The Response of Monoecious and Dioecious Cultivars of Agricultural Hemp to an Organic Fertiliser Derived from Black Soldier Fly Frass
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Plant Cultivation and Harvest
2.3. The Effect of HexaFrass Application Rate on Hemp Shoot Growth
2.4. A Comparison of HexaFrass with Other Organic Fertilisers
2.5. The Effect of HexaFrass on Monoecious and Dioecious Hemp Cultivars
2.6. The Effect of HexaFrass on Macro-Nutrient Content of Hemp Shoots
2.7. Statistical Analysis
2.8. Consistency of Effects Among Repeat Trials and Between Monoecious and Dioecious Cultivars
3. Results
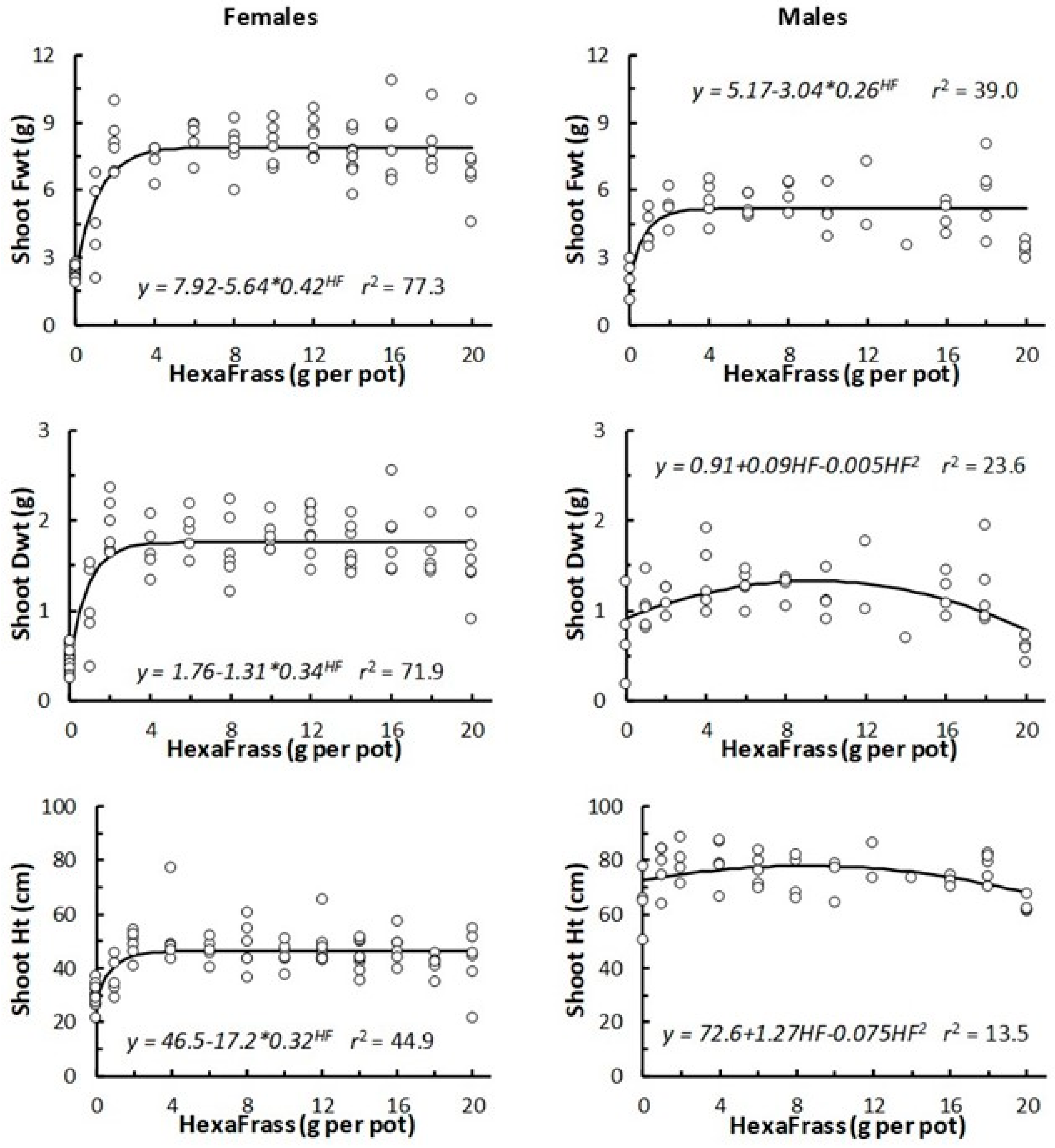
3.1. The Effect of HexaFrass Application Rate on Hemp Shoot Growth
3.2. The Response of Hemp to HexaFrass Compared with That Obtained with Other Organic Fertilisers
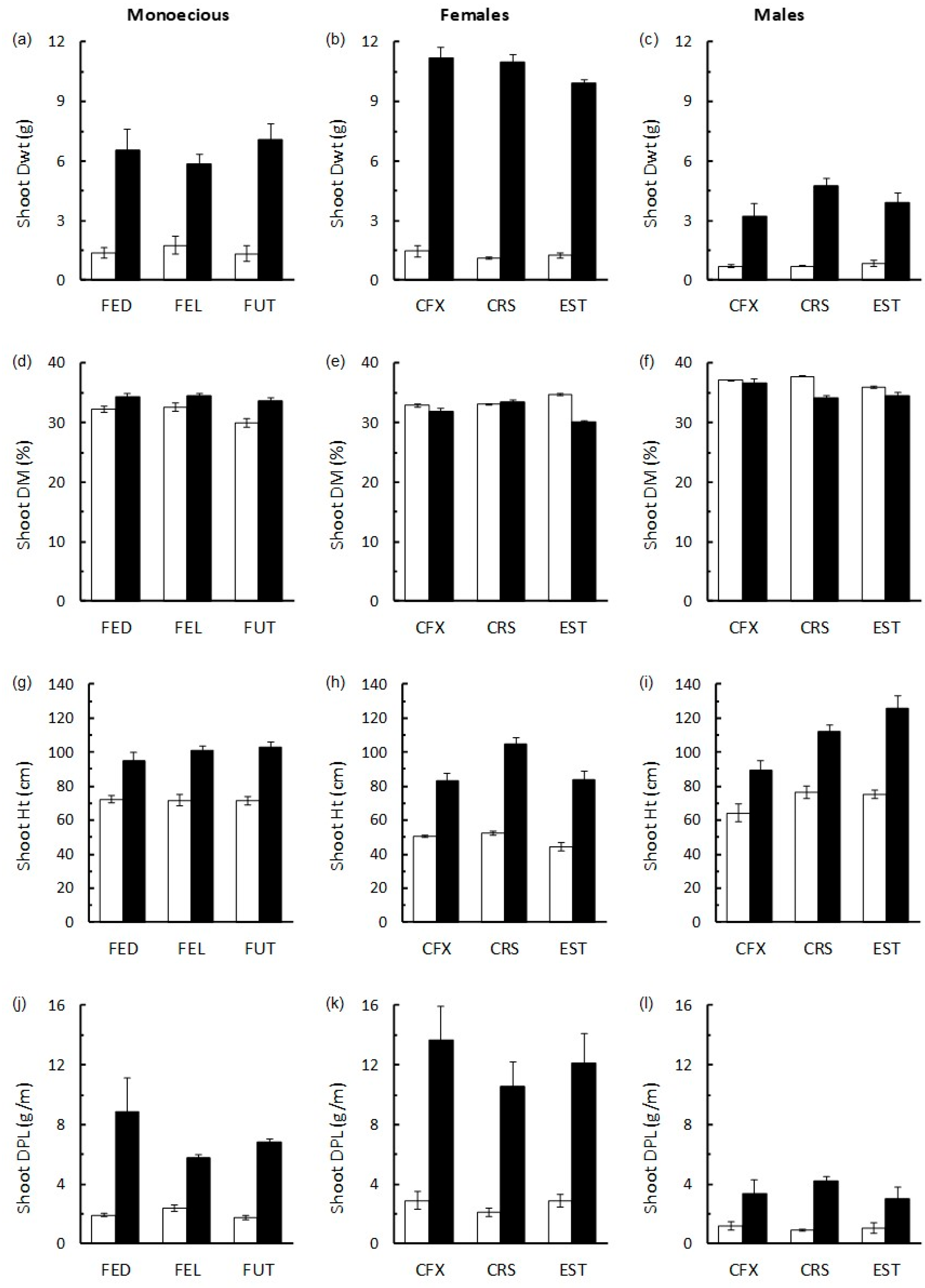
3.3. The Effects of HexaFrass on Shoot Growth in Monoecious and Dioecious Hemp Cultivars
3.4. The Effect of HexaFrass on Macro-Nutrient Content of Hemp Shoots
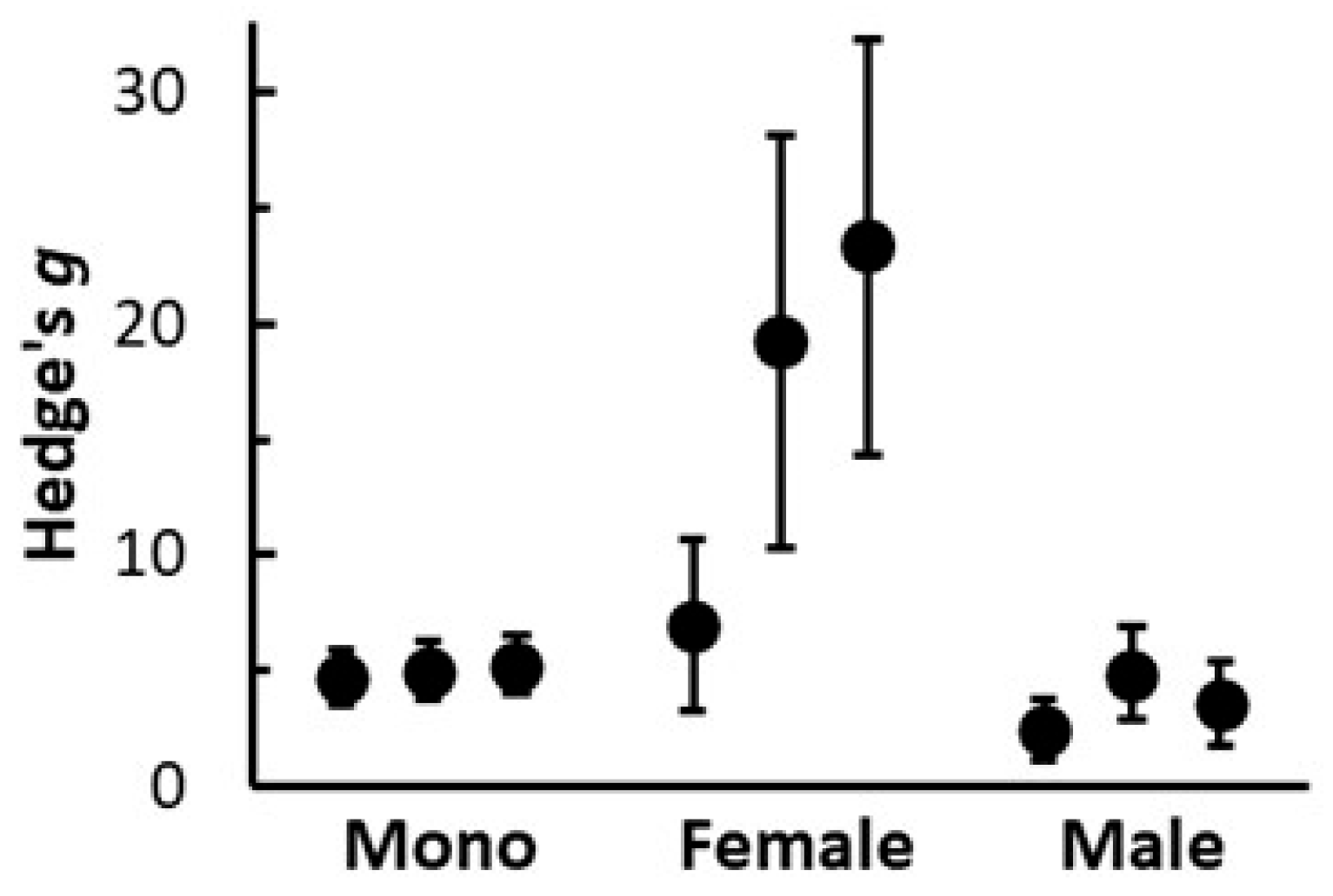
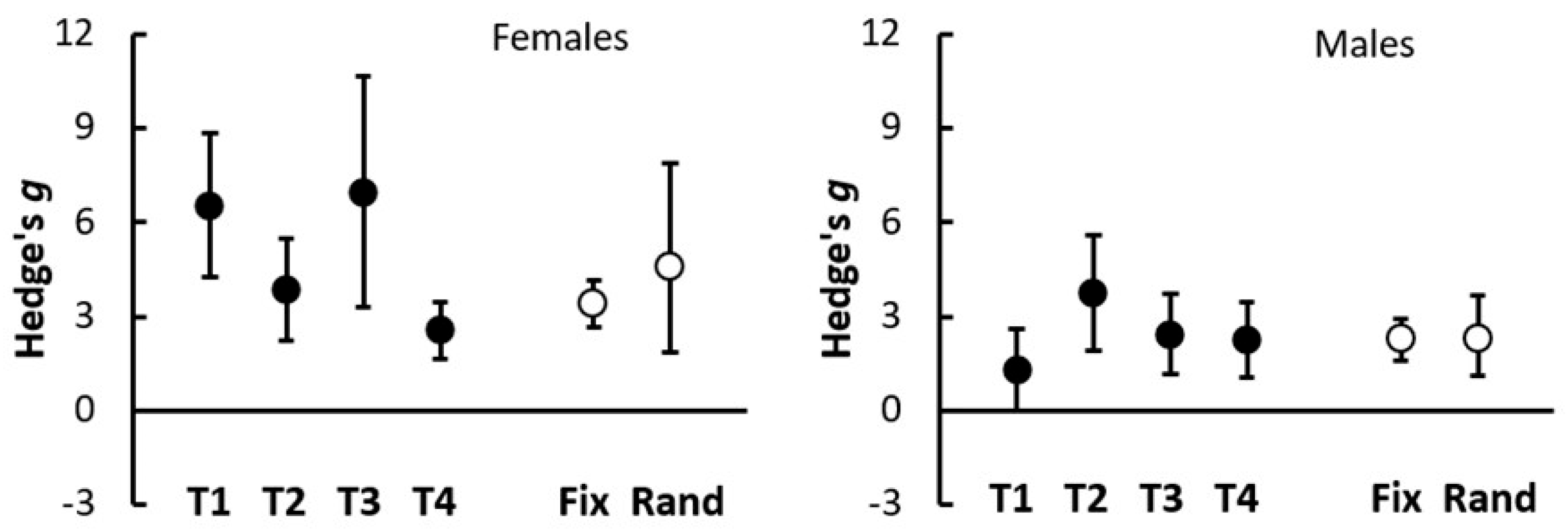
3.5. Consistency of HexaFrass Effects on Hemp Shoot Growth
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Henn, D.; Humphreys, J.; Gibbons, J.; Styles, D. Land use and environmental quality in Ireland over two decades of contrasting agricultural trends. Biol. Environ. Proc. R. Ir. Acad. 2023, 123B, 39–53. [Google Scholar] [CrossRef]
- Ryan, M.; Hennessy, T.; Buckley, C.; Dillon, E.J.; Donnellan, T.; Hanrahan, K.; Moran, B. Developing farm-level sustainability indicators for Ireland using the Teagasc National Farm Survey. Ir. J. Agric. Food Res. 2016, 55, 112–125. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Assefa, S. The principal role of organic fertilizer on soil properties and agricultural productivity—A review. Agric. Res. Technol. Open Access J. 2019, 22, 556192. [Google Scholar] [CrossRef]
- Azarbad, H. Conventional vs. organic agriculture—Which one promotes better yields and microbial resilience in rapidly changing climates? Front. Microbiol. 2022, 13, 903500. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Smitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- van Huis, A.; Rumpold, B.A.; van der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Lomonaco, G.; Franco, A.; De Smet, J.; Scieuzo, C.; Salvia, R.; Falabella, P. Larval frass of Hermetia illucens as organic fertilizer: Composition and beneficial effects on different crops. Insects 2024, 15, 293. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect frass composition and potential use as an organic fertilizer in circular economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, J.; Kim, J.; Kim, M.; Kim, W.; Park, K.; Bae, S.; Jeong, G. Potential usage of food waste as a natural fertilizer after digestion by Hermetial Illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2009, 19, 171–174. [Google Scholar]
- Quilliam, R.S.; Nuku-Adeku, C.; Maquart, P.; Little, D.; Newton, R.; Murray, F. Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed 2020, 6, 315–322. [Google Scholar] [CrossRef]
- Borkent, S.; Hodge, S. Glasshouse evaluation of the Black Soldier Fly waste product HexaFrass™ as an organic fertilizer. Insects 2021, 12, 977. [Google Scholar] [CrossRef]
- Hodge, S.; Conway, J. The effects of insect frass fertilizer and biochar on the shoot growth of chicory and plantain, two forage herbs commonly used in multispecies swards. Agronomy 2022, 12, 2459. [Google Scholar] [CrossRef]
- Tanga, C.M.; Beesigamukama, D.; Kassie, M.; Egonyu, P.J.; Ghemoh, C.J.; Nkoba, K.; Subramanian, S.; Anyega, A.O.; Ekesi, S. Performance of black soldier fly frass fertiliser on maize (Zea mays L.) growth, yield, nutritional quality, and economic returns. J. Insects Food Feed 2022, 8, 185–196. [Google Scholar] [CrossRef]
- Rodgers, E.; Nicolson, E.; Lauder, S.; Hodge, S. Response of pasture grasses to organic fertilizer produced from Black Soldier Fly frass. Plants 2024, 13, 943. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring Black Soldier Fly frass as novel fertilizer for improved growth, yield, and nitrogen use efficiency of maize under field conditions. Front. Plant Sci. 2020, 11, 574592. [Google Scholar] [CrossRef]
- Carroll, A.; Fitzpatrick, M.; Hodge, S. The effects of two organic soil amendments, biochar and insect frass fertilizer, on shoot growth of cereal seedlings. Plants 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Wu, X.; Guo, X.-Q.; Chen, Y.-B.; Wang, J.-L.; Xu, X.-Y. Effects of black soldier fly frass on rice growth and soil physical and chemical properties. J. Plant Nutr. Fertil. 2021, 27, 1874–1882. [Google Scholar]
- Gärttling, D.; Schulz, H. Compilation of Black Soldier Fly frass analyses. J. Soil Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture: A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.; de Boer, W.; Dicke, M. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from yellow mealworm (Tenebrio molitor) as plant fertilizer and defense priming agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed insect frass as a future organic fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karanastasi, E.; Athanassiou, C.G. Plant health promoting potential of insect frass: Just a soil fertiliser or much more besides? J. Insects Food Feed 2025, 11, 1131–1136. [Google Scholar] [CrossRef]
- McClatchie, M.; Matterne, V.; Rovira Buendia, N.; Andonova, M.; Lohwasser, U.; Waalen, W.; Bantis, F.; Knez, M.; Milešević, J.; Orahovac, A.; et al. Underutilised crops in Europe: An interdisciplinary approach towards sustainable practices. Archaeometry 2025. [Google Scholar] [CrossRef]
- Melzer, R.; McCabe, P.F.; Schilling, S. Evolution, genetics and biochemistry of plant cannabinoid synthesis: A challenge for biotechnology in the years ahead. Curr. Opin. Biotechnol. 2022, 75, 102684. [Google Scholar] [CrossRef]
- Schilling, S.; Dowling, C.A.; Shi, J.; Ryan, L.; Hunt, D.J.L.; O’Reilly, E.; Perry, A.S.; Kinnane, O.; McCabe, P.F.; Melzer, R. The cream of the crop: Biology, breeding, and applications of Cannabis sativa. Annu. Plant Rev. 2021, 4, 471–528. [Google Scholar]
- Arrigoni, A.; Pelosato, R.; Melià, P.; Ruggieri, G.; Sabbadini, S.; Dotelli, G. Life cycle assessment of natural building materials: The role of carbonation, mixture components and transport in the environmental impacts of hempcrete blocks. J. Clean. Prod. 2017, 149, 1051–1061. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial hemp (Cannabis sativa L.) agronomy and utilization: A review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Petzel, E.A.; Nelson, K.; Shiba, S.; Brake, D.W. Influences of industrial hemp variety and botanical part on measures of in situ ruminal nutrient disappearance. J. Anim. Sci. 2025, 103, 334. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons Ltd.: Chichester, UK, 2009; p. 421. [Google Scholar]
- Łochyńskaa, M.; Frankowski, J. Impact of silkworm excrement organic fertilizer on hemp biomass yield and composition. J. Ecol. Eng. 2019, 20, 63–71. [Google Scholar] [CrossRef]
- Łochyńskaa, M.; Frankowski, J. The effects of silkworm excrement organic fertilizer on the hemp yield. J. Nat. Fibers 2022, 19, 847–857. [Google Scholar] [CrossRef]
- Atoloye, I.A.; Adesina, I.; Shahbazi, A.; Bhowmik, A. Response of cannabidiol hemp (Cannabis sativa L.) varieties grown in the southeastern United States to nitrogen fertilization. Open Agric. 2022, 7, 373–381. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal rate of organic fertilizer during the vegetative-stage for cannabis grown in two coir-based substrates. HortScience 2017, 52, 1307–1312. [Google Scholar] [CrossRef]
- Vera, C.; Malhi, S.; Raney, J.; Wang, Z. The effect of N and P fertilization on growth, seed yield and quality of industrial hemp in the Parkland region of Saskatchewan. Can. J. Plant Sci. 2004, 84, 939–947. [Google Scholar] [CrossRef]
- James, M.S.; Vann, M.C.; Suchoff, D.H.; McGinnis, M.; Whipker, B.E.; Edmisten, K.L.; Gatiboni, L.C. Hemp yield and cannabinoid concentrations under variable nitrogen and potassium fertilizer rates. Crop Sci. 2023, 63, 1555–1565. [Google Scholar] [CrossRef]
- Podder, S.; Shafian, S.; Thomason, W.E.; Wilson, T.B.; Fike, J.H. Hemp seed yield responses to nitrogen fertility rates. Crops 2024, 4, 145–155. [Google Scholar] [CrossRef]
- Yang, Y.; Zha, W.; Tang, K.; Deng, G.; Du, G.; Liu, F. Effect of Nitrogen Supply on Growth and Nitrogen Utilization in Hemp (Cannabis sativa L.). Agronomy 2021, 11, 2310. [Google Scholar] [CrossRef]
- Salomon, M.J.; Cavagnaro, T.R.; Burton, R.A. Potential of black soldier fly larvae frass (BSFL) as a novel fertilizer: Impacts on tomato growth, nutrient uptake, and mycorrhizal formation. Plant Soil 2025, 2025, 1–18. [Google Scholar] [CrossRef]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Eichhorn Bilodeau, S.; Lyu, D.; Ahmed, M.B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the yield gap for cannabis: A meta-analysis of factors determining cannabis yield. Front. Plant Sci. 2019, 10, 495. [Google Scholar] [CrossRef]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Analysis of chemical and phytotoxic properties of frass derived from Black Soldier Fly-based bioconversion of biosolids. Sustainability 2023, 15, 11526. [Google Scholar] [CrossRef]
- González-Lara, H.; Parra-Pacheco, B.; Aguirre-Becerra, H.; Feregrino-Perez, A.A.; Garcia-Trejo, J.F. Effects of using thermocomposted frass from Black Soldier Fly larvae as a germination substrate on the phytotoxicity, germination index, growth and antioxidant contents in in Kale (Brassica oleracea). Agronomy 2024, 14, 1392. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect frass as a novel organic soil fertilizer for the cultivation of spinach (Spinacia oleracea): Effects on soil properties, plant physiological parameters, and nutrient status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Foscari, A.; Costa, L.D.; Tulli, F.; Uboni, C.; Fellet, G. Frass from Tenebrio molitor as alternative to NPK-mineral fertilization: Results from a germination test and pot experiment on sunflower. Ital. J. Agron. 2024, 19, 10010. [Google Scholar] [CrossRef]
- Iommelli, P.; Zicarelli, F.; Amato, R.; Musco, N.; Sarubbi, F.; Bailoni, L.; Lombardi, P.; Di Bennardo, F.; Infascelli, F.; Tudisco, R. The effects of hemp hay (Canapa sativa L.) in the diets of grazing goats on milk production and fatty acid profile. Animals 2024, 14, 2373. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Cheng, C.; Lv, J.; Lambo, M.T.; Zhang, G.; Li, Y.; Zhang, Y. Nutritional values of industrial hemp byproducts for dairy cattle. Animals 2022, 12, 3488. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Brym, Z.; Oyola, L.A.M.; Sharma, L.K. Nitrogen fertilization impact on hemp (Cannabis sativa L.) crop production: A review. Agron. J. 2023, 115, 1557–1570. [Google Scholar] [CrossRef]
- Trubanova, N.; Isobe, S.; Shirasawa, K.; Watanabe, A.; Kelesidis, G.; Melzer, R.; Schilling, S. Genome-specific association study (GSAS) for exploration of variability in hemp (Cannabis sativa). Sci. Rep. 2025, 15, 8371. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, H.M.G.; van den Berg, W. Nitrogen fertilization and sex expression affect size variability of fibre hemp (Cannabis Sativa L.). Oecologia 1995, 103, 462–470. [Google Scholar] [CrossRef]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Edmeades, D.C. The effects of liquid fertilisers derived from natural products on crop, pasture, and animal production: A review. Aust. J. Agric. Res. 2002, 53, 965–976. [Google Scholar] [CrossRef]
- Hodge, S.; Merfield, C.N.; Liu, W.Y.Y.; Tan, H.W. Seedling responses to organically-derived plant growth promoters: An effects-based approach. Plants 2021, 10, 660. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Randhawa, P.; Jenkins, V. How well do fertilizer enhancers work? J. Plant Nutr. 2018, 41, 832–845. [Google Scholar] [CrossRef]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK, 2010; p. 192. [Google Scholar]
- Houben, D.; Daoulas, G.; Dulaurent, A.-M. Assessment of the short-term fertilizer potential of mealworm frass using a pot experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Anedo, E.O.; Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Haukeland, S.; Cheseto, X.; Nyongesa, M.; Pwaipwai, P.; Subramanian, S.; Tenkouano, A.; et al. Unpacking the benefits of black soldier fly frass fertilizer towards nematode suppression and potato production. Front. Plant Sci. 2025, 16, 1509643. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Del Vecchio, L.; Dellapina, C.; Moliterni, V.M.C.; Caligiani, A.; Cirlini, M. Black Soldier Fly larvae grown on hemp fiber: Nutritional composition and production of potential bioactive peptides. Macromol 2024, 4, 135–149. [Google Scholar] [CrossRef]
- Yakti, W.; Förster, N.; Müller, M.; Mewis, I.; Ulrichs, C. Hemp waste as a substrate for Hermetia illucens (L.) (Diptera: Stratiomyidae) and Tenebrio molitor L. (Coleoptera: Tenebrionidae) rearing. Insects 2023, 14, 183. [Google Scholar] [CrossRef] [PubMed]
| Variable | Sex | CONT | HF | MG | CM | LSD | F | p |
|---|---|---|---|---|---|---|---|---|
| Fresh Wt (g) | Fem | 1.62 a | 7.34 c | 10.13 d | 5.22 b | 2.70 | 21.71 | <0.001 |
| Mal | 0.66 a | 3.41 b | 7.25 c | 3.52 b | 2.94 | 9.40 | <0.001 | |
| Dry Wt (g) | Fem | 0.51 a | 2.45 c | 3.25 d | 1.67 b | 0.87 | 22.56 | <0.001 |
| Mal | 0.22 a | 1.02 ab | 2.31 c | 1.06 b | 0.96 | 8.92 | <0.001 | |
| Height (cm) | Fem | 20.8 a | 53.3 c | 52.3 bc | 40.8 b | 13.8 | 17.75 | <0.001 |
| Mal | 38.2 a | 68.1 bc | 76.7 c | 55.7 b | 18.0 | 9.59 | <0.001 | |
| Leaf Pairs | Fem | 4.6 a | 8.1b | 8.8 b | 7.3 b | 1.99 | 11.74 | <0.001 |
| Mal | 4.0 a | 5.8 ab | 8.0 c | 6.7 bc | 2.23 | 6.46 | 0.003 | |
| Dry Matter (%) | Fem | 31.8 | 33.4 | 32.0 | 30.1 | 5.63 | 0.87 | 0.470 |
| Mal | 32.7 | 29.7 | 32.3 | 27.9 | 5.58 | 1.94 | 0.154 | |
| DPL (g/m) | Fem | 1.94 a | 4.65 b | 6.22 c | 3.64 b | 1.75 | 12.62 | <0.001 |
| Mal | 0.52 a | 1.49 b | 2.92 c | 1.65 b | 0.99 | 10.96 | <0.001 | |
| SPAD | Fem | 36.3 | 31.7 | 39.8 | 36.5 | 7.82 | 2.50 | 0.086 |
| Mal | 24.1 | 25.6 | 27.3 | 30.9 | 9.82 | 1.08 | 0.386 |
| Tissue | Nutrient | Females | Males | LSD | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 g HF | 4 g HF | 0 g HF | 4 g HF | HF | Sex | Int | |||
| Leaf | N (%) | 1.49 | 1.50 | 1.76 | 1.46 | 0.59 | 0.539 | 0.586 | 0.367 |
| P (%) | 0.28 a | 0.94 b | 0.29 a | 1.10 c | 0.17 | <0.001 | 0.063 | 0.135 | |
| Ca (%) | 5.64 | 4.99 | 6.64 | 6.59 | 2.49 | 0.330 | 0.078 | 0.679 | |
| K (%) | 3.33 b | 4.23 c | 2.37 a | 3.60 b | 0.47 | <0.001 | <0.001 | 0.238 | |
| Mg (%) | 1.53 a | 1.88 b | 1.82 b | 2.10 b | 0.31 | 0.002 | 0.012 | 0.742 | |
| Dwt (g) | 0.69 a | 1.46 c | 0.48 a | 1.07 b | 0.28 | <0.001 | 0.002 | 0.325 | |
| Stem | N (%) | 0.65 b | 0.44 a b | 0.53 a b | 0.35 a | 0.25 | 0.027 | 0.155 | 0.838 |
| P (%) | 0.14 a | 0.67 b | 0.11 a | 0.54 b | 0.24 | <0.001 | 0.180 | 0.464 | |
| Ca (%) | 0.84 | 0.84 | 0.94 | 0.86 | 0.33 | 0.649 | 0.568 | 0.675 | |
| K (%) | 3.13 | 4.17 | 3.03 | 3.75 | 0.65 | 0.047 | 0.443 | 0.700 | |
| Mg (%) | 0.33 a | 0.31 a | 0.47 b | 0.32 a | 0.11 | 0.033 | 0.093 | 0.070 | |
| Dwt (g) | 0.31 a | 0.91 b | 0.34 a | 0.81 b | 0.23 | <0.001 | 0.730 | 0.377 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavanagh, G.; Schilling, S.; Melzer, R.; Hodge, S. The Response of Monoecious and Dioecious Cultivars of Agricultural Hemp to an Organic Fertiliser Derived from Black Soldier Fly Frass. Insects 2025, 16, 918. https://doi.org/10.3390/insects16090918
Kavanagh G, Schilling S, Melzer R, Hodge S. The Response of Monoecious and Dioecious Cultivars of Agricultural Hemp to an Organic Fertiliser Derived from Black Soldier Fly Frass. Insects. 2025; 16(9):918. https://doi.org/10.3390/insects16090918
Chicago/Turabian StyleKavanagh, Gordon, Susanne Schilling, Rainer Melzer, and Simon Hodge. 2025. "The Response of Monoecious and Dioecious Cultivars of Agricultural Hemp to an Organic Fertiliser Derived from Black Soldier Fly Frass" Insects 16, no. 9: 918. https://doi.org/10.3390/insects16090918
APA StyleKavanagh, G., Schilling, S., Melzer, R., & Hodge, S. (2025). The Response of Monoecious and Dioecious Cultivars of Agricultural Hemp to an Organic Fertiliser Derived from Black Soldier Fly Frass. Insects, 16(9), 918. https://doi.org/10.3390/insects16090918








