Simple Summary
Millet is the most consumed cereal in Niger, but its seasonal production and year-round demand create a strong need for safe long-term storage. Although pesticides are commonly used in warehouses, concerns over misuse and safety highlight the need for safer alternatives. This study evaluated the effectiveness of (i) hermetic bags, (ii) pesticide (Phostoxin or aluminum phosphide), and (iii) regular polypropylene bags without chemicals in controlling Trogoderma granarium, an emerging pest of stored millet in large warehouses. Infested millet from the national grain reserve was stored using these treatments and analyzed at the start and after six months. The control treatment showed significant increases in pest numbers, resulting in a 19.15% loss of grain weight. In contrast, both hermetic bags and Phostoxin treatments prevented further infestation. Overall, hermetic bags proved to be an effective, safer, and chemical-free storage option.
Abstract
Millet (Pennisetum glaucum (L.) R. Br.) is the most widely consumed cereal in Niger. Although its production lasts six months, consumption is year-round, requiring effective and safe storage solutions. Post-harvest losses of millet can reach up to 17% after several months of storage. Chemical pesticides are commonly used in large warehouses, but concerns over misuse and health risks have driven interest in safer alternatives. This study assessed the effectiveness of traditional and improved storage methods in controlling Trogoderma granarium Everts, an emerging pest of millet in large warehouse facilities. Three storage methods were tested: (i) a hermetic Purdue Improved Crop Storage (PICS) bag; (ii) chemical pesticide Phostoxin; and (iii) an untreated ordinary polypropylene bag as a control. Naturally infested millet obtained from a large warehouse was assessed for each treatment at the start and end of a six-month storage period. Two insect pests were identified, T. granarium and Tribolium sp. Initial infestation levels were 60 larvae and 0.47 adults per 500 g. After six months, the control showed a 1.7-fold increase in T. granarium larvae, a 2.96-fold increase in its adults, and a 3.19-fold increase in Tribolium sp. adults, resulting in 19.15% weight loss. In contrast, PICS and Phostoxin treatments maintained initial pest levels with no weight loss. These results suggest that hermetic storage is a safe, effective, and chemical-free alternative for preserving millet in large warehouses.
1. Introduction
In many African countries, cereals such as millet, sorghum, wheat, maize, and rice form the foundation of the rural diet, especially among low-income populations [1,2,3]. These cereals play a critical role in ensuring food and nutrition security across sub-Saharan Africa [4]. Among them, millet is particularly important in arid and semi-arid areas, including the Sahel, where it serves as a major source of energy [5,6,7].
In Niger, millet is an important cereal. Over the past several decades, millet production in Niger has experienced significant growth in both cultivated area and yields. For instance, production increased from 776,000 tons on 1,640,100 hectares in 1961 to 581,300 tons on 1,692,900 hectares in 1975, and then to 3,321,753 tons on 7,358,247 hectares in 2014 [8]. In 2024, millet production was recorded at 3,161,868 tons over 6,736,592 hectares [9], representing an increase of 307% in output and 311% in cultivated area compared to 1961. Although production has increased significantly over the years, supply remains limited due to various factors, including post-harvest storage losses.
In the dry Sahelian zone, post-harvest storage is essential, as it bridges the gap between the annual harvest and continuous household consumption [3,10]. However, achieving food self-sufficiency is often constrained by low production and poor post-harvest management practices [6]. Insects are one of the major challenges to effective grain storage. Millet stocks are particularly vulnerable to numerous pests including Corcyra cephalonica (Stainton), Tribolium castaneum (Herbst, 1797), Tribolium confusum Jacquelin du Val, Sitotroga cerealella (Olivier), Oryzeaphilus mercator (Fauvel), Lasioderma serricorne (Fabricius), Rhyzopertha dominica (Fabricius), Sitophilus oryzae (Linnaeus), Ephestia cautella (Walker), Cryptolestes ferrugineus (Stephens), and Trogoderma granarium Everts [11,12,13,14,15]. In Niger, a study identified C. cephalonica and Tribolium spp. as key pests, causing an estimated post-harvest loss of 17.1% [16].
Trogoderma granarium, also known as the khapra beetle, is a destructive pest of stored products. This pest is considered one of the most serious threats to stored cereals and legumes globally, especially in tropical and subtropical regions [17,18]. Native to the Indian subcontinent, T. granarium has spread to parts of Africa, Asia, Europe, and South America [19]. It is a quarantine pest in many parts of the Western Hemisphere, continental Europe, and Asia [20]. It has been found in groundnut stocks in the Maradi and Zinder regions of Niger [21] and more recently in large grain warehouses in both regions [22]. These large storage facilities comprise government food reserves or stocks held by development agencies to support emergency responses during food shortages and crises.
The larva of T. granarium feeds aggressively on stored grains, often leaving behind only the outer pericarp. Its presence contaminates food products with exuviae and excreta, compromising food quality. The pest is particularly difficult to control because its larvae can hide in crevices and enter diapause for extended periods, even years [20,23]. Furthermore, repeated use of synthetic pesticides has led to increasing insecticide resistance, with T. granarium showing high tolerance to several chemical treatments, temperature, and growth regulators [11,24,25,26]. Despite this, large warehouse managers and farmers continue to rely on unapproved chemical treatments for grain protection [27,28,29], highlighting the urgent need for safer and more sustainable storage solutions.
Significant improvements in post-harvest grain protection have been achieved in the Sahelian region due to the introduction and widespread adoption of hermetic storage technologies such as the Purdue Improved Crop Storage (PICS) bag [30,31]. Farmers and large-scale users, such as the Office des Produits Vivriers du Niger (OPVN), have used the technology for cowpea storage [32]. A study in Niger comparing five commonly used storage technologies in sub-Saharan Africa confirmed that PICS bags were effective in protecting stored cowpeas and maize [29,33,34]. No study has investigated the use of hermetic bags for storing millet. In this context, the current study was conducted to evaluate the effectiveness of hermetic storage against T. granarium on grain stored in large warehouses in south-central Niger.
Though T. granarium has recently been reported on millet in large warehouses, data on its impact and control remain limited. Though PICS bags are effective in storing cereals, their efficacy against T. granarium on millet has not been studied in the Sahel and particularly in Niger. This study addresses this gap by evaluating the damage caused by this pest and testing whether hermetic storage can minimize losses. Beyond reducing pest damage, hermetic storage offers significant food safety benefits by eliminating pesticide residues and improves worker safety by reducing exposure to hazardous chemicals commonly used in warehouses. The study thus contributes to both improved grain protection and safer storage practices in Niger.
2. Materials and Methods
The study was conducted in the laboratory of the Biodiversity Center at the University of Maradi (UDDM) in Niger from 25 August 2023, to 25 February 2024. The experiment compared three post-harvest storage methods: (i) PICS hermetic bag with two liners fitted into one woven bag [29]; (ii) Fumigation with Phostoxin or aluminum phosphide (1 tablet per bag), a pesticide containing aluminum phosphide applied in millet stored in a standard polypropylene bag lined with a 50 μm thick plastic liner (positive control); (iii) Ordinary polypropylene bag without pesticide, used as a negative control. This study used 50 kg bags, the standard size commonly employed in warehouses for grain storage. The PICS bags were obtained from the manufacturer, Lela Agro Industries (Kano, Nigeria), while the Phostoxin tablets, manufactured by Degesch America Inc. (Weyers Cave, VA, USA) and regular PP bags were purchased from local input dealers’ shops.
Millet grains used in the experiment were obtained from the National Food Security Agency, Office des Produits Vivriers du Niger (OPVN). In the Sahel, cowpeas in warehouses are treated with chemicals, but millet is not. The spread of T. granarium highlights the need for more effective control methods. The millet used in this study had been stored untreated for three years and was already naturally infested. All millet was thoroughly mixed to homogenize the levels of insect pest infestation. Each treatment consisted of four replicates, with 40 kg of millet per bag, resulting in a total of 12 bags. Once filled, the bags were stored inside the laboratory under ambient temperature and relative humidity conditions for the duration of the experiment.
Infestation levels were evaluated at both the beginning and end of the experiment using twelve 500 g samples (three samples per replicate). Each 500 g sample was manually sorted, and the density of pests was determined by counting all live larvae and adult insects present. From each of these, three 100-grain sub-samples (n = 36 per treatment) were analyzed to determine the total weight, number of damaged kernels, and the weight of damaged kernels and impurities. The level of impurities was assessed using 500 g samples, which were sorted to separate non-edible organic and inorganic materials. Each fraction was then weighed, and its proportion calculated relative to the original sample. Insect mortality, grain damage and weight loss were calculated using the following formulas
To monitor environmental conditions, EL-USB-2 dataloggers (Lascar, Whiteparish, Wiltshire, UK) were placed both inside the experimental room and within one bag per treatment. These devices recorded temperature and relative humidity every hour throughout the experiment. For the PICS hermetic bags, oxygen (O2) and carbon dioxide (CO2) concentrations were measured at the start of the experiment, as well as at the end of the first and second months (between 27 August and 27 October 2023). These gas levels were monitored using a Mocon PAC Check Model 325 headspace analyzer (Mocon, Minneapolis, MN, USA).
Data analysis was conducted using the Statistical Package for the Social Sciences (SPSS) version 22.0. Analysis of variance (ANOVA) was used to assess treatment effects. When ANOVA results were significant, means were compared using the Student-Newman-Keuls test at a 5% significance level. When the data did not follow a normal distribution, the Kruskal-Wallis test was used to compare group means.
3. Results
The relative humidity ranged from 21.16% to 67.73%, and the average daily temperature recorded was between 26.18 °C and 37.89 °C during the experimental period. In the PICS bag, the oxygen concentration varied over time. At the closing of the bags, an average oxygen concentration of 20.73 ± 0.34% was noted but decreased to 16.98 ± 1.42% after one month and rose to 18.96 ± 0.69% after two months. The CO2 levels were undetectable at the beginning of the experiment but increased to 5.08 ± 1.99% after one month and then decreased to 2.16 ± 0.78% after the second month. Collected millet samples revealed the presence of two insect pest species: T. granarium and Tribolium sp. (Table 1, Figure 1).

Table 1.
Infestation and proportion of impurities per 500 g of naturally infested millet stored for six months.
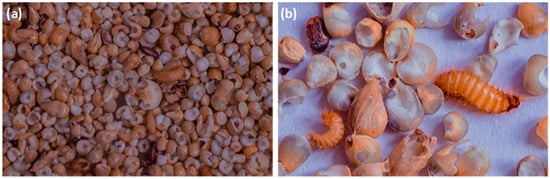
Figure 1.
Millet (a) infested by Trogoderma granarium and Tribolium sp., (b) damaged by larva of T. granarium in control after six months of storage.
The insect population increased in the control while it decreased in the treatments. After 6 months of storage, larvae and pupae of T. granarium decreased by 98.2% in the Phostoxin treatment and by 98.8% in the PICS bag treatment (Table 1). In contrast, in the control, the population of larvae and pupae was 1.7 times higher than that of the initial level. The Adult T. granarium decreased by 39.3% and 71.4% in the Phostoxin and PICS bag treatments, respectively, compared to the initial infestation level. Conversely, in the control, it increased by 2.96 times compared to the initial. For Tribolium sp., the insect population declined to 0.25 and 0.08 adults per 500 g in the Phostoxin and PICS bag treatments, respectively, but increased 3.2-fold in the control. The initial impurity content was 4.08%. After six months, impurity levels remained stable in the Phostoxin and PICS treatments, while the control showed a 4.95 percentage points increase.
The number of kernels damaged by insect pests varied over time (p < 0.05) (Table 2). This ratio increased by 3.17% in the control treatment, whereas in the Phostoxin and the PICS bag treatments, it remained similar to levels observed at the beginning of the experiment. At the end of the experiment, the weight of 100 seeds varied by treatment (p < 0.001) (Table 2). After 6 months of storage in the PICS bag and Phostoxin treatments, the weight of 100 seeds remained comparable to the initial values at the start of the experiment. In the control treatment, however, the weight of 100 seeds after 6 months was 19.15% lower compared to levels measured at the beginning of the study.

Table 2.
Weight and damage of insect pests per 100 seeds of naturally infested millet stored for six months.
4. Discussion
This study was conducted under ambient Sahelian conditions, which reflect typical environments for grain storage by farmers, traders, and warehouses. Although rarely reported in smallholder settings in Niger, T. granarium is a well-known pest of stored cereals and legumes across Sahelian Africa [12,22,35]. Under Sahelian conditions, millet was found to be susceptible to infestation by T. granarium and Tribolium sp. The detection of T. granarium in large warehouses is consistent with its known association with centralized storage systems and international grain flows. As a polyphagous, quarantine pest with long-lived diapausing larvae, T. granarium presents persistent risks in storage environments, particularly where cracks and crevices allow for hidden infestations [36,37,38,39]. In contrast, smallholder storage in Niger typically reports pests such as C. cephalonica, S. cerealella, and Sitophilus spp. [12,16,40,41,42]. Tribolium sp., the second pest identified, is commonly observed on cereals but is generally considered a secondary pest of limited economic importance, as it primarily feeds on pre-damaged seeds [43,44].
In untreated control bags, populations of T. granarium and Tribolium sp. increased significantly over a six-month period. These infestations contributed to an increase in damaged grains, leading to greater weight loss. Although these losses are substantial, they are lower than those typically reported for cowpea storage in the Sahel, which often exceeds 35–70% loss [29,45,46]. Nevertheless, the findings confirm that millet is susceptible to significant deterioration when stored without effective protection, particularly in large-scale facilities where pest introductions and spread are more challenging to control. Comparable studies have reported substantial crop losses due to storage pests. Trogoderma granarium has been shown to cause weight losses ranging from 2% to 70%, depending on the type of stored product [47,48]. In India, losses of up to 61% due to T. granarium were observed on untreated millet after six months of storage [49].
Phostoxin fumigation proved highly effective, significantly reducing the density of T. granarium larvae, pupae, and adults. Similarly, Tribolium sp. populations were reduced. These results confirm the fumigant’s potency when applied under controlled conditions, especially with a tight containment system using a waterproof plastic liner. Studies have shown that Phostoxin is effective in controlling T. granarium in stored grains, including millet [12,22]. However, the use of Phostoxin presents serious safety concerns. It is toxic to humans and animals and may leave harmful chemical residues in the grain treated [50,51,52,53,54]. Additional concerns include workers’ safety (applicators) and the development of insect resistance to this pesticide due to repeated applications over long storage durations [52,55,56,57,58,59], a common practice in these large food security warehouses.
PICS bags offered a safer, chemical-free, and equally effective alternative. Oxygen depletion and CO2 buildup inside the bags created modified atmospheres that killed insects through suffocation and desiccation [60,61]. After six months, the millet stored in PICS bags retained its physical quality, with no significant increases in seed damage or weight loss. The PICS technology has been widely demonstrated to protect a variety of crops [62,63]. In addition to eliminating pest threats without the use of chemicals, the PICS bag provides economic and financial benefits for smallholder farmers and grain traders [31,64]. Beyond pest control, PICS bags support improved food safety by avoiding pesticide residues and enhancing worker safety by eliminating exposure to harmful fumigants. The broad efficacy and cost-effectiveness of hermetic bags compared to pesticides across various crops make it a highly suitable option as a safe and sustainable post-harvest management approach in the Sahel [29,65,66]. Scaling the use of PICS bags for millet storage in large warehouses will not be a challenge as the same technology has been used by food security agencies (e.g., OPVN) for cowpea storage [32].
5. Conclusions
This study confirms that millet stored under ambient Sahelian conditions is susceptible to infestation by T. granarium and Tribolium sp., particularly in large-scale storage environments. In the absence of control measures, pest populations increased significantly over six months, resulting in measurable losses in grain quality, including increased seed damage and weight loss. Both Phostoxin fumigation and hermetic storage using PICS bags were effective in suppressing pest populations and preserving millet quality. However, Phostoxin poses considerable risks to human health and the environment due to misuse and overuse, chemical residues, and the potential for insect resistance. In contrast, PICS bags maintained grain quality without the use of synthetic chemicals, offering a safer and more sustainable alternative. The findings underscore the potential of hermetic storage as a practical and scalable solution for improving post-harvest grain protection in large-scale warehouses in the Sahel. PICS technology not only reduces losses from insect pests but also enhances food safety and reduces occupational exposure to hazardous pesticides.
PICS hermetic bags should be promoted as a safe and effective method for storing millet, particularly in large warehouses, including those used by food security agencies. The use of chemical fumigants, such as Phostoxin, should be restricted to well-managed environments with appropriate safety measures to minimize health risks and the development of resistance. Strengthening the capacity of farmers, traders, and warehouse managers and workers through targeted training will support the adoption of safer storage practices. Ultimately, further research is necessary to assess the long-term effect of hermetic storage on the life cycle of T. granarium, particularly the larvae that enter diapause for extended periods. Additionally, understanding policies that incentivize millet value chain actors to use chemical-free storage methods is crucial for enhancing food safety and security.
Author Contributions
Conceptualization, H.Y.D.B., I.B.B., M.M.R. and D.B.; methodology, I.B.B., H.Y.D.B., M.M.R. and D.B.; software, H.Y.D.B., I.B.B., M.M.R. and D.B.; validation, H.Y.D.B., I.B.B., M.M.R. and D.B.; formal analysis, H.Y.D.B., I.B.B. and D.B.; investigation, H.Y.D.B. and I.B.B.; resources, I.B.B.; data curation, I.B.B. and H.Y.D.B.; writing—original draft preparation, H.Y.D.B.; writing—review and editing, I.B.B., D.B. and M.M.R.; visualization, H.Y.D.B., I.B.B. and D.B.; supervision, I.B.B.; project administration, I.B.B.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Raw data are not publicly available but may be obtained upon request.
Acknowledgments
We would like to thank the Office des Produits Vivriers du Niger (OPVN) for facilitating access to the millet used in this experiment. We also thank the United Nations World Food Program for providing financial assistance to Habibou Yahaya Dan Bawa for his studies.
Conflicts of Interest
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: author Dieudonne Baributsa is a co-founder of PICS Global Inc., a company that commercializes PICS bags around the world, and hence declares a potential conflict of interest. He contributed to the “Conceptualization, Methodology, Software, Validation, formal analysis, Writing—review and editing, visualization” of this study. Dieudonne Baributsa’s participation had no effect on the objectivity and authenticity of the study. PICS Global did not have any role in the funding, study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Habibou Yahaya Dan Bawa, Boukary Baoua Ibrahim, and Mahamane Moctar Rabé have no conflicts of interest to declare.
References
- Fall, R.; Cisse, M.; Sarr, F.; Brabet, C.; Dieme, E. Pratiques Culturales et Gestion Post-Récolte Du Sorgho Au Sénégal. Int. J. Biol. Chem. Sci. 2020, 14, 1001–1013. [Google Scholar] [CrossRef]
- Guèye, M.T.; Seck, D.; Jean-Paul, W.; Lognay, G. Lutte Contre Les Ravageurs Des Stocks de Céréales et de Légumineuses Au Sénégal et En Afrique Occidentale: Synthèse Bibliographique. Biotechnol. Agron. Soc. Environ. 2011, 15, 183–194. [Google Scholar]
- Oura, A.; Waongo, A.; Yamkoulga, M.; Traore, F.; Ilboudo, Z.; Sanon, A. Stockage Post Récolte Des Céréales et Statut Du Grand Capucin Des Grains, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) Au Burkina Faso. J. Appl. Biosci. 2022, 178, 18609–18623. [Google Scholar]
- Songre-Ouattara, L.T.; Bationo, F.; Parkouda, C.; Dao, A.; Bassole, I.H.N.; Diawara, B. Qualité Des Grains et Aptitude à La Transformation: Cas Des Variétés de Sorghum bicolor, Pennisetum glaucum et Zea mays En Usage En Afrique de l’Ouest. Int. J. Biol. Chem. Sci. 2016, 9, 2819. [Google Scholar] [CrossRef]
- Aubert, M.; Bouhsina, Z.; Egg, J.; Lançon, F. Habitudes Alimentaire et Stabilisation Du Marché Des Céréales Au Sahel: Une Analyse Comparative Niger et Mali. In Proceedings of the Intégration des marchés et sécurité alimentaire dans les pays en développement, Clermont-Ferrand, France, 3–4 November 2008; Cerdi, Université d’Auvergne Habitudes: Clermont-Ferrand, France, 2008; pp. 1–16. [Google Scholar]
- Lo, M.; Diome, T.; Thiaw, C.; Sembene, M. Evaluation of Quantitative and Qualitative Losses on Millet and White Rice in Storage Grains Caused by Corcyra cephalonica (Stainton) in Senegal. Adv. Entomol. 2021, 09, 30–43. [Google Scholar] [CrossRef]
- Nambiar, V.S.; Dhaduk, J.J.; Sareen, N.; Shahu, T.; Desai, R. Potential Functional Implications of Pearl Millet (Pennisetum glaucum) in Health and Disease. J. Appl. Pharm. Sci. 2011, 1, 62–67. [Google Scholar]
- Hamadou, M.; Idrissa, S.; Mahamadou, C.; Oumarou, S.; Valentin, K. Potentialités Fourragères Du Mil (Pennisetum glaucum (L.) R. Br): Revue de Littérature. J. Anim. Plant Sci. 2017, 34, 5424–5447. [Google Scholar]
- MAE. Rapport d’évaluation de La Campagne Agricole d’hivernage 2023 et Perspectives Alimentaires 2023/2024; Ministère de l’Agriculture et de l’Elevage: Niamey, Niger, 2024. [Google Scholar]
- Waongo, A.; Yamkoulga, M.; Dabire-Binso, C.; Ba, M.; Sanon, A. Conservation Post-Récolte Des Céréales En Zone Sud-Soudanienne Du Burkina Faso: Perception Paysanne et Évaluation Des Stocks. Int. J. Biol. Chem. Sci. 2013, 7, 1157–1167. [Google Scholar] [CrossRef][Green Version]
- Adjalian, E.; Noudogbessi, J.-P.; Kossou, D.; Sohounhl, D. État et Perspectives de Lutte Contre Sitotroga cerealella (Olivier, 1789), Déprédateur Des Céréales Au Bénin: Synthèse Bibliographique. J. Appl. Biosci. 2014, 79, 6955–6967. [Google Scholar] [CrossRef][Green Version]
- Alagbe, T.O.; Loko, Y.L.E.; Djègbè, I.; Gandjala, J.; Gavoedo, D.; Tamò, M. Post-Harvest Conservation Practices, Related Insect Pests of Stored Pearl Millet (Pennisetum glaucum (L) R. Br.), and Their Management in Northern Benin. J. Basic Appl. Zool. 2025, 86, 7. [Google Scholar] [CrossRef]
- Issan, B.; Merouane, T. Étude de l’activité biologique de l’huile essentielle et de l’extrait hydrométhanoïque des clous de girofle (Syzygium aromaticum) à l’égard du bioagresseur des denrées stockées Trogoderma granarium. Master’s Thesis, Université Abdelhamid Ibn Badis-Mostaganem, Mostaganem, Algérie, 2020. [Google Scholar]
- Lale, N.E.S.; Yusuf, B.A. Insect Pests Infesting Stored Pearl Millet Pennisetum glaucum (L.) R. Br. in Northeastern Nigeria and Their Damage Potential. Cereal Res. Commun. 2000, 28, 181–186. [Google Scholar] [CrossRef]
- Meenatchi, R.; Loganathan, M. Millet Storage and Pest Management. In Handbook of Millets—Processing, Quality, and Nutrition Status; Springer: Singapore, 2022; pp. 49–61. [Google Scholar]
- Baoua, I.B.; Amadou, L.; Abdourahmane, M.; Bakoye, O.; Baributsa, D.; Murdock, L.L. Grain Storage and Insect Pests of Stored Grain in Rural Niger. J. Stored Prod. Res. 2015, 64, 8–12. [Google Scholar] [CrossRef]
- Riaz, T.; Shakoori, F.R.; Ali, S.S. Effect of Temperature on the Development, Survival, Fecundity and Longevity of Stored Grain Pest, Trogoderma granarium. Pak. J. Zool. 2014, 46, 1485–1489. [Google Scholar]
- Viljoen, J.H. The Occurrence of Trogoderma (Coleoptera: Dermestidae) and Related Species in Southern Africa with Special Reference to T. granarium and Its Potential to Become Established. J. Stored Prod. Res. 1990, 26, 43–51. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Subramanyam, B. A Review of Stored-Product Entomology Information Sources. Am. Entomol. 2009, 55, 174–183. [Google Scholar] [CrossRef]
- Arthur, F.H.; Ghimire, M.N.; Myers, S.W.; Phillips, T.W. Evaluation of Pyrethroid Insecticides and Insect Growth Regulators Applied to Different Surfaces for Control of Trogoderma granarium (Coleoptera: Dermestidae) the Khapra Beetle. J. Econ. Entomol. 2018, 111, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Bakoye, O.; Baoua, I.; Sitou, L.; Moctar, M.R.; Amadou, L.; Njoroge, A.W.; Murdock, L.L.; Baributsa, D. Groundnut Production and Storage in the Sahel: Challenges and Opportunities in the Maradi and Zinder Regions of Niger. J. Agric. Sci. 2019, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bawa, H.Y.D.; Baoua, I.B.; Rabé, M.M.; Baributsa, D. Hermetic Bags Effectively Manage Emerging and Common Pests of Stored Cowpeas in Niger. Insects 2025, 16, 196. [Google Scholar] [CrossRef]
- Wilches, D.M.; Laird, R.A.; Floate, K.D.; Fields, P.G. A Review of Diapause and Tolerance to Extreme Temperatures in Dermestids (Coleoptera). J. Stored Prod. Res. 2016, 68, 50–62. [Google Scholar] [CrossRef]
- Bell, C.H.; Wilson, S.M. Phosphine Tolerance and Resistance in Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Stored Prod. Res. 1995, 31, 199–205. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Pant, N.C. Nature of Growth Inhibitors for Trogoderma granarium Everts (Coleoptera: Dermestidae) in Lentil (Lens esculenta Moench.) and French Bean (Phaseolus vulgaris L.). J. Stored Prod. Res. 1969, 5, 379–388. [Google Scholar] [CrossRef]
- Boukouvala, M.; Kavallieratos, N. Effect of Six Insecticides on Egg Hatching and Larval Mortality of Trogoderma granarium Everts (Coleoptera: Dermestidae). Insects 2020, 11, 263. [Google Scholar] [CrossRef]
- Anaduaka, E.G.; Uchendu, N.O.; Asomadu, R.O.; Ezugwu, A.L.; Okeke, E.S.; Chidike Ezeorba, T.P. Widespread Use of Toxic Agrochemicals and Pesticides for Agricultural Products Storage in Africa and Developing Countries: Possible Panacea for Ecotoxicology and Health Implications. Heliyon 2023, 9, e15173. [Google Scholar] [CrossRef]
- Apeh, A.C.; Apeh, C.C.; Ukwuaba, S.I.; Agbugba, I.K.; Onyeaka, H. Exploring Data Sources and Farmers’ Perceptions Regarding Agrochemical Use and Food Safety in Nigeria. JSFA Rep. 2024, 4, 304–315. [Google Scholar] [CrossRef]
- Baoua, I.B.; Amadou, L.; Margam, V.; Murdock, L.L. Comparative Evaluation of Six Storage Methods for Postharvest Preservation of Cowpea Grain. J. Stored Prod. Res. 2012, 49, 171–175. [Google Scholar] [CrossRef]
- Baributsa, D.; Concepcion Ignacio, M.C. Developments in the Use of Hermetic Bags for Grain Storage. In Advances in Postharvest Management of Cereals and Grains; Maier, D.E., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 171–198. [Google Scholar]
- Rabé, M.M.; Baoua, I.B.; Baributsa, D. Adoption and Profitability of the Purdue Improved Crop Storage Technology for Grain Storage in the South-Central Regions of Niger. Agronomy 2021, 11, 2470. [Google Scholar] [CrossRef]
- Baributsa, D.; Abdoulaye, T.; Lowenberg-DeBoer, J.; Dabiré, C.; Moussa, B.; Coulibaly, O.; Baoua, I. Market Building for Post-Harvest Technology through Large-Scale Extension Efforts. J. Stored Prod. Res. 2014, 58, 59–66. [Google Scholar] [CrossRef]
- Bakoye, O.N.; Ibrahim, B.; Seyni, H.; Amadou, L.; Murdock, L.L.; Baributsa, D. Comparative Study of Cowpea Storage Technologies in the Sahel Region of Niger. Insects 2020, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- De Groote, H.; Kimenju, S.C.; Likhayo, P.; Kanampiu, F.; Tefera, T.; Hellin, J. Effectiveness of Hermetic Systems in Controlling Maize Storage Pests in Kenya. J. Stored Prod. Res. 2013, 53, 27–36. [Google Scholar] [CrossRef]
- Gómez, C. Cowpea: Post-Harvest Operations; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Boukouvala, M.C. Population Growth of the Khapra Beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae) on Different Commodities. J. Stored Prod. Res. 2016, 69, 72–77. [Google Scholar] [CrossRef]
- Castañé, C.; Agustí, N.; del Estal, P.; Riudavets, J. Survey of Trogoderma spp. in Spanish Mills and Warehouses. J. Stored Prod. Res. 2020, 88, 101661. [Google Scholar] [CrossRef]
- Gourgouta, M.; Baliota, G.V.; Morrison, W.R.; Domingue, M.J.; Athanassiou, C.G. Comparative Capture of Trogoderma granarium (Coleoptera: Dermestidae) and T. variabile in Floor Traps in Single Species Releases with Previously Captured Conspecific or Heterospecific Individuals. J. Econ. Entomol. 2021, 114, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Scheff, D.S.; Arthur, F.H.; Myers, S.W.; Domingue, M.J. Efficacy Determination of Commercial Deltamethrin-Treated Storage Bags on Trogoderma granarium Everts Adults and Larvae. Agronomy 2020, 10, 814. [Google Scholar] [CrossRef]
- Demis, E.; Yenewa, W. Review on Major Storage Insect Pests of Cereals and Pulses. Asian J. Adv. Res. 2022, 5, 41–56. [Google Scholar]
- Kabore, A.; Sawadogo, R.S.W.; Koussoube, S.; Boly, A.; Sanou, A.; Waongo, A.; Traore, F.; Ba, M.N.; Sanon, A.; Adama, K.; et al. Evaluation of Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) Damage and Insecticidal Efficacy of Six Plant Powders on the Three Main Cereals Produced in Burkina Faso. J. Stored Prod. Postharvest Res. 2024, 15, 24–30. [Google Scholar]
- Swamy, S.V.S.G.; Wesley, B.J. Incidence and Diversity of Stored Product Insects in Processed Millets. Indian J. Entomol. 2021, 83, 660–664. [Google Scholar] [CrossRef]
- Ahmady, A.; Rahmatzi, N.; Hazim, Z.; Mousa, M.A.; Zaitoun, A.A. Effect of Temperature on Stored Product Pests Tribolium confusum Jaquelin Du Val (Coleoptera: Tenebrionidae) and Callosobruchus maculatus (F.)(Coleoptera: Chrysomelidae: Bruchidae). J. Entomol. Zool. Stud. 2016, 4, 166–172. [Google Scholar]
- Kumar, J.; Marin, G.; Arivoli, S.; Tennyson, S. Assessment of Piper longum L. (Piperaceae) Leaves Toxicity on the Adults of Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae). Futur. J. Pharm. Sci. 2024, 10, 134. [Google Scholar] [CrossRef]
- FAO. Amélioration De La Gestion Des Pertes Après-Récolte Dans Les Filières Céréales Et Légumineuses Au Burkina Faso; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Komadan, M.; Souley, K.; Séraphin, M.; Yabi, I. Gestion Des Stocks Alimentaires Dans Les Communes de Lokossa et de Dogbo Au Sud-BENIN. Rev. Espac. Géographique Société Marocaine 2022, 65, 105–126. [Google Scholar]
- Ahmedani, M.S.; Khaliq, A.; Tariq, M.; Anwar, M.; Naz, S. Khapra Beetle (Trogoderma granarium Everts): A Serious Threat to Food Security and Safety. Pak. J. Agri. Sci. 2007, 44, 481–493. [Google Scholar]
- Amit, S.; Chand1, P.; Vishwakarma, R.; Chandan, K.S. Khapra Beetle (Trogoderma granarium Everts): A Food Security Threat. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 14–19. [Google Scholar]
- Yadav, S.D.; Pandey, S.; Tripathi, A.; Khan, M.; Sahni, P.; Bhardwaj, R.; Kumar, M.; Singh, R. Evaluation of Some Plant Products against Trogoderma granarium Everts and Rhyzopertha dominica Fabricius in Pearl Millet and Their Effects on Nutritional Composition and Organoleptic Characteristics. J. Stored Prod. Res. 2025, 113, 102680. [Google Scholar] [CrossRef]
- Çakın, Ö.; Tazegul, G.; Gümüş, A.; Cengiz, M.; Ramazanoğlu, A. Incidental Aluminum Phosphide Poisoning: Case Report and Current Management. Folia Med. 2018, 60, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.L.; Dageri, A.; Aslan, T.N. Nanopesticides for Managing Primary and Secondary Stored Product Pests: Current Status and Future Directions. Heliyon 2025, 11, e42341. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Qureshi, A.W.; Arshad, S.; Shehzadi, A.; Kamran, A.; Noreen, S. Biochemical Toxic Response of Phosphine on Human Health Estimated From Enzymatic Variance in Trogoderma granarium. Dose Response 2022, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Petkovska, L.; Babulovska, A.; Simonovska, N.; Kostadinovski, K.; Brezovska, J.; Zafirova, B. Fatal Acute Aliminum Phosphide Poisoning-Case Report and Literature Review with Reference to Current Treatment Protocols and Outcome. Georgian Med. News 2021, 320, 111–115. [Google Scholar]
- Tonato, O.; Dannon, E.A.; Hounsou, S.; Chougourou, D.C.; Tamò, M. Assessing Insecticide Residues in Stored Maize in Southern and Central Benin. J. Stored Prod. Res. 2025, 111, 102529. [Google Scholar] [CrossRef]
- Pimentel, M.A.G.; Faroni, L.R.D.; Tótola, M.R.; Guedes, R.N.C. Phosphine Resistance, Respiration Rate and Fitness Consequences in Stored-product Insects. Pest Manag. Sci. 2007, 63, 876–881. [Google Scholar] [CrossRef]
- Lindgren, D.L.; Vincent, L.E. Relative Toxicity of Hydrogen Phosphide to Various Stored-Product Insects. J. Stored Prod. Res. 1966, 2, 141–146. [Google Scholar] [CrossRef]
- Yadav, S.K.; Srivastava, C. The Toxicity of Phosphine against Susceptible and Resistant Strains of Trogoderma granarium (Coleoptera: Dermestidae). Afr. Entomol. 2021, 29, 414–422. [Google Scholar] [CrossRef]
- Ugwu, J.; Amos Omoloye, A.; Nigeria Aduloju, I. Pesticide-Handling Practices among Smallholder Vegetable Farmers in Oyo State, Nigeria. Sci. Res. J. 2015, 3, 40–47. [Google Scholar]
- Dahiru, B.; Abdullahi, G.; Bukar, N. Pesticides Use among Grain Merchants in Mubi Grain Markets of Adamawa State, Nigeria. Agrosearch 2014, 14, 1–13. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Yaninek, J.S.; Baributsa, D. Hand Warmer-Induced Hypoxia Accelerates Pest Control in Hermetic Storage. Insects 2024, 15, 821. [Google Scholar] [CrossRef]
- Murdock, L.L.; Margam, V.; Baoua, I.; Balfe, S.; Shade, R.E. Death by Desiccation: Effects of Hermetic Storage on Cowpea Bruchids. J. Stored Prod. Res. 2012, 49, 166–170. [Google Scholar] [CrossRef]
- Oyewole, O.S.; Abdulbaki, M.K.; Adisa, A.; Adebiyi, A.; Raji, M.A.; Balogun, B.; Ajao, T.O.; Ibitoye, O.; Aremu, M.B. Purdue Improved Crop Storage (PICS) Bags: An Innovative Hermetic Storage Technology for Postharvest Crop Management in Developing Countries. African J. Agric. Sci. Food Res. 2025, 20, 83–103. [Google Scholar] [CrossRef]
- Coffi, H.; Nyabicha, J.; Ouma, J.O. The Use of Hermetic Bags for on Farm Storage of Grains and Pulses Against Insect Pests. Outlooks Pest Manag. 2016, 27, 231–235. [Google Scholar] [CrossRef]
- Aker, J.C.; Dillon, B.; Welch, C.J. Demand, Supply and Long-Term Adoption: Evidence from a Storage Technology in West Africa. J. Dev. Econ. 2023, 165, 103129. [Google Scholar] [CrossRef]
- Deressa, T.; Diro, D.; Getachew, G.; Demissie, G. Performance Evaluation of Metal Silo and PICS Bag for Maize Grain Storage against Insect Pest Infestation and Grain Quality. EAS J. Nutr. Food Sci. 2025, 7, 1–9. [Google Scholar] [CrossRef]
- Foy, C.; Wafula, M. Scaling Up of Hermetic Bag Technology (PICS) in Kenya: Review of Successful Scaling of Agricultural Technologies; United States Agency for International Development: Washington, DC, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).