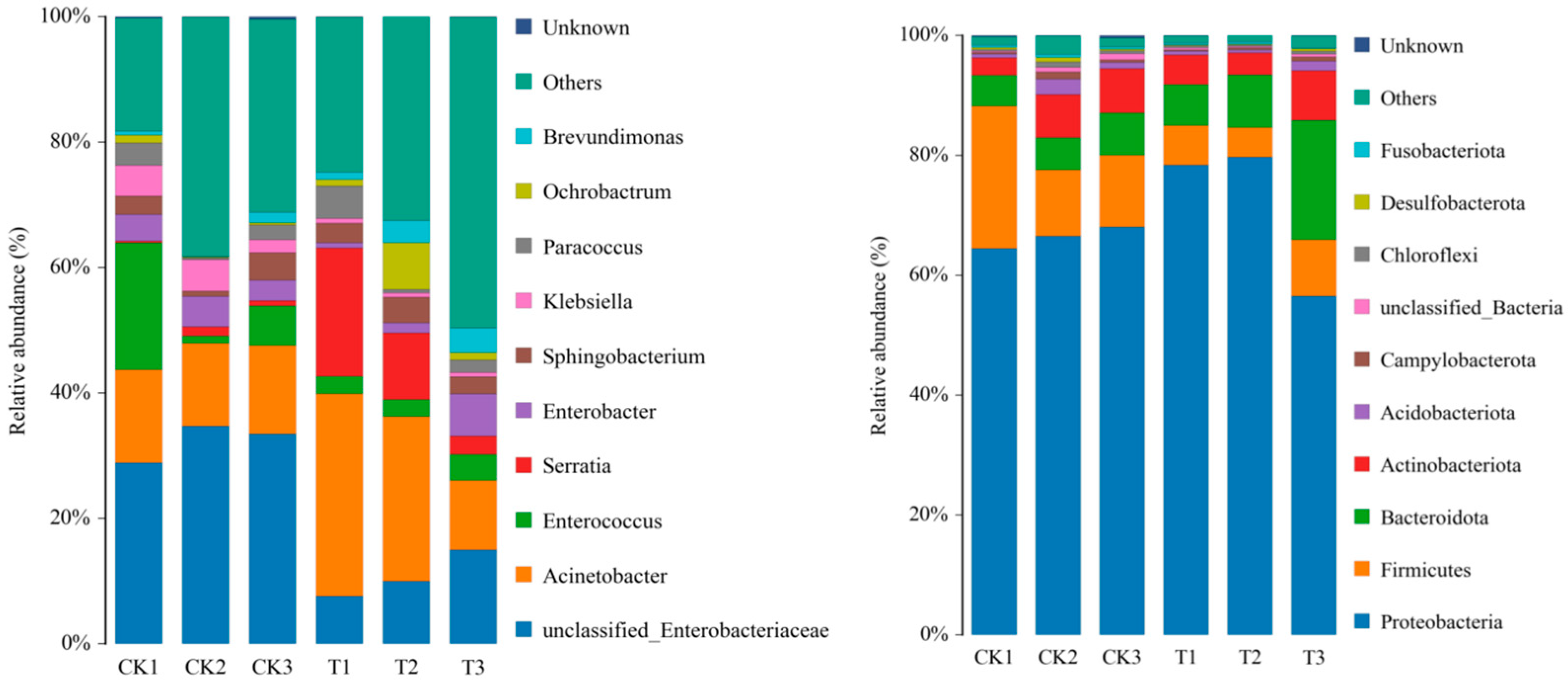
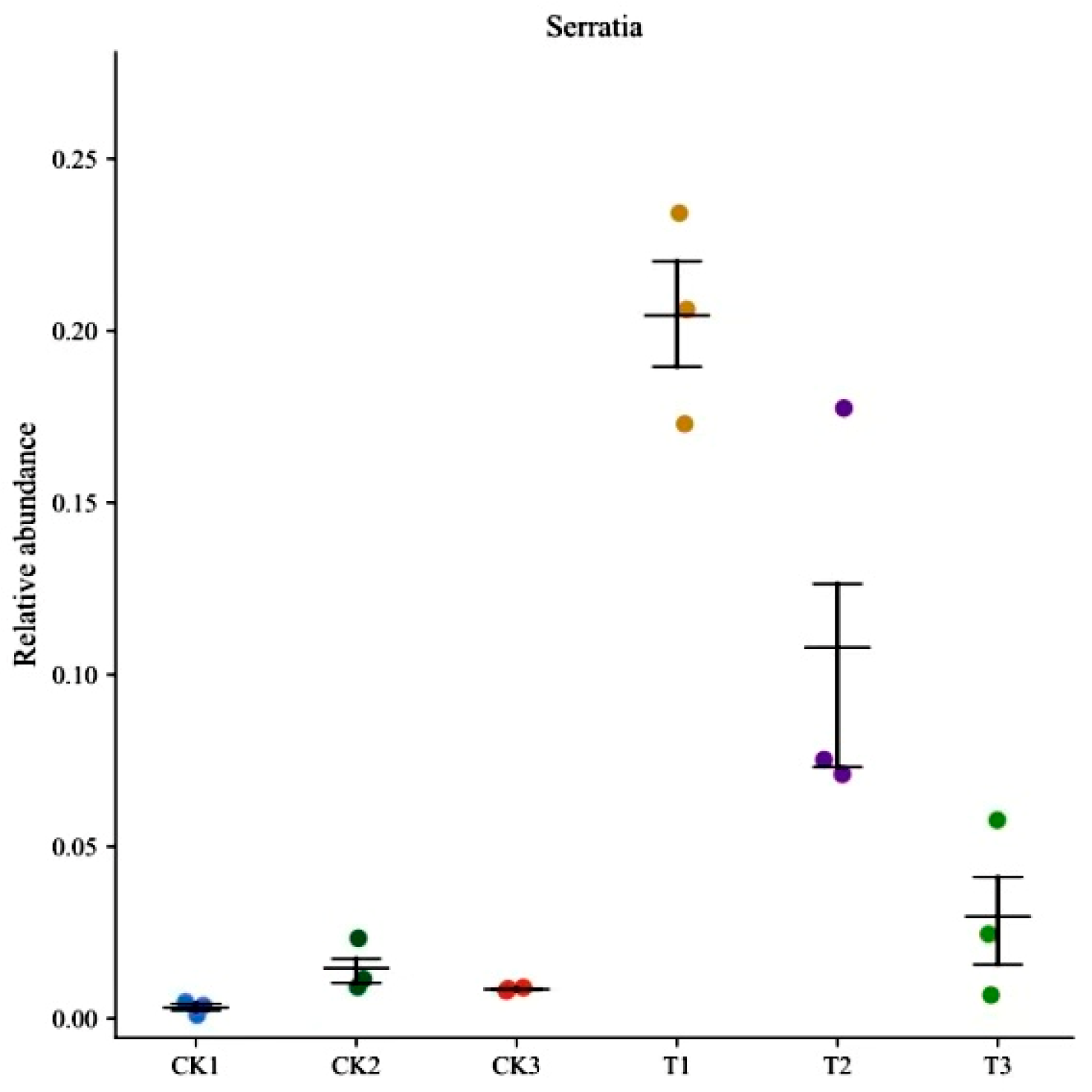
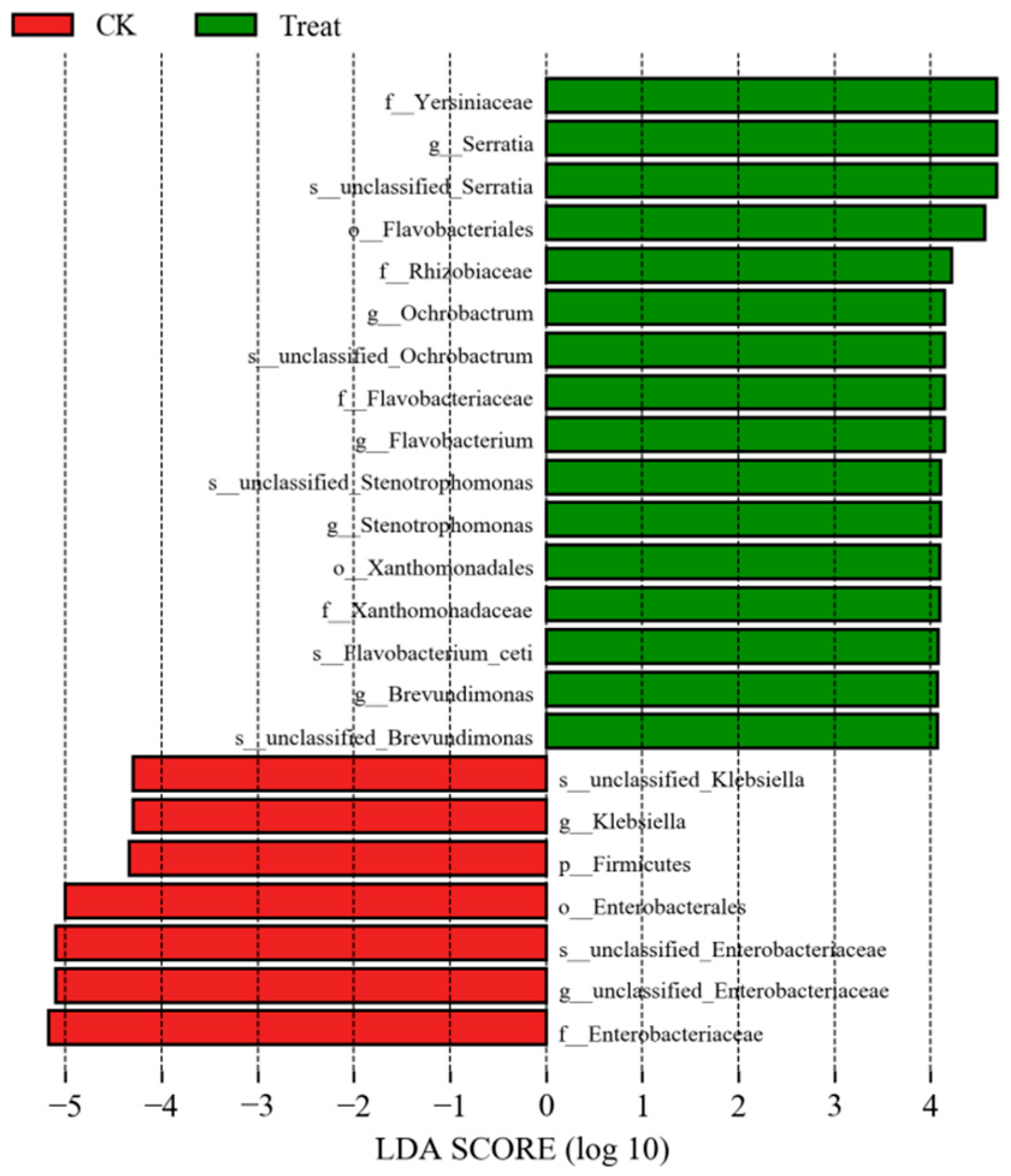
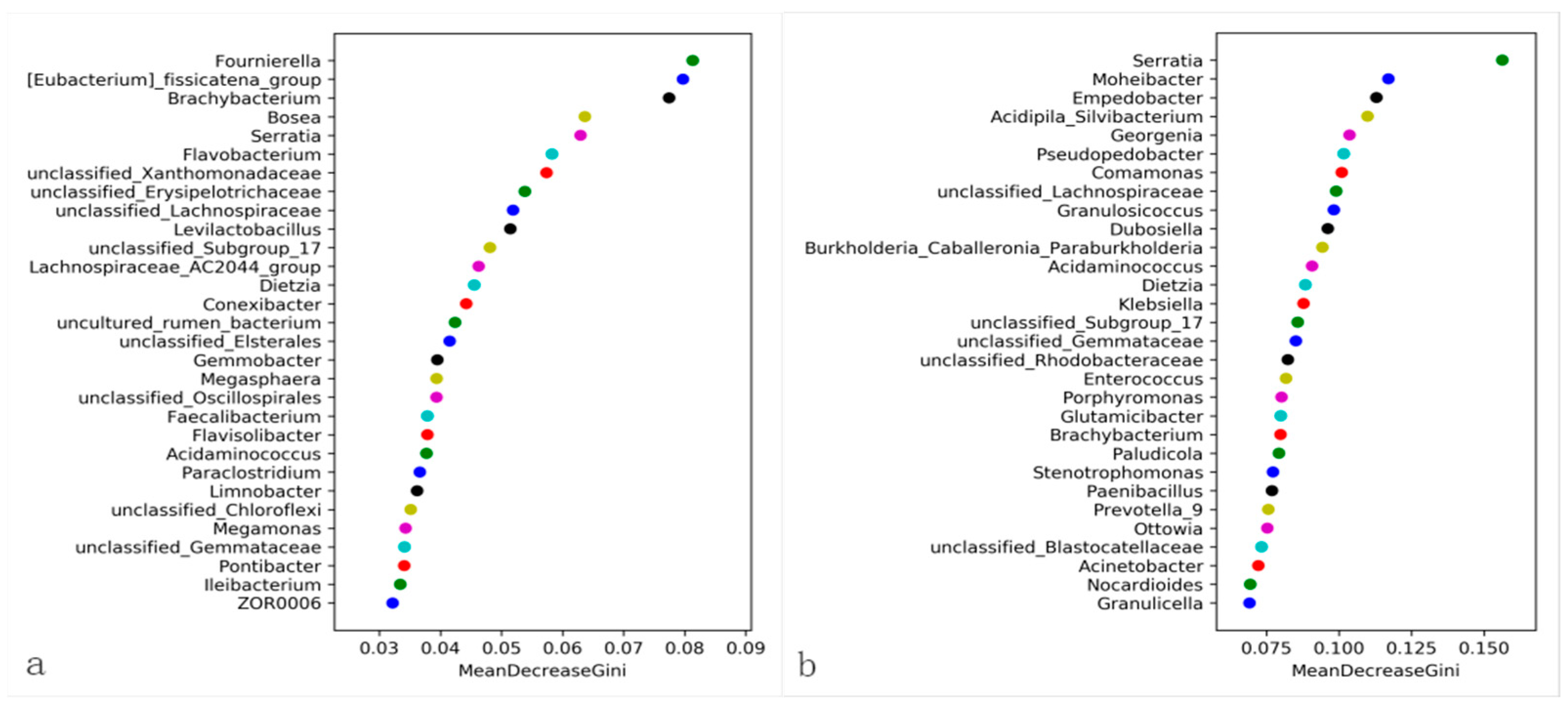
3.4. Changes in the Midgut Microbiota of FAW after Infection with S. marcescens
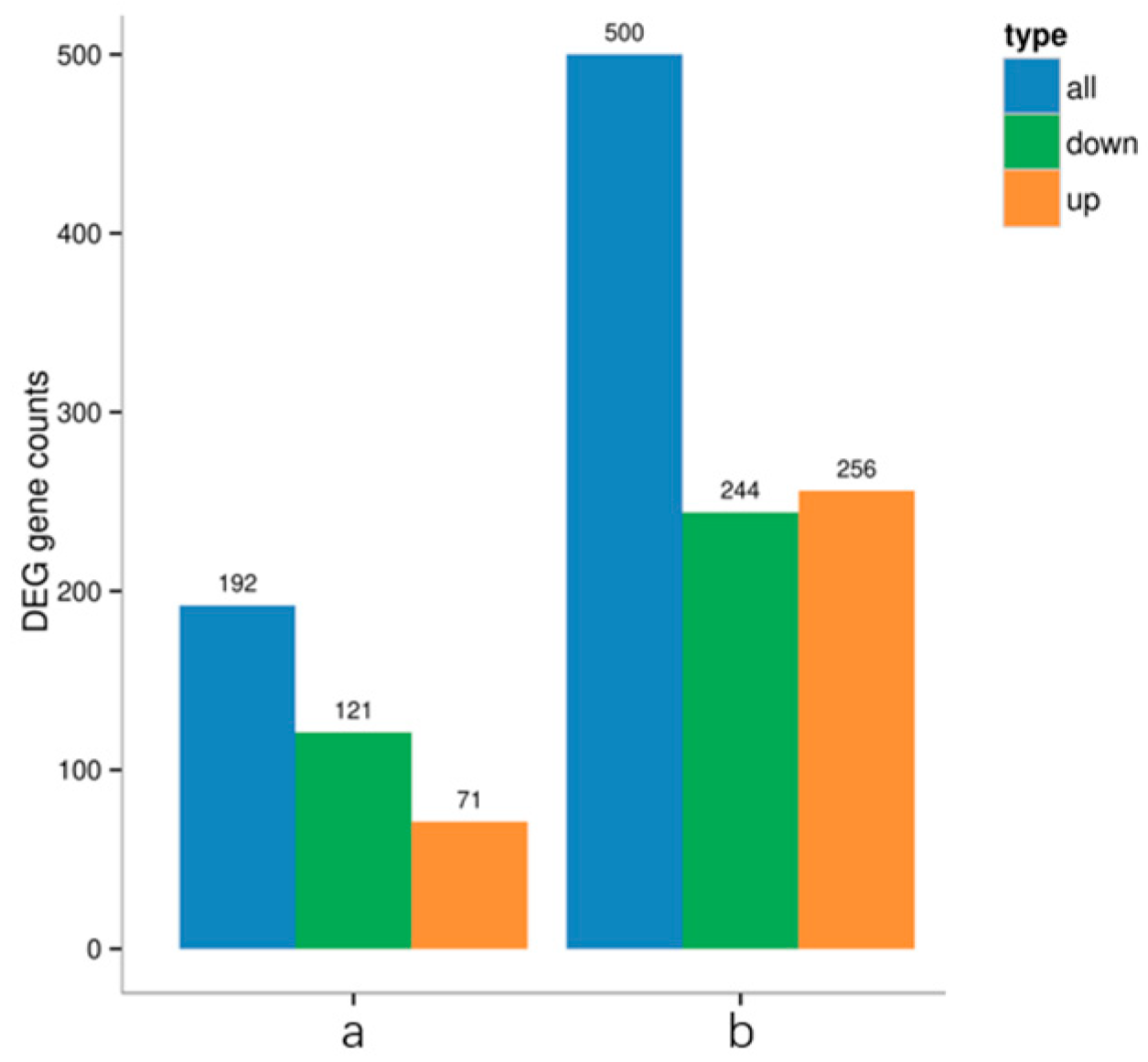
3.5. Assembly of Fall Armyworm Transcriptome Data
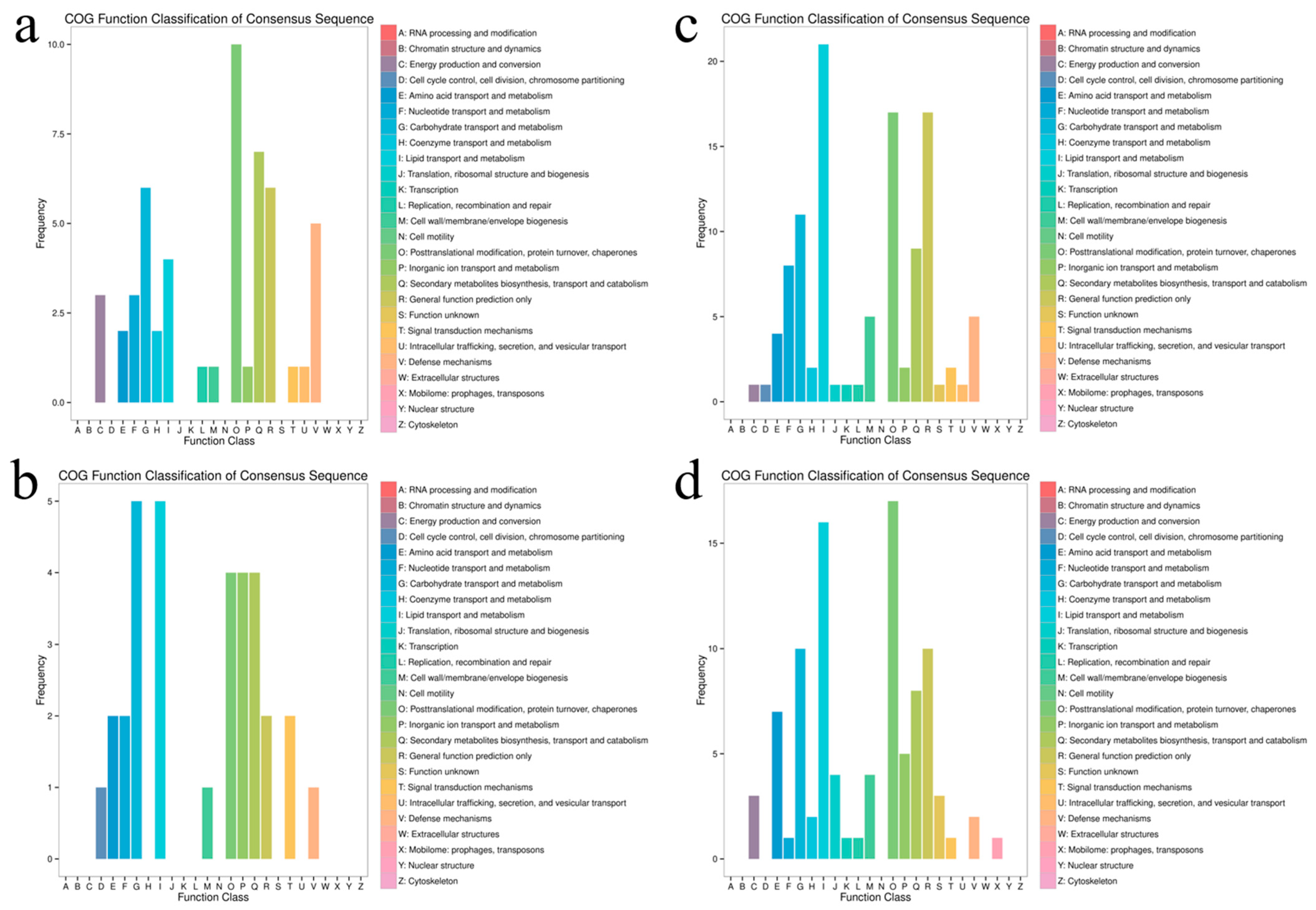
3.6. Functional Annotation Analysis of Differentially Expressed Gene COG
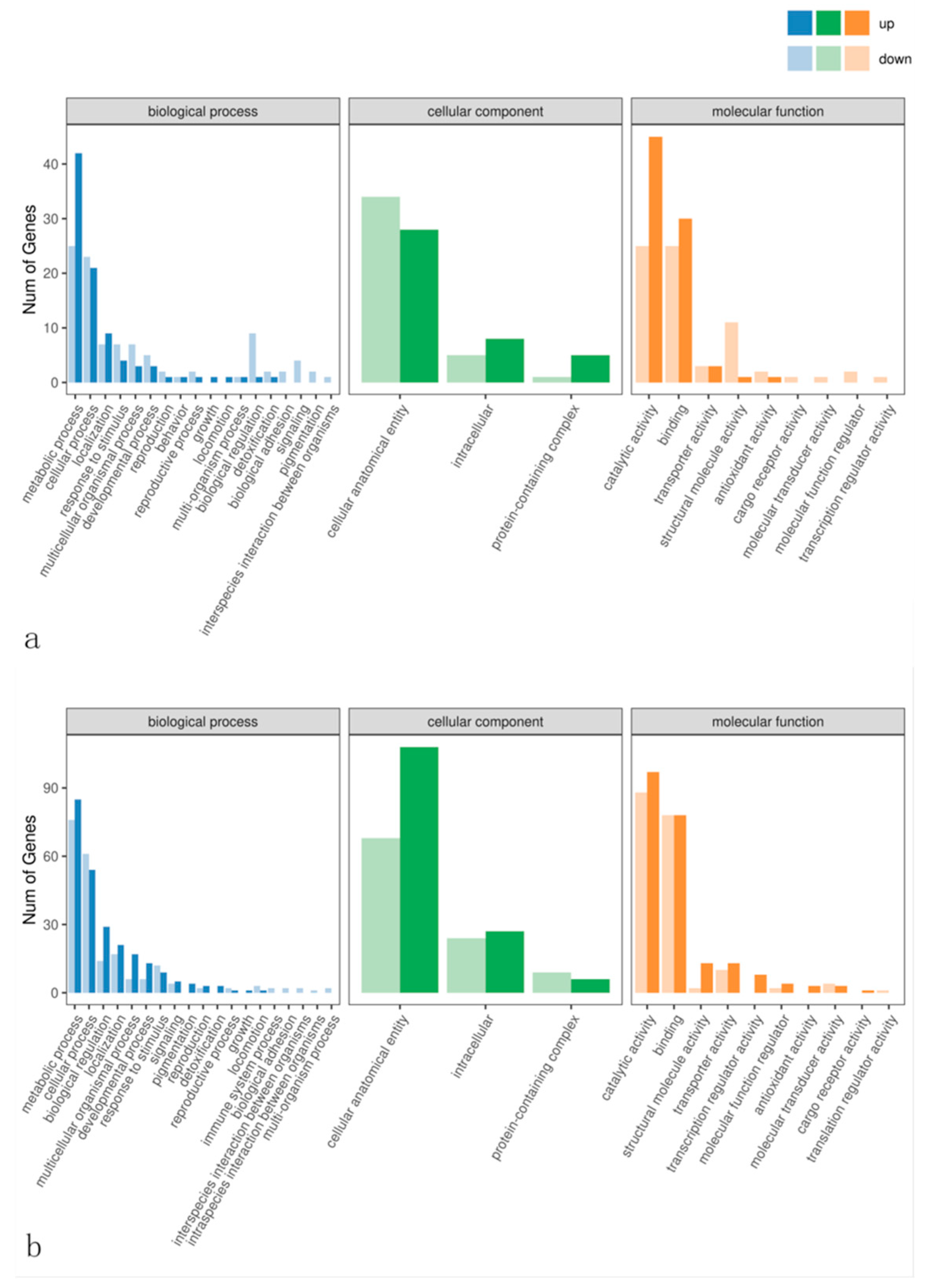
3.7. Functional Enrichment Analysis of Differentially Expressed Gene GO
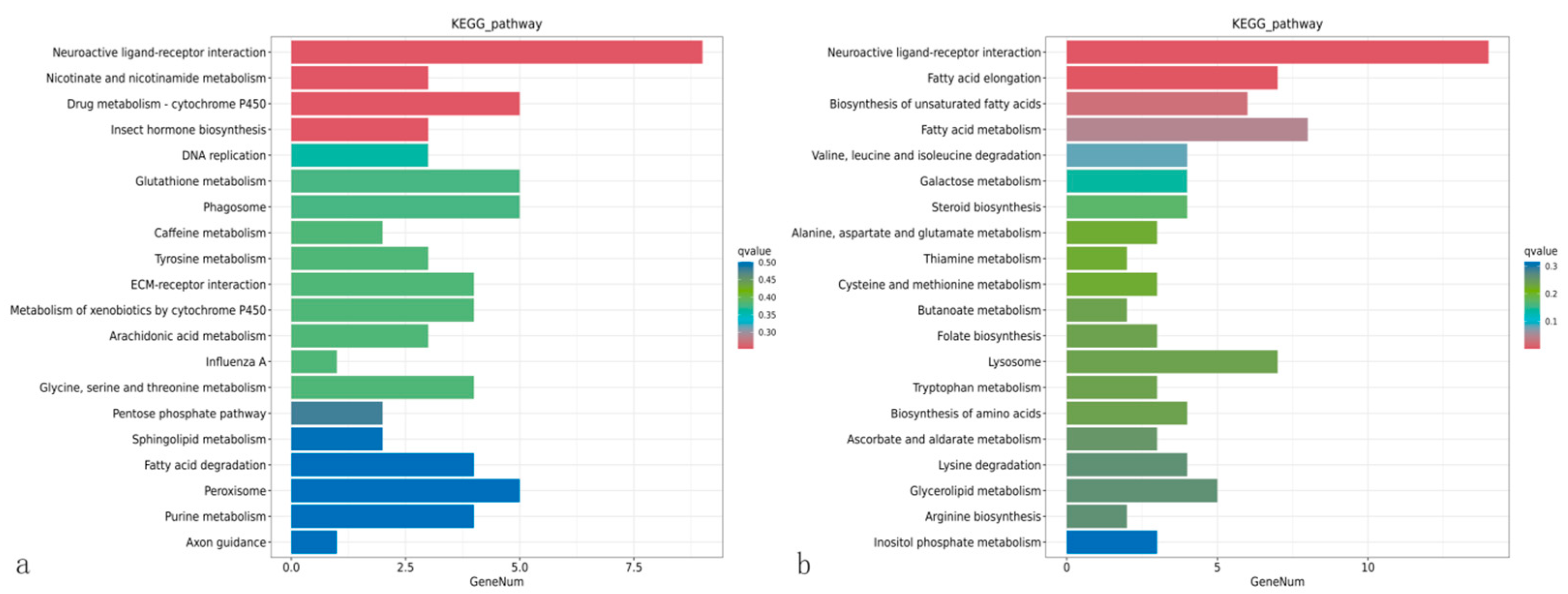
3.8. Enrichment Analysis of the KEGG Pathway of Differentially Expressed Genes
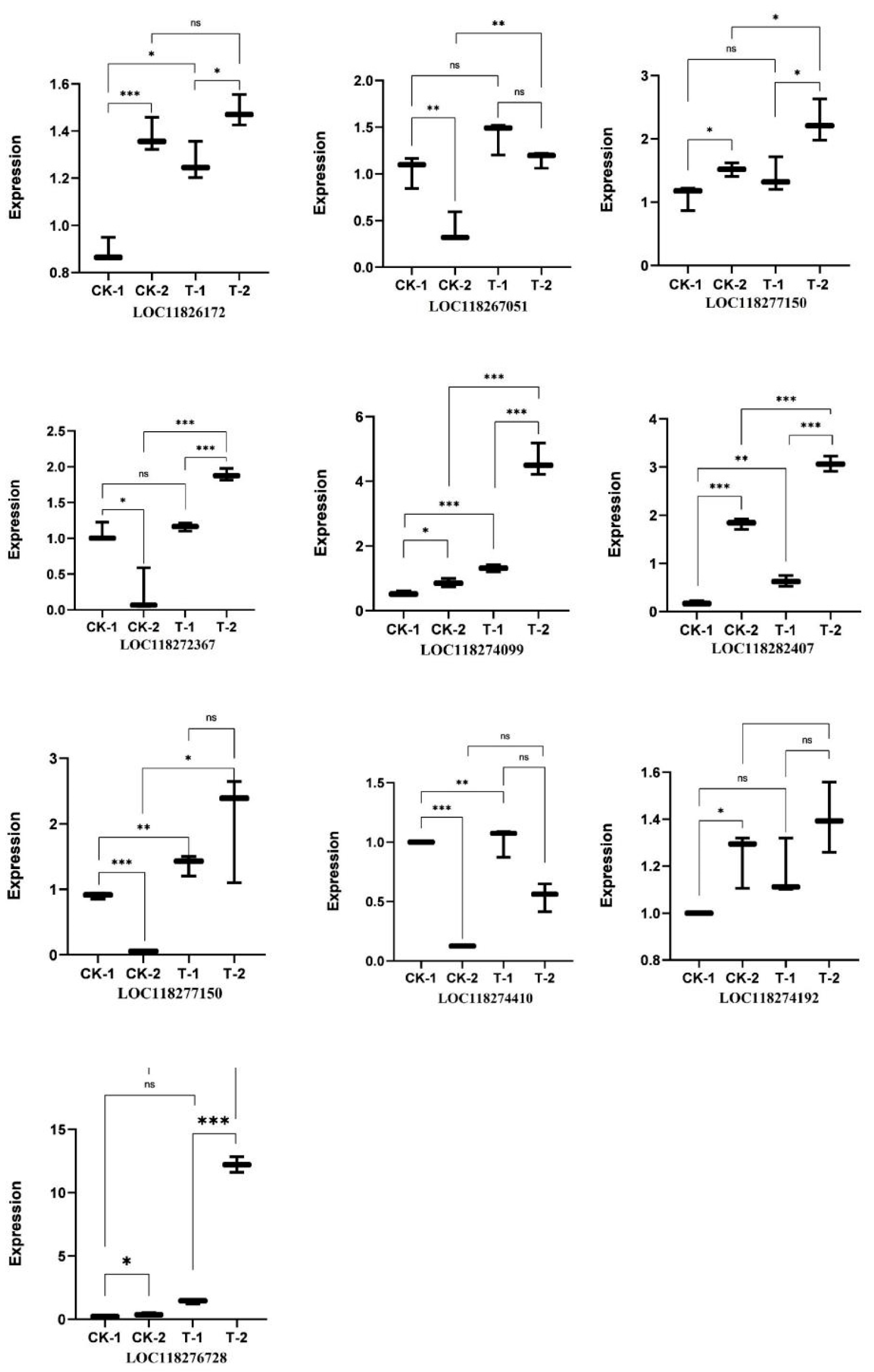
3.9. The qRT-PCR Verification
4. Discussion
4.1. Pathogenicity of S. marcescens ZJ9 and Its Infection Route in FAW
4.2. S. marcescens Infection Elicits Hemocytic Immune Response and Functional Impairment in FAW
4.3. S. marcescens Disrupts the Midgut Physical Barrier and Induces Microbiota Dysbiosis in FAW
4.4. Transcriptomic Impacts of S. marcescens ZJ9 on Metabolism and Immunity in the FAW Midgut
4.5. The Application Potential of S. marcescens in Suppressing FAW Growth by Disrupting Gut Microecological Balance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAW | Spodoptera frugiperda |
| qRT-PCR | Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction |
| LC50 | Lethal Concentration 50% |
| PL | Plasmatocyte |
| GR | Granulocyte |
| SP | Spherulocyte |
| PM | Peritrophic Membranes |
| COG | Cluster of Orthologous Groups of Proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Chen, H.; Yang, X.L.; Chen, A.D.; Li, Y.C.; Wang, D.H.; Liu, J.; Hu, T. lmmigration timing and origin of the first fall armyworms (Spodoptera frugiperda) detected in China. Chin. J. Appl. Entomol. 2020, 57, 1270–1278. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.-A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van der Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Èntomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Èntomol. 2023, 68, 299–317. [Google Scholar] [CrossRef]
- Hussain, A.G.; Wennmann, J.T.; Goergen, G.; Bryon, A.; Ros, V.I. Viruses of the Fall Armyworm Spodoptera frugiperda: A Review with Prospects for Biological Control. Viruses 2021, 13, 2220. [Google Scholar] [CrossRef]
- dos Santos, K.B.; Neves, P.; Meneguim, A.M.; dos Santos, R.B.; dos Santos, W.J.; Boas, G.V.; Dumas, V.; Martins, E.; Praça, L.B.; Queiroz, P.; et al. Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biol. Control 2009, 50, 157–163. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Abbas, A.; Ullah, F.; Hafeez, M.; Han, X.; Dara, M.Z.N.; Gul, H.; Zhao, C.R. Biological Control of Fall Armyworm, Spodoptera Frugiperda. Agronomy 2022, 12, 2704. [Google Scholar] [CrossRef]
- Dillon, R.J.; Vennard, C.T.; Buckling, A.; Charnley, A.K. Diversity of locust gut bacteria protects against pathogen invasion. Ecol. Lett. 2005, 8, 1291–1298. [Google Scholar] [CrossRef]
- Barbole, R.S.; Sharma, S.; Patil, Y.; Giri, A.P.; Joshi, R.S. Chitinase inhibition induces transcriptional dysregulation altering ecdysteroid-mediated control of Spodoptera frugiperda development. iScience 2024, 27, 109280. [Google Scholar] [CrossRef]
- Rabadiya, D.; Behr, M. The biology of insect chitinases and their roles at chitinous cuticles. Insect Biochem. Mol. Biol. 2024, 165, 104071. [Google Scholar] [CrossRef]
- Lin, D.-J.; Zhou, J.-X.; Ali, A.; Fu, H.-Y.; Gao, S.-J.; Jin, L.; Fang, Y.; Wang, J.-D. Biocontrol efficiency and characterization of insecticidal protein from sugarcane endophytic Serratia marcescens (SM) against oriental armyworm Mythimna separata (Walker). Int. J. Biol. Macromol. 2024, 262, 129978. [Google Scholar] [CrossRef] [PubMed]
- Jupatanakul, N.; Pengon, J.; Selisana, S.M.G.; Choksawangkarn, W.; Jaito, N.; Saeung, A.; Bunyong, R.; Posayapisit, N.; Thammatinna, K.; Kalpongnukul, N.; et al. Serratia marcescens secretes proteases and chitinases with larvicidal activity against Anopheles dirus. Acta Trop. 2020, 212, 105686. [Google Scholar] [CrossRef]
- Aggarwal, C.; Paul, S.; Nain, V.; Tripathi, V.; Paul, B.; Khan, A. Comparative response of Spodoptera litura challenged per os with Serratia marcescens strains differing in virulence. J. Invertebr. Pathol. 2021, 183, 107562. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Mason, C.J.; Carlson, J.E.; Tien, M.; Chen, H.-Y.; Harding, S.; Tsai, C.-J.; Hoover, K. Host-plant induced changes in microbial community structure and midgut gene expression in an invasive polyphage (Anoplophora glabripennis). Sci. Rep. 2018, 8, 9620. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, G.M.; Emam, M.T.H.; Hammad, M.A.; Mahmoud, S.H. Gene Cloning, Heterologous Expression, and In Silico Analysis of Chitinase B from Serratia marcescens for Biocontrol of Spodoptera frugiperda Larvae Infesting Maize Crops. Molecules 2024, 29, 1466. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Mason, C.J.; Felton, G.W.; Hoover, K. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci. Rep. 2019, 9, 2792. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef]
- Salem, H.; Bauer, E.; Strauss, A.S.; Vogel, H.; Marz, M.; Kaltenpoth, M. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141838. [Google Scholar] [CrossRef]
- Salem, H.; Bauer, E.; Kirsch, R.; Berasategui, A.; Cripps, M.; Weiss, B.; Koga, R.; Fukumori, K.; Vogel, H.; Fukatsu, T.; et al. Drastic Genome Reduction in an Herbivore’s Pectinolytic Symbiont. Cell 2017, 171, 1520–1531. [Google Scholar] [CrossRef]
- Lehane, M.J. Peritrophic Matrix Structure and Function. Annu. Rev. Èntomol. 1997, 42, 525–550. [Google Scholar] [CrossRef]
- Lü, D.; Dong, Y.; Yan, Z.; Liu, X.; Zhang, Y.; Yang, D.; He, K.; Wang, Z.; Wang, P.; Yuan, X.; et al. Dynamics of gut microflora across the life cycle of Spodoptera frugiperda and its effects on the feeding and growth of larvae. Pest Manag. Sci. 2022, 79, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Buchon, N.; Lemaitre, B.; McFall-Ngai, M.J. Microbiota-Induced Changes in Drosophila melanogaster Host Gene Expression and Gut Morphology. mBio 2014, 5, e01117-14. [Google Scholar] [CrossRef]
- Zhao, L.-N.; Ma, Y.; Yang, X.; Iqbal, A.; Ruan, C.-C.; Zang, L.-S. Identification of Serratia marcescens isolated from Antheraea pernyi eggs and determination of bacterial pathogenicity and transmission pathway. J. Invertebr. Pathol. 2020, 169, 107297. [Google Scholar] [CrossRef]
- Inglis, G.D.; Lawrence, A.M. Effects of Serratia marcescens on the F1 Generation of Laboratory-Reared Heliothis virescens (Lepidoptera: Noctuidae). J. Econ. Èntomol. 2001, 94, 362–366. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.-W. Insecticidal Serralysin of Serratia marcescens Is Detoxified in M3 Midgut Region of Riptortus pedestris. Front. Microbiol. 2022, 13, 913113. [Google Scholar] [CrossRef]
- Qu, S.; Wang, S. Interaction of entomopathogenic fungi with the host immune system. Dev. Comp. Immunol. 2018, 83, 96–103. [Google Scholar] [CrossRef]
- Fu, J.; Wang, J.; Huang, X.; Guan, B.; Feng, Q.; Deng, H. Composition and diversity of gut microbiota across developmental stages of Spodoptera frugiperda and its effect on the reproduction. Front. Microbiol. 2023, 14, 1237684. [Google Scholar] [CrossRef]
- Bai, S.; Yao, Z.; Raza, M.F.; Cai, Z.; Zhang, H. Regulatory mechanisms of microbial homeostasis in insect gut. Insect Sci. 2020, 28, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Lachat, J.; Lextrait, G.; Jouan, R.; Boukherissa, A.; Yokota, A.; Jang, S.; Ishigami, K.; Futahashi, R.; Cossard, R.; Naquin, D.; et al. Hundreds of antimicrobial peptides create a selective barrier for insect gut symbionts. Proc. Natl. Acad. Sci. USA 2024, 121, e2401802121. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Kikuchi, Y. Endosymbiotic Bacteria in Insects: Their Diversity and Culturability. Microbes Environ. 2009, 24, 195–204. [Google Scholar] [CrossRef]
- Sprouse, P. Evaluation of Structure-Property Relationships Within Insect Cuticle to Identify Motifs for Biomaterial Design. Ph.D. Thesis, The University of Kansas, Lawrence, KS, USA, May 2014. [Google Scholar]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef]
- Terra, W.R.; Barroso, I.G.; Dias, R.O.; Ferreira, C. Molecular physiology of insect midgut. Adv. Insect Physiol. 2019, 56, 117–163. [Google Scholar] [CrossRef]
- Zeng, T.; Jaffar, S.; Xu, Y.; Qi, Y. The Intestinal Immune Defense System in Insects. Int. J. Mol. Sci. 2022, 23, 15132. [Google Scholar] [CrossRef]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28. [Google Scholar] [CrossRef]
- Majumder, J.; Ghosh, D.; Agarwala, B.K. Haemocyte Morphology and Differential Haemocyte Counts of Giant Ladybird Beetle, Anisolemnia dilatata (F.) (Coleoptera:Coccinellidae): A Unique Predator of Bamboo Woolly Aphids. Curr. Sci. 2017, 112, 160. [Google Scholar] [CrossRef]
- Sutter, G.R.; Raun, E.S. Histopathology of European-corn-borer larvae treated with Bacillus thuringiensis. J. Invertebr. Pathol. 1967, 9, 90–103. [Google Scholar] [CrossRef]
- Caccia, S.; Casartelli, M.; Tettamanti, G. The amazing complexity of insect midgut cells: Types, peculiarities, and functions. Cell Tissue Res. 2019, 377, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.E.; Merkushova, A.V.; Savina, I.A.; Nizhnikov, A.A.; Antonets, K.S. The man, the plant, and the insect: Shooting host specificity determinants in Serratia marcescens pangenome. Front. Microbiol. 2023, 14, 1211999. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota–host interactions in insects. J. Exp. Biol. 2021, 224, jeb207696. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Montero, G.; Wood, M.; Horn, H.A.; Gemperline, E.; Li, L.; Currie, C.R.; Cavanaugh, C.M. Symbiont-Mediated Protection of Acromyrmex Leaf-Cutter Ants from the Entomopathogenic Fungus Metarhizium anisopliae. mBio 2021, 12, e0188521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Zhang, X.; Zhang, K.; Liu, W.; Zhang, R.; Zhang, Z. Enterobacter hormaechei in the intestines of housefly larvae promotes host growth by inhibiting harmful intestinal bacteria. Parasites Vectors 2021, 14, 598. [Google Scholar] [CrossRef]
- Khan, S.A.; Kojour, M.A.M.; Han, Y.S. Recent trends in insect gut immunity. Front. Immunol. 2023, 14, 1272143. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, L. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. npj Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wen, S.; Wang, G.; Zhang, X.; Di, T.; Du, G.; Chen, B.; Zhang, L. Reconstruction of Gut Bacteria in Spodoptera frugiperda Infected by Beauveria bassiana Affects the Survival of Host Pest. J. Fungi 2023, 9, 906. [Google Scholar] [CrossRef]
- Muñoz-Benavent, M.; Pérez-Cobas, A.E.; García-Ferris, C.; Moya, A.; Latorre, A. Insects’ potential: Understanding the functional role of their gut microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Goodman, R.M.; Handelsman, J. Census of the Bacterial Community of the Gypsy Moth Larval Midgut by Using Culturing and Culture-Independent Methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef]
- Broderick, N.; Robinson, C.J.; McMahon, M.D.; Holt, J.; Handelsman, J.; Raffa, K.F. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009, 7, 11. [Google Scholar] [CrossRef]
- Yasika, Y.; Shivakumar, M.S. A comprehensive account of functional role of insect gut microbiome in insect orders. J. Nat. Pestic. Res. 2024, 11, 100110. [Google Scholar] [CrossRef]
- Xiang, T.; Zhou, W.; Xu, C.; Xu, J.; Liu, R.; Wang, N.; Xu, L.; Zhao, Y.; Luo, M.; Mo, X.; et al. Transcriptomic Analysis Reveals Competitive Growth Advantage of Non-pigmented Serratia marcescens Mutants. Front. Microbiol. 2022, 12, 793202. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Lu, X. Diversity and Functional Roles of the Gut Microbiota in Lepidopteran Insects. Microorganisms 2022, 10, 1234. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, H.; Lai, Y.; Chen, Q.; Yu, X.-Q.; Wang, X. Gut Microbiota Dysbiosis Influences Metabolic Homeostasis in Spodoptera frugiperda. Front. Microbiol. 2021, 12, 727434. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Q.; Katsevich, E.; Sabatti, C. Exploratory Gene Ontology Analysis with Interactive Visualization. Sci. Rep. 2019, 9, 7793. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Osire, T.; Fu, W.; Yi, G.; Yang, S.-T.; Yang, T.; Rao, Z. Comparative transcriptome analysis reveals metabolic regulation of prodigiosin in Serratia marcescens. Syst. Microbiol. Biomanuf. 2021, 1, 323–335. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Song, X.; Zhang, H.; Ma, S.; Wang, J.; Li, W.; Lv, R.; Liu, X.; Ma, S.; et al. Salmonella Typhimurium reprograms macrophage metabolism via T3SS effector SopE2 to promote intracellular replication and virulence. Nat. Commun. 2021, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Raymann, K.; Coon, K.L.; Shaffer, Z.; Salisbury, S.; Moran, N.A. Pathogenicity of Serratia marcescens Strains in Honey Bees. mBio 2019, 10. [Google Scholar] [CrossRef]
- Stanley, D.; Miller, J.; Tunaz, H. Eicosanoid Actions in Insect Immunity. J. Innate Immun. 2009, 1, 282–290. [Google Scholar] [CrossRef]
- Leclerc, V.; Reichhart, J. The immune response of Drosophila melanogaster. Immunol. Rev. 2004, 198, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Blenau, W.; Thamm, M. Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies. Lessons from Drosophila melanogaster and Apis mellifera. Arthropod Struct. Dev. 2011, 40, 381–394. [Google Scholar] [CrossRef] [PubMed]


| Gene Number | Upstream Primer | Downstream Primer |
|---|---|---|
| LOC118261729 | CGTCGCCAAAGTGAAAGTGG | TTCGCATCGATGTAGGGCTC |
| LOC118267051 | TAGCAGCCAGAGGTTTCCTG | AACAAGCCTTGAGTACCGCA |
| LOC118272367 | GACTAGGTCACGCTGGCATT | AGACCGCTTGTGAACGTGAT |
| LOC118274099 | CCCACCATCAACCCCAGATT | CAGCGAAACCACTGAGGACT |
| LOC118274192 | AGAAGAGAACTCGGACGGGA | AGCACACCTGGTTAGCGAAA |
| LOC118274410 | CGCATTGTCACAAAATGACACC | AGTGATGTCGGCTTTGCCTA |
| LOC118276728 | AGTACAGCGTGAACCAAGGG | TTAACTTCGACGGCAACGGA |
| LOC118277150 | ACGTTGCAGCTGCTGATTTG | TGTGCTACGGGAGAGTTTGC |
| LOC118282336 | CAGTATCTGGCGTCCTCGTC | CACCGAGAGTTGTCTCGCAA |
| LOC118282407 | AGCGATCATGAAATTGTTGGTGT | ACCTGTGCCGTCACTTTGAT |
| LOC118279579 | TTGGTAGGCACGCTACACAG | CGGTGTCAGGCAGAAGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zou, Y.; Chen, Y.; Liu, J.; Ye, Y.; Huang, X.; Wu, Z. Changes in Gut Microbiota, Midgut Structure, and Gene Expression of Spodoptera frugiperda Infected by Serratia marcescens. Insects 2025, 16, 933. https://doi.org/10.3390/insects16090933
Guo Y, Zou Y, Chen Y, Liu J, Ye Y, Huang X, Wu Z. Changes in Gut Microbiota, Midgut Structure, and Gene Expression of Spodoptera frugiperda Infected by Serratia marcescens. Insects. 2025; 16(9):933. https://doi.org/10.3390/insects16090933
Chicago/Turabian StyleGuo, Yibo, Yue Zou, Youyang Chen, Jiaxin Liu, Yingying Ye, Xinglong Huang, and Zhengwei Wu. 2025. "Changes in Gut Microbiota, Midgut Structure, and Gene Expression of Spodoptera frugiperda Infected by Serratia marcescens" Insects 16, no. 9: 933. https://doi.org/10.3390/insects16090933
APA StyleGuo, Y., Zou, Y., Chen, Y., Liu, J., Ye, Y., Huang, X., & Wu, Z. (2025). Changes in Gut Microbiota, Midgut Structure, and Gene Expression of Spodoptera frugiperda Infected by Serratia marcescens. Insects, 16(9), 933. https://doi.org/10.3390/insects16090933







