Occurrence, Biological Characteristics, and Annual Dynamics of Atherigona orientalis (Schiner 1968) (Diptera: Muscidae) in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution and Hosts Survey of the Atherigona orientalis in Hunan Province
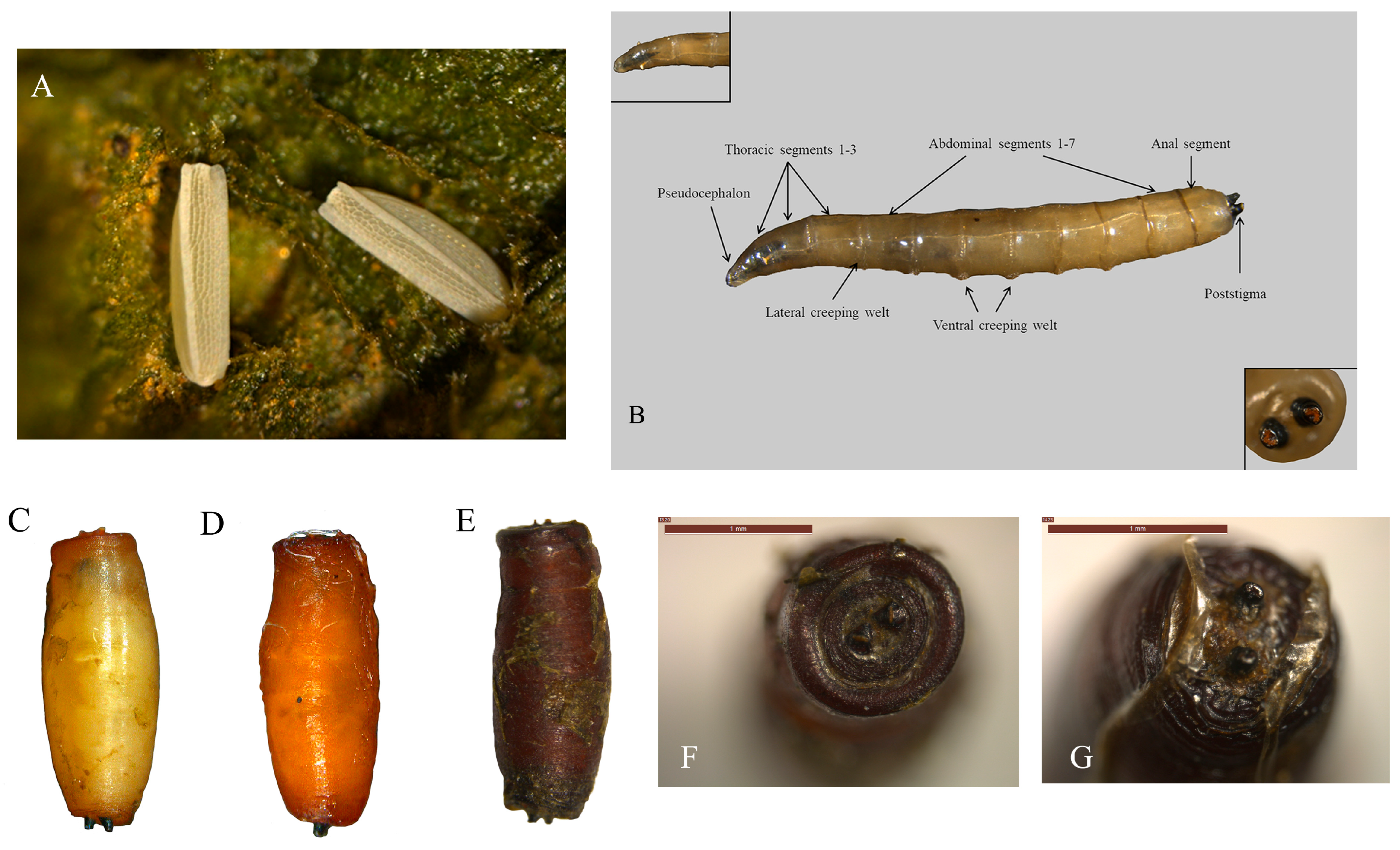
2.2. Morphological Characterization of Different Developmental Stages of Atherigona orientalis
2.3. Developmental Duration of Each Insect State of Atherigona orientalis
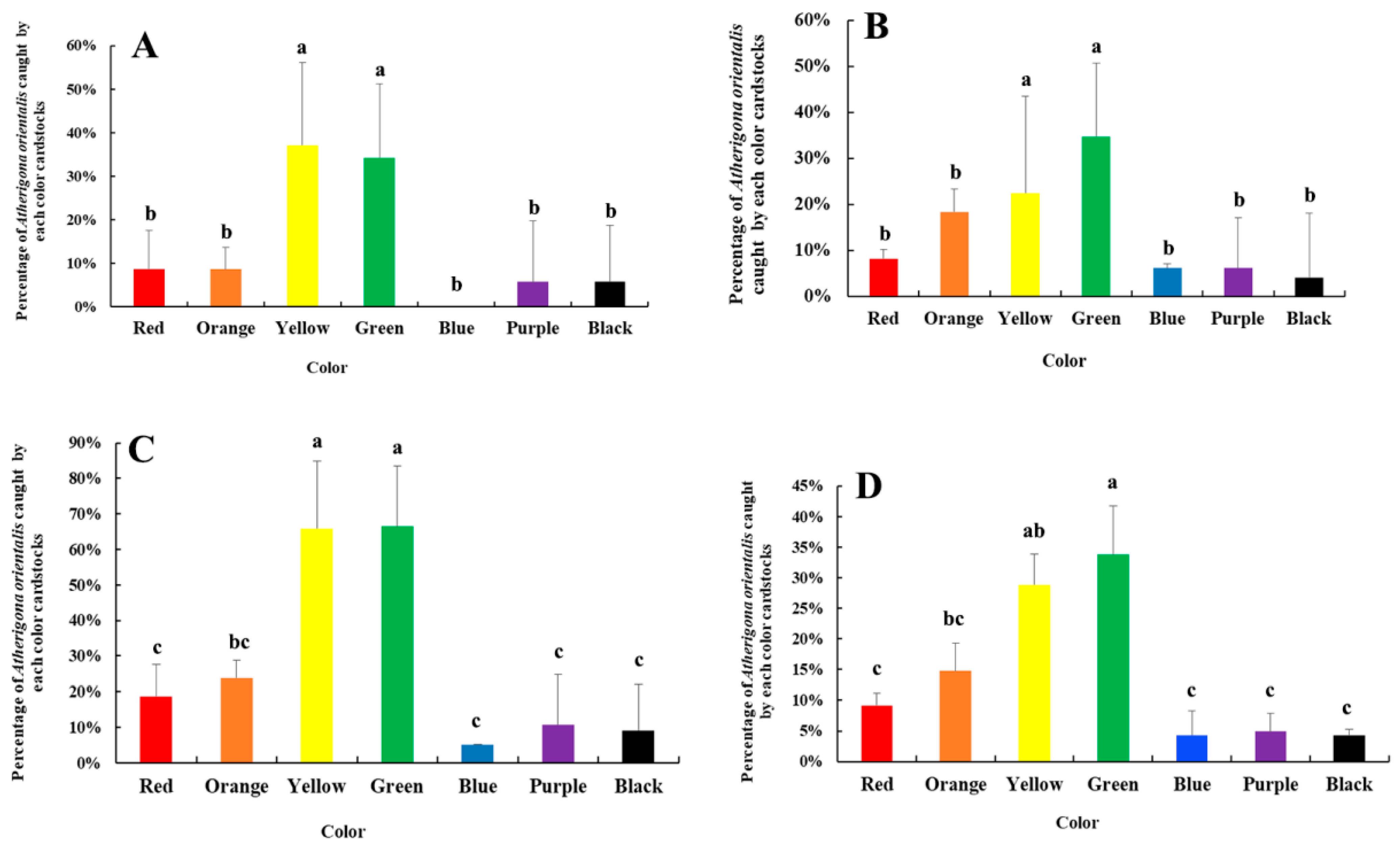
2.4. The Color Selections of Adult Atherigona orientalis
2.5. Monitoring of Atherigona orientalis Population Dynamics
3. Result
3.1. Distribution and Host Ranges of Atherigona orientalis in Hunan Province
3.2. Morphological Characteristics of Atherigona orientalis in Various Stages
Key Morphological Characteristics of Atherigona orientalis Adults
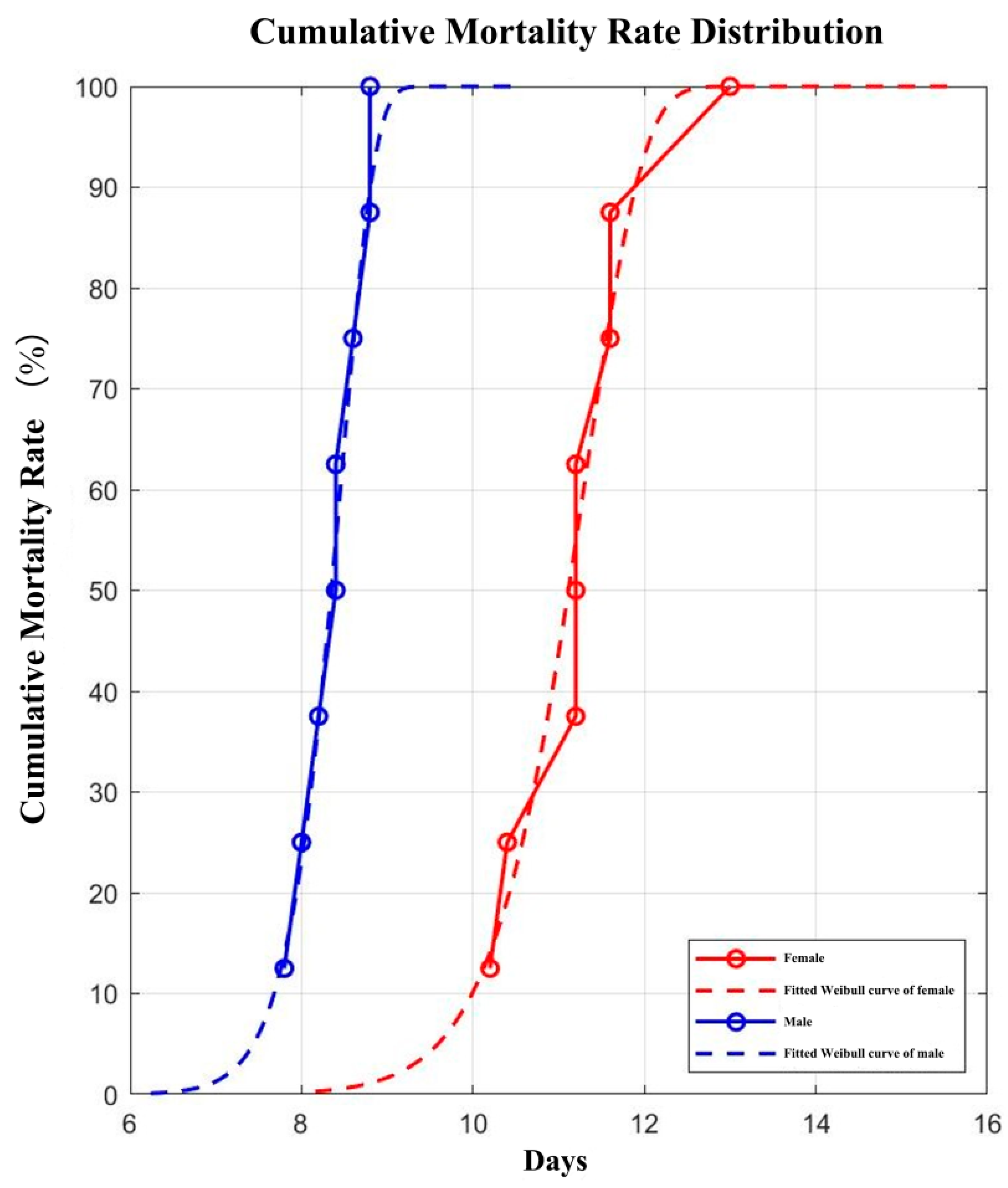
3.3. The Development Duration of Atherigona orientalis in Different Stages
3.4. The Color Selections of Adult Atherigona orientalis
3.5. Dynamics Monitoring of Atherigona orientalis Around Changsha City
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Huang, D.; Wang, Q.; Wu, J.; Wang, K. Invasions by alien plant species of the agro-pastoral ecotone in northern China: Species-specific and environmental determinants. J. Nat. Conserv. 2016, 34, 133–144. [Google Scholar] [CrossRef]
- Prospero, S.; Cleary, M. Effects of Host Variability on the Spread of Invasive Forest Diseases. Forests 2017, 8, 80. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Campolo, O.; Canale, A.; Kapranas, A.; Liedo, P.; De Meyer, M.; Nestel, D.; Ruiu, L.; Scolari, F.; et al. Management of the Mediterranean fruit fly, Ceratitis capitata: Past, present, and future. Entomol. Gen. 2023, 43, 1241–1263. [Google Scholar] [CrossRef]
- Jing, W.; Huang, C.; Li, C.-y.; Zhou, H.-x.; Ren, Y.-l.; Li, Z.-y.; Xing, L.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Mankad, A.; Kennedy, U.; Carter, L. Biological control of pests and a social model of animal welfare. J. Environ. Manag. 2019, 247, 313–322. [Google Scholar] [CrossRef]
- Wan, F.-H.; Yang, N.-W. Invasion and Management of Agricultural Alien Insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef]
- Sarles, L.; Verhaeghe, A.; Francis, F.; Verheggen, F.J. Semiochemicals of Rhagoletis fruit flies: Potential for integrated pest management. Crop Prot. 2015, 78, 114–118. [Google Scholar] [CrossRef]
- Staton, T.; Williams, D.T. A meta-analytic investigation of the potential for plant volatiles and sex pheromones to enhance detection and management of Lepidopteran pests. Bull. Entomol. Res. 2023, 113, 725–734. [Google Scholar] [CrossRef]
- Qian, Q.; Cui, J.; Miao, Y.; Xu, X.; Gao, H.; Xu, H.; Lu, Z.; Zhu, P. The Plant Volatile-Sensing Mechanism of Insects and Its Utilization. Plants 2024, 13, 185. [Google Scholar] [CrossRef]
- Nath, A.; Elaiyabharathi, T.; Srinivasan, T.; Santhanakrishnan, V.P.; Sritharan, N.; Murugan, M. Food baited traps in fruit fly management: Present status and future prospects. Indian J. Exp. Biol. 2025, 63, 7–17. [Google Scholar] [CrossRef]
- Segura, D.F.; Belliard, S.A.; Teresa Vera, M.; Bachmann, G.E.; Josefina Ruiz, M.; Jofre-Barud, F.; Fernandez, P.C.; Liza Lopez, M.; Shelly, T.E. Plant Chemicals and the Sexual Behavior of Male Tephritid Fruit Flies. Ann. Entomol. Soc. Am. 2018, 111, 239–264. [Google Scholar] [CrossRef]
- Benelli, G.; Daane, K.M.; Canale, A.; Niu, C.-Y.; Messing, R.H.; Vargas, R.I. Sexual communication and related behaviours in Tephritidae: Current knowledge and potential applications for Integrated Pest Management. J. Pest Sci. 2014, 87, 385–405. [Google Scholar] [CrossRef]
- Cui, Z.; Si, P.; Liu, L.; Chen, S.; Wang, Y.; Li, X.; Zhou, J.-J.; Zhou, Q. Push-pull strategy for integrated control of Bactrocera minax (Diptera, Tephritidae) based on olfaction and vision. J. Appl. Entomol. 2022, 146, 1243–1251. [Google Scholar] [CrossRef]
- Pilkington, L.J.; Messelink, G.; van Lenteren, J.C.; Le Mottee, K. “Protected Biological Control”—Biological pest management in the greenhouse industry. Biol. Control 2010, 52, 216–220. [Google Scholar] [CrossRef]
- Parolin, P.; Bresch, C.; Poncet, C.; Desneux, N. Functional characteristics of secondary plants for increased pest management. Int. J. Pest Manag. 2012, 58, 368–376. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, E. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwon, Y.J. Taxonomy of the genus Atherigona Rondani (Diptera: Muscidae) from Korea. Entomol. Res. 2018, 48, 187–197. [Google Scholar] [CrossRef]
- Roditakis, E.; Kremi, K.; Mylona, K.; Georgousis, V.; Avtzis, D.N.; Simoglou, K.B. First report of the pepper fruit fly Atherigona orientalis (Schiner 1968) (Diptera: Muscidae) infesting commercial pepper crops in Greece. Insects 2023, 14, 393. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwon, Y.J. First finding of a quarantine pest, Atherigona (Acritochaeta) orientalis Schiner (Diptera: Muscidae), in Korea. Entomol. Res. 2016, 46, 185–189. [Google Scholar] [CrossRef]
- Herawani, F.; Rauf, A.; Santoso, S. Status of infestation and biology of pepper fruit fly, Atherigona orientalis (Schiner) (Diptera: Muscidae). J. Hama Dan Penyakit Tumbuh. Trop. 2019, 19, 64. [Google Scholar] [CrossRef]
- Ogbalu, O.K. The effects of different traditional sources of nutrients on the infestation of pepper fruits by the pepper fruitfly, Atherigona orientalis (Schiner), in Nigeria. J. Agron. Crop Sci. 2010, 182, 65–71. [Google Scholar] [CrossRef]
- Skidmore, P. The Biology of the Muscidae of the World; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Cabrera-Cánoves, P.; Pujade-Villar, J.; Pont, A.C. The first record of the pantropical filth fly Atherigona orientalis Schiner from mainland Europe and another record of Synthesiomyia nudiseta (van der Wulp) from Spain (Diptera: Muscidae). Entomol. Mon. Mag. 2019, 155, 277–280. [Google Scholar] [CrossRef]
- Pont, A.C. The World Distribution, Host Range and Abundance of Atherigona orientalis Schiner, 1968 (Insecta, Diptera, Muscidae); A Report Prepared for the Bureau of Rural Resources; Department of Primary Industries and Energy: Canberra, Australia, 1992. [Google Scholar]
- Lou, Q.Z.; Li, J.; Shang, J.C.; Cui, J.Y.; Gu, Q.; Wang, J.C.; Sun, X.X.; Zhang, J.P. First record of Atherigona orientails (Diptera: Muscidae) feeding on rotten pear fruits in Hebei Province and its morphological and molecular identification. Plant Quar. 2018, 32, 29–34. [Google Scholar]
- Grzywacz, A.; Pape, T.; Hudson, W.G.; Gomez, S. Morphology of immature stages of Atherigona reversura (Diptera: Muscidae), with notes on the recent invasion of North America. J. Nat. Hist. 2013, 47, 1055–1067. [Google Scholar] [CrossRef]
- Xie, G.A.; Qian, W.; Fen, Y. Taxonomic study of genus Acritochaeta (Diptera: Muscidae) With description of a new species from China. J. Vector Biol. Control 2013, 24, 157–160. [Google Scholar]
- Mouttet, R.; Taddei, A. First record of Atherigona orientalis Schiner, 1868 (Diptera: Muscidae) in France. EPPO Bull. 2024, 54, 13022. [Google Scholar] [CrossRef]
- Pont, A.C. A review of the Oriental species of Atherigona Rondani (Diptera: Muscidae) of economic importance. In Controlof Sorghum Shoot Fly; Oxford & IBH Pub. Co.: New Delhi, India, 1972; pp. 27–104. [Google Scholar]
- Couri, S.; Araujo, D. The immature stages of Atherigona orientalis Schiner (Diptera: Muscidae). Proc. Biol. Soc. Wash. 1992, 105, 490–493. [Google Scholar]
- Ferrar, P. A Guide to the Breeding Habits and Immature Stages of Diptera Cyclorrhapha (Part 1: Text; Part 2: Figures); Brill: Leiden, The Netherlands, 2015. [Google Scholar]
- Wu, S.; Tang, L.; Zhang, X.; Xing, Z.; Lei, Z.; Gao, Y. A decade of a thrips invasion in China: Lessons learned. Ecotoxicology 2018, 27, 1032–1038. [Google Scholar] [CrossRef]
- Yadav, S.K.; Bhowmik, S.; Yadav, P.C.; Sharma, K.C. Identification and control of Trogoderma granarium (Coleoptera: Dermestidae), a potential threat to stored products and international trade. Int. J. Trop. Insect Sci. 2022, 42, 999–1017. [Google Scholar] [CrossRef]
- Mugala, T.; Visser, D.; Malan, A.P.; Addison, P. Review of Liriomyza huidobrensis (Blanchard, 1926) (Diptera: Agromyzidae) on potatoes in South Africa, with special reference to biological control using entomopathogens and parasitoids. Afr. Entomol. 2022, 30, e11455. [Google Scholar] [CrossRef]
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Wakil, W.; Eleftheriadou, N.; Ghazanfar, M.U.; El-Shafie, H.; Simmons, A.M.; Dimase, M.; Smith, H.A.; Chandler, D. Integrated management system of the whitefly Bemisia tabaci: A review. Entomol. Gen. 2024, 44, 1117–1133. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, Y.; Wang, X.; He, J.; Zhou, Q. Identification and sex expression profiles of candidate chemosensory genes from Atherigona orientalis via the antennae and leg transcriptome analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 50, 101222. [Google Scholar] [CrossRef]
- Ogbalu, O.K.; Manuel, R.B.B. Abscission of Pepper Fruits By Dipterous Pest, Atherigona orientalis (Schiner) In Traditional and Mono-Crop Farms in Port Harcourt, Niger Delta, Nigeria. J. Agric. Vet. Sci. 2014, 7, 31–36. [Google Scholar]
- Cheng, P.; Song, W.; Gong, X.; Liu, Y.; Xie, W.; Huang, L.; Hong, Y. Proteomic Approaches of Trichoderma hamatum to Control Ralstonia solanacearum Causing Pepper Bacterial Wilt. Int. J. Agric. Biol. 2015, 17, 1101–1109. [Google Scholar] [CrossRef]
- Coulibaly, S.; Kamsu-Foguem, B.; Kamissoko, D.; Traore, D. Explainable deep convolutional neural networks for insect pest recognition. J. Clean. Prod. 2022, 371, 133638. [Google Scholar] [CrossRef]
- Preti, M.; Verheggen, F.; Angeli, S. Insect pest monitoring with camera-equipped traps: Strengths and limitations. J. Pest Sci. 2021, 94, 203–217. [Google Scholar] [CrossRef]
- Bohart, G.E.; Gressitt, J.L. Filth-inhabiting flies of Guam. Bernice P Bish. Mus. Bull. 1951, 204, 1–152. [Google Scholar]
- Grzywacz, A.; Pape, T. Larval morphology of Atherigona orientalis (Schiner) (Diptera: Muscidae)—A species of sanitary and forensic importance. Acta Trop. 2014, 137, 174–184. [Google Scholar] [CrossRef]
- Wang, Y.; Andongma, A.A.; Dong, Y.; Chen, Z.; Xu, P.; Ren, X.; Krosch, M.N.; Clarke, A.R.; Niu, C. Rh6 gene modulates the visual mechanism of host utilization in fruit fly Bactrocera minax. Pest Manag. Sci. 2019, 75, 1621–1629. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Wang, Y.; Cao, Z.; Cao, S.; Wei, B.; Liu, Y.; Lienard, M.A.; Niu, C. Specific transcription factors regulate the expression of Rh6 in Bactrocera minax and Bactrocera dorsalis (Diptera: Tephritidae). Int. J. Biol. Macromol. 2025, 305, 141201. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chen, Y.-P.; Yang, E.-C. Chromatic cues to trap the oriental fruit fly, Bactrocera dorsalis. J. Insect Physiol. 2007, 53, 509–516. [Google Scholar] [CrossRef]
- Haynes, K.J.; Walter, J.A. Advances in understanding the drivers of population spatial synchrony. Curr. Opin. Insect Sci. 2022, 53, 100959. [Google Scholar] [CrossRef]
- Ims, R.A.; Henden, J.-A.; Killengreen, S.T. Collapsing population cycles. Trends Ecol. Evol. 2008, 23, 79–86. [Google Scholar] [CrossRef]
- Kennedy, G.G.; Storer, N.P. Life systems of polyphagous arthropod pests in temporally unstable cropping systems. Annu. Rev. Entomol. 2000, 45, 467–493. [Google Scholar] [CrossRef]
- Du, J.; Fang, J.; Xu, W.; Shi, P. Analysis of dry/wet conditions using the standardized precipitation index and its potential usefulness for drought/flood monitoring in Hunan Province, China. Stoch. Environ. Res. Risk Assess. 2013, 27, 377–387. [Google Scholar] [CrossRef]
- Madeira, N.R.; Amaro, G.B.; Melo, R.A.C.; Ribeiro, C.S.C.; Reifschneider, F.J.B. Rootstock compatibility for sweet pepper hybrids production under greenhouse. Hortic. Bras. 2016, 34, 470–474. [Google Scholar] [CrossRef][Green Version]
- Sadi, M.; Arabkoohsar, A. Techno-economic analysis of off-grid solar-driven cold storage systems for preventing the waste of agricultural products in hot and humid climates. J. Clean. Prod. 2020, 275, 124143. [Google Scholar] [CrossRef]


| Family | Host Plants | Percentage of Flies Emerging from Host Plants | ||||
|---|---|---|---|---|---|---|
| Atherigona orientalis | Bactrocera tau | Bactrocera latifrons | Bactrocera dorsalis | Bactrocera minax | ||
| Solanaceae | Capsicum annuum | 1392 (99.43%) | 3 (0.20%) | 5 (0.37%) | - | - |
| Lycopersicon esculentum | 117 (100%) | - | - | - | - | |
| Solanum melongena | 72 (100%) | - | - | - | - | |
| Cucurbitaceae | Momordica charantia | 694 (77.50%) | 201 (22.40%) | - | 1 (0.10%) | - |
| Luffa cylindrica | 163 (57.00%) | 123 (43.00%) | - | - | - | |
| Cucumis melo | 61 (76.25%) | 19 (23.75%) | - | - | - | |
| Cucurbita pepo | 11 (84.62%) | 2 (15.38%) | - | - | - | |
| Benincasa hispida | 11 (100%) | - | - | - | - | |
| Cucurbita moschata | 6 (2.64%) | 221 (97.36%) | - | - | - | |
| Lagenaria sciceraria | 3 (100%) | - | - | - | - | |
| Cucumis sativus | - | - | - | - | - | |
| Rosaceae | Amygdalus persica | 44 (100%) | - | - | - | - |
| Prunus salicina | - | - | - | - | - | |
| Prunus persica | - | - | - | - | - | |
| Pyrus spp. | 1 (100%) | - | - | - | - | |
| Rutaceae | Citrus reticulata | 42 (91.30%) | - | - | 1 (2.20%) | 3 (6.50%) |
| Actinidiaceae | Actinidia chinensis | 1 (100%) | - | - | - | - |
| Ebenaceae | Diospyros kaki | 3 (100%) | - | - | - | - |
| Moraceae | Ficus carica | - | - | - | - | - |
| Rhamnaceae | Ziziphus jujuba | - | - | - | 3 (100%) | - |
| Stage | Number | Development Time (Days) | Mean Developmental Time (Days) |
|---|---|---|---|
| Egg | 80 | 2–3 | 2.19 ± 0.25 |
| Larva | 60 | 5–7 | 5.56 ± 0.49 |
| Pupa | 50 | 6–8 | 6.16 ± 0.38 |
| Female adult | 40 | 3–15 | 11.3 ± 2.43 |
| Male adult | 40 | 1–12 | 8.38 ± 1.98 |
| Male | Female | |
|---|---|---|
| η | 8.4856 | 11.3475 |
| β | 22.5861 | 17.6779 |
| LT50W | 8.3490 | 11.1146 |
| LT50O | 8.400 | 11.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Luo, Y.; Qin, J.; Wang, X.; Ning, S.; He, J.; Zhou, Q. Occurrence, Biological Characteristics, and Annual Dynamics of Atherigona orientalis (Schiner 1968) (Diptera: Muscidae) in China. Insects 2025, 16, 931. https://doi.org/10.3390/insects16090931
Zhou Z, Luo Y, Qin J, Wang X, Ning S, He J, Zhou Q. Occurrence, Biological Characteristics, and Annual Dynamics of Atherigona orientalis (Schiner 1968) (Diptera: Muscidae) in China. Insects. 2025; 16(9):931. https://doi.org/10.3390/insects16090931
Chicago/Turabian StyleZhou, Zihao, Yujie Luo, Jiawei Qin, Xintong Wang, Shuaijun Ning, Jing He, and Qiong Zhou. 2025. "Occurrence, Biological Characteristics, and Annual Dynamics of Atherigona orientalis (Schiner 1968) (Diptera: Muscidae) in China" Insects 16, no. 9: 931. https://doi.org/10.3390/insects16090931
APA StyleZhou, Z., Luo, Y., Qin, J., Wang, X., Ning, S., He, J., & Zhou, Q. (2025). Occurrence, Biological Characteristics, and Annual Dynamics of Atherigona orientalis (Schiner 1968) (Diptera: Muscidae) in China. Insects, 16(9), 931. https://doi.org/10.3390/insects16090931






