3.3. Description
Zoobank: urn:lsid:zoobank.org:act:98F98919-E673-4C0A-8FE7-11CCCD2AF8BE
Type specimens and type locality. ♂—holotype (in 70% ethanol); 3♂4♀—paratypes (in 70% ethanol). Fengya Valley, Pan’an County, Jinhua City, Zhejiang Province, China (29°04′45″ N 120°37′39″ E, 542 m), in leaf litter and on rocks near signal station (
Figure 1), 17.VII.2024, coll. Jia-Yong Zhang, Chen-Yang Shen, Zhi-Qiang Guo, and Jie-Hong Ji. All type specimens deposited in the Animal Herbarium, Zhejiang Normal University, Jinhua, China.
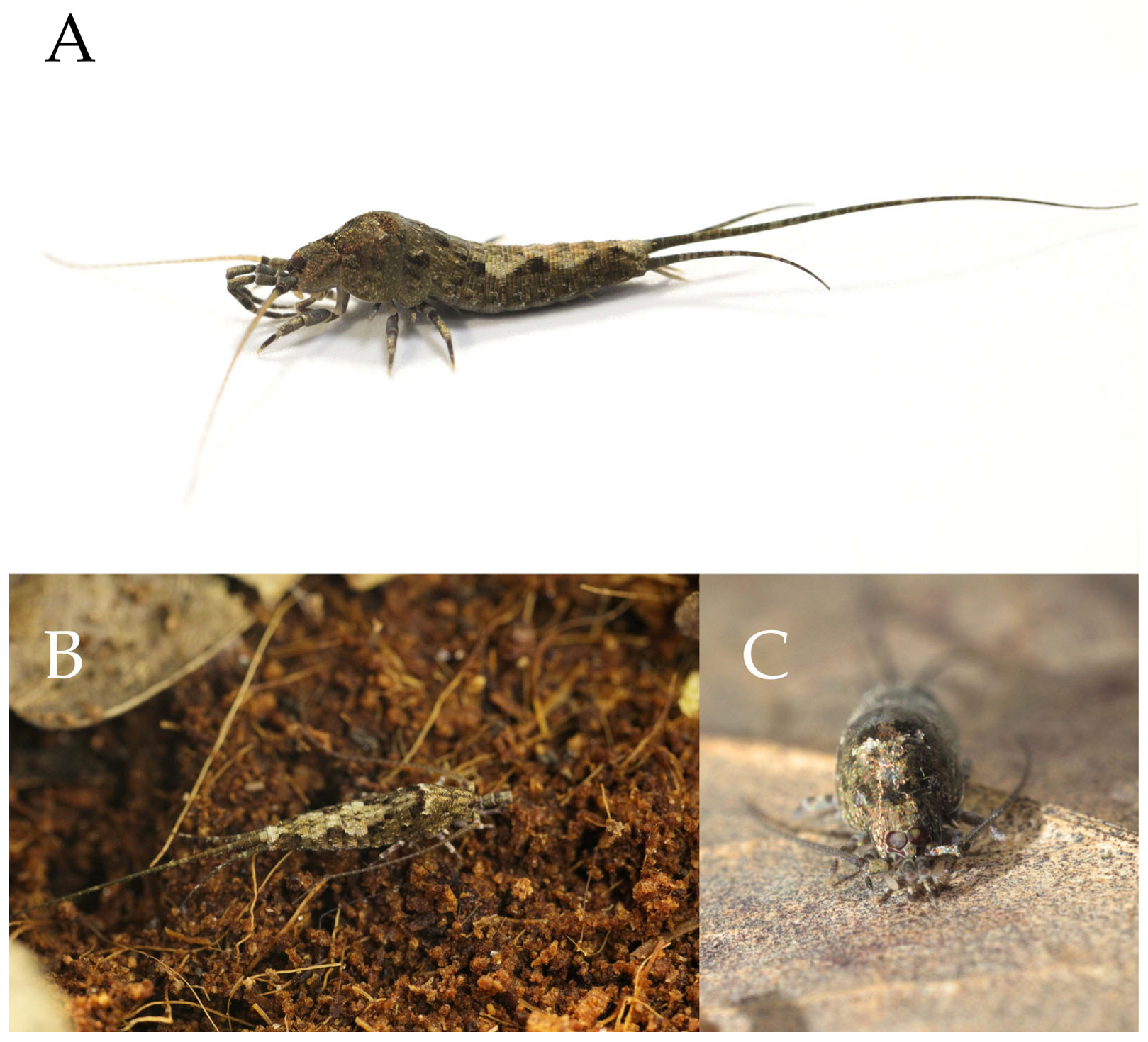
Figure 1.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n. (A) P. (V.) elegans sp. n. in situ. (B) Ditto. (C) Habitat.
Figure 1.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n. (A) P. (V.) elegans sp. n. in situ. (B) Ditto. (C) Habitat.
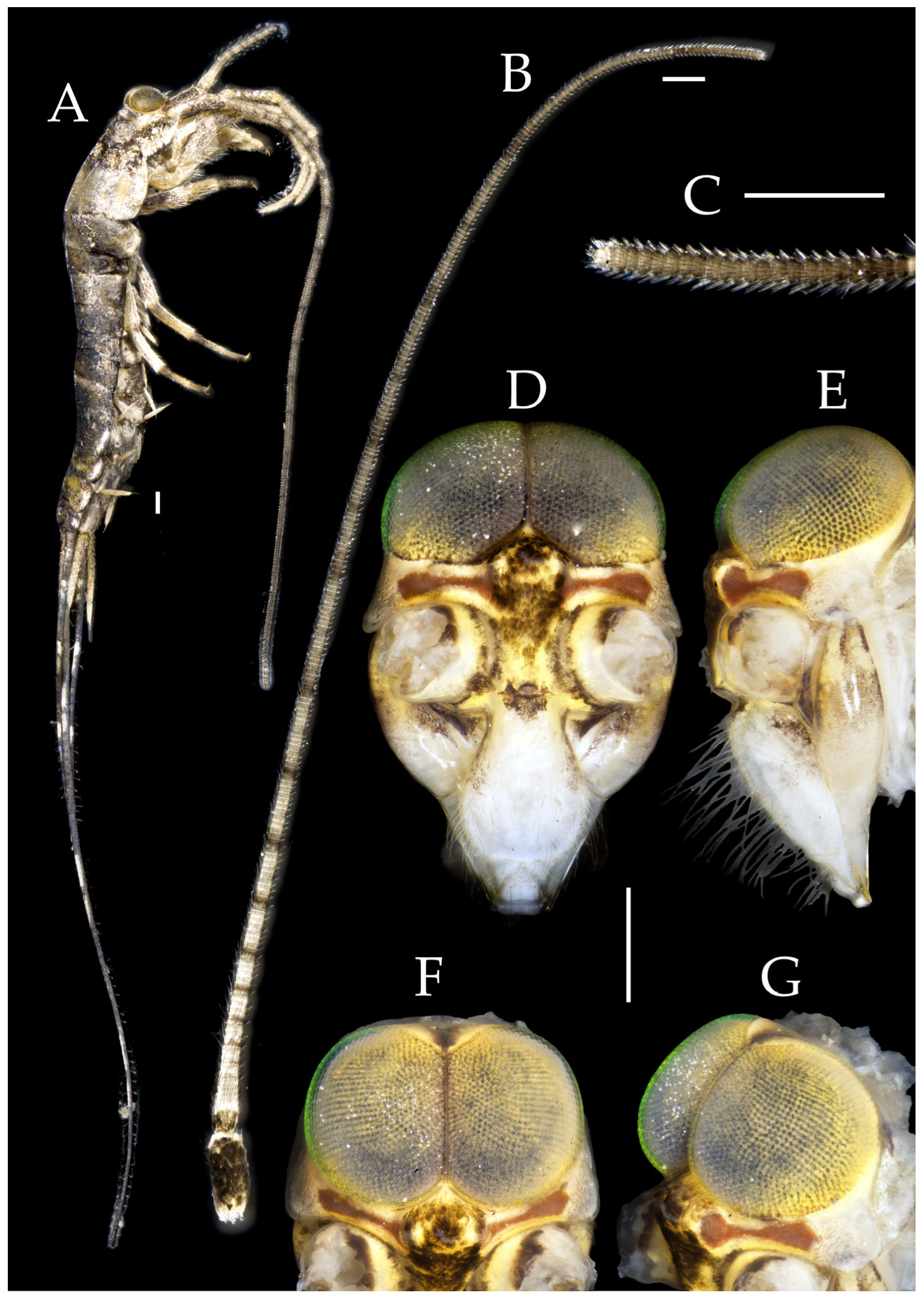
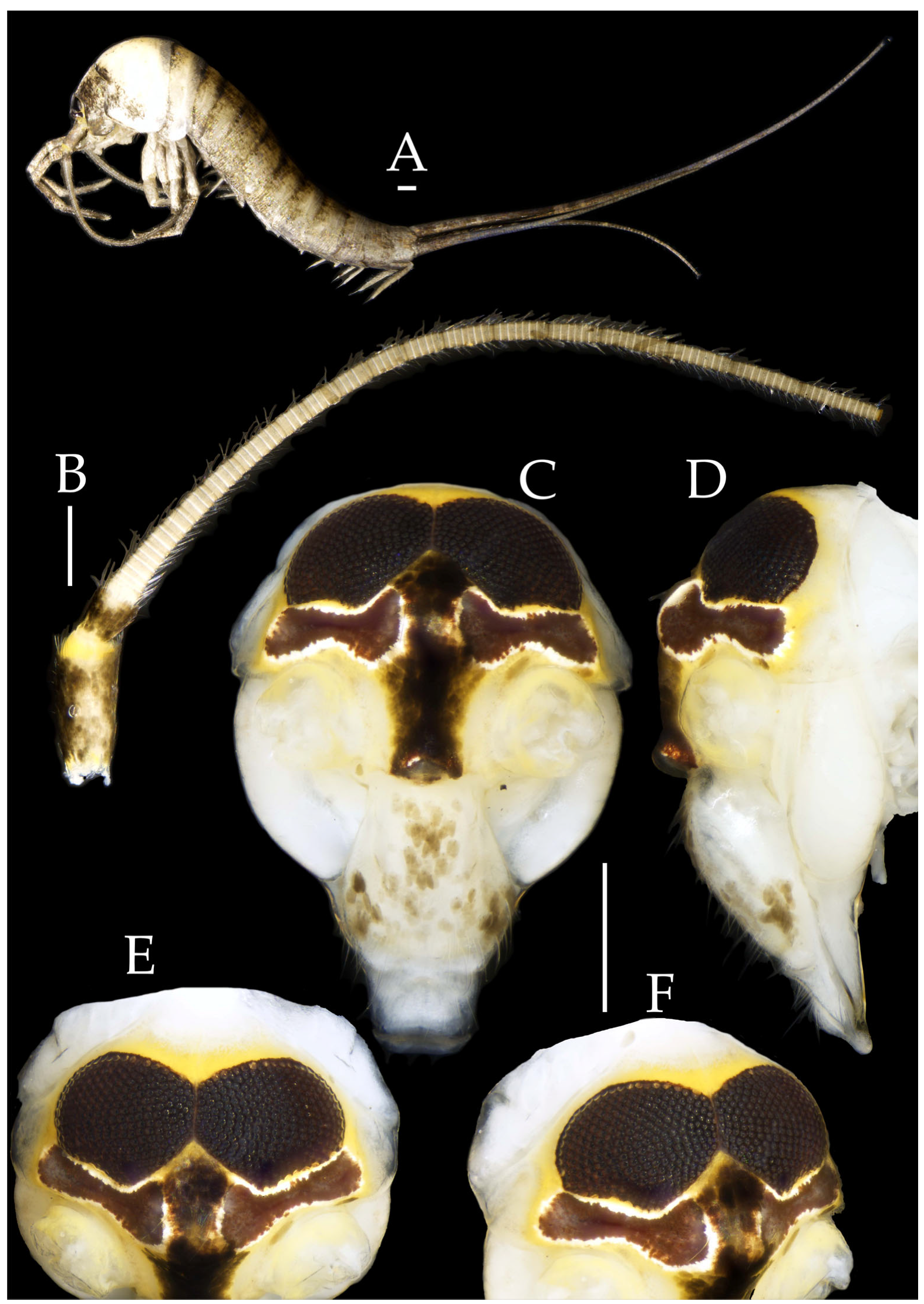
Description. Male body length 10.4–11.2 mm, female 11.9 mm. Body covered with scales, light brown, with several white longitudinal stripes, black markings, and a few green markings; caudal filament and cerci with several well-developed white annulate setae. Antennae and median caudal filament approximately 1.6 times body length. Lateral cerci length approximately two-thirds of body length (
Figure 1A,B,
Figure 2A, and
Figure 5A).
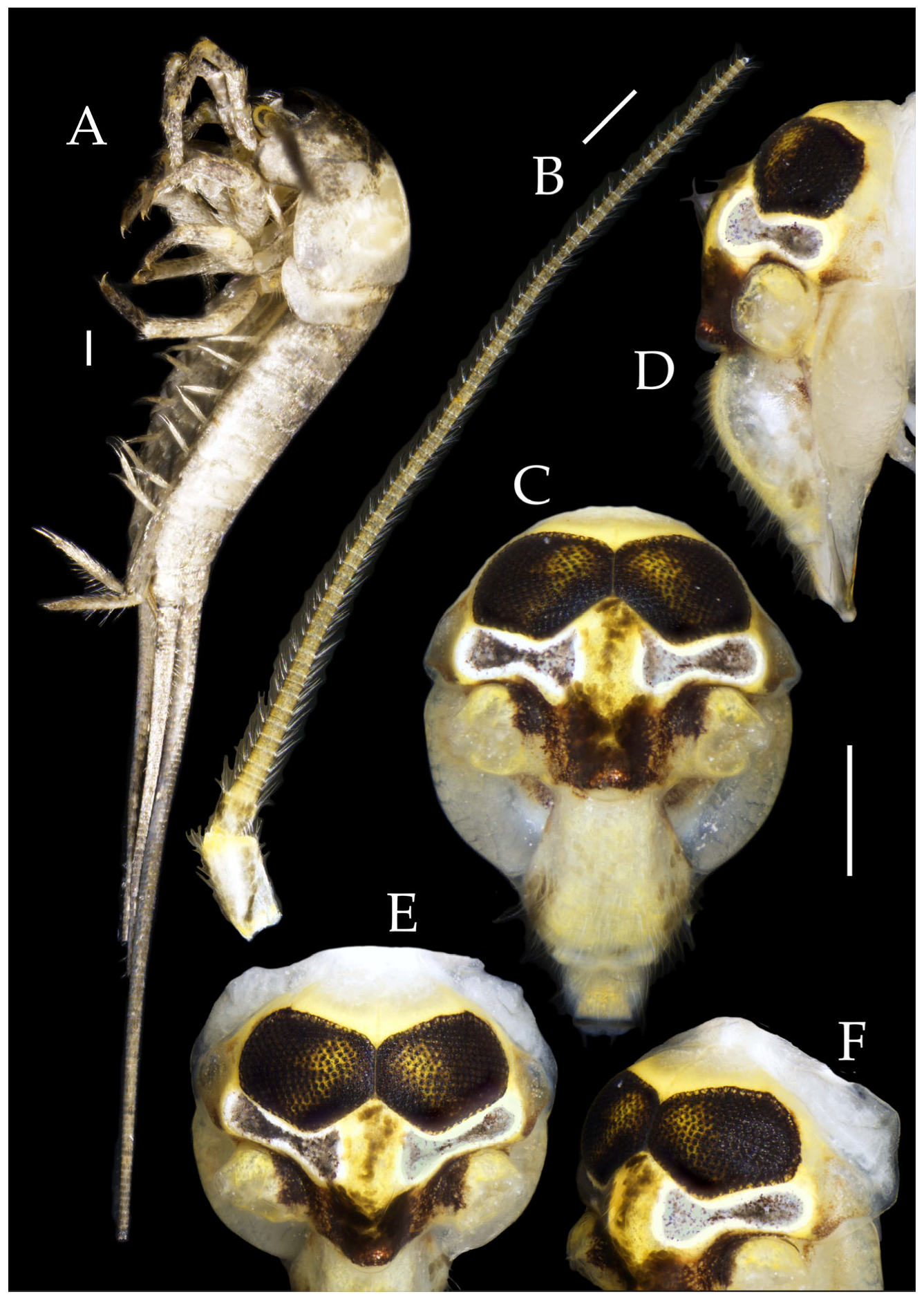
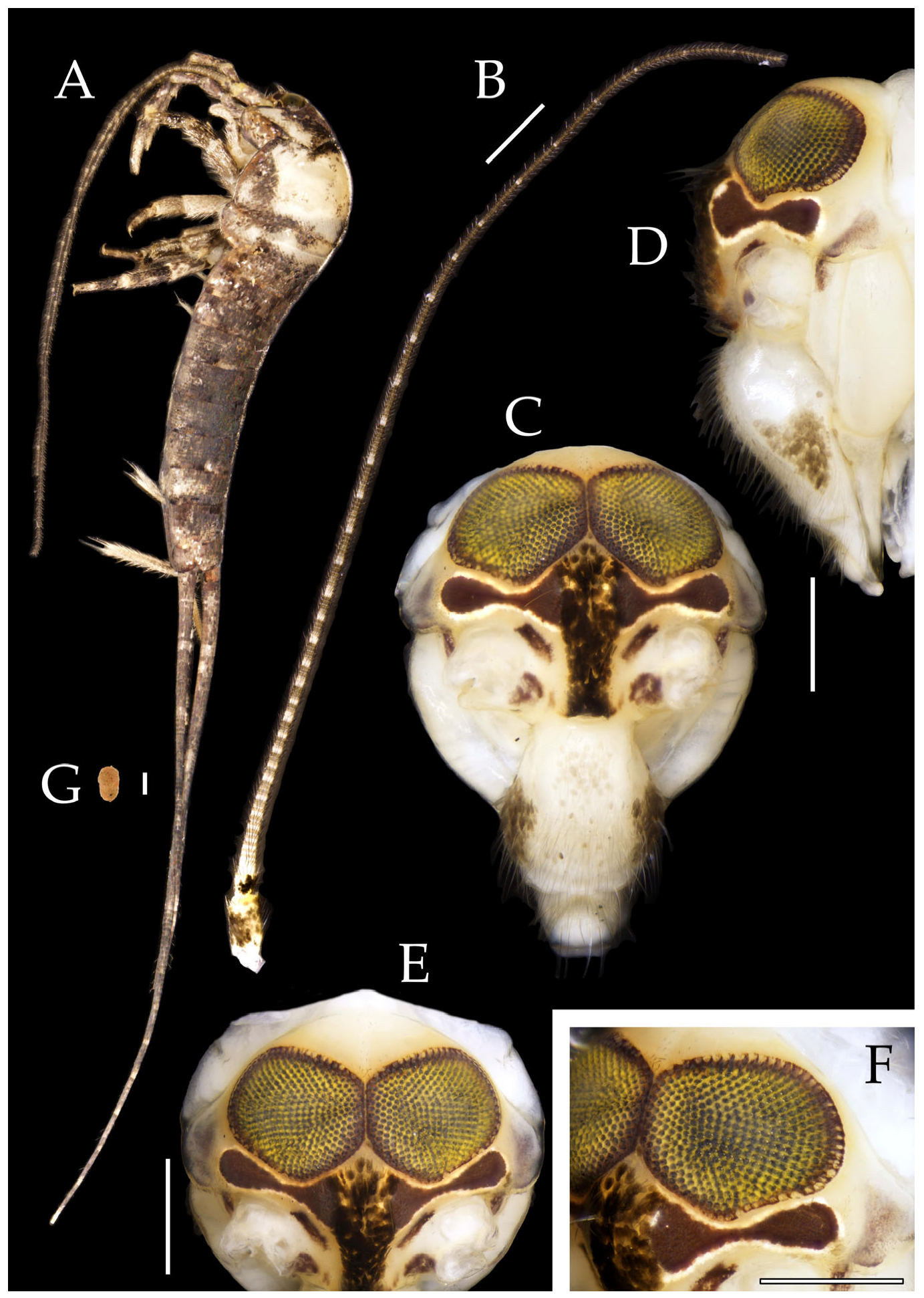
Head. Compound eyes well-developed, strongly convex, bright yellowish-green (
Figure 2D–G and
Figure 5B,C). Ratios of eye length to width and contact line length to eye length given in
Table 1. Paired ocelli subinferior to compound eyes, reddish-brown, dumbbell-shaped. Ratios of ocellus length to width and distance between inner margins of ocelli to combined width of eyes given in
Table 1. Vertex straight, with deep chestnut markings. Frons convex, bearing fine setae, without long bristles. Genae bearing black setae. Clypeus and labrum bearing transparent long setae (
Figure 2D,E and
Figure 5B,C).
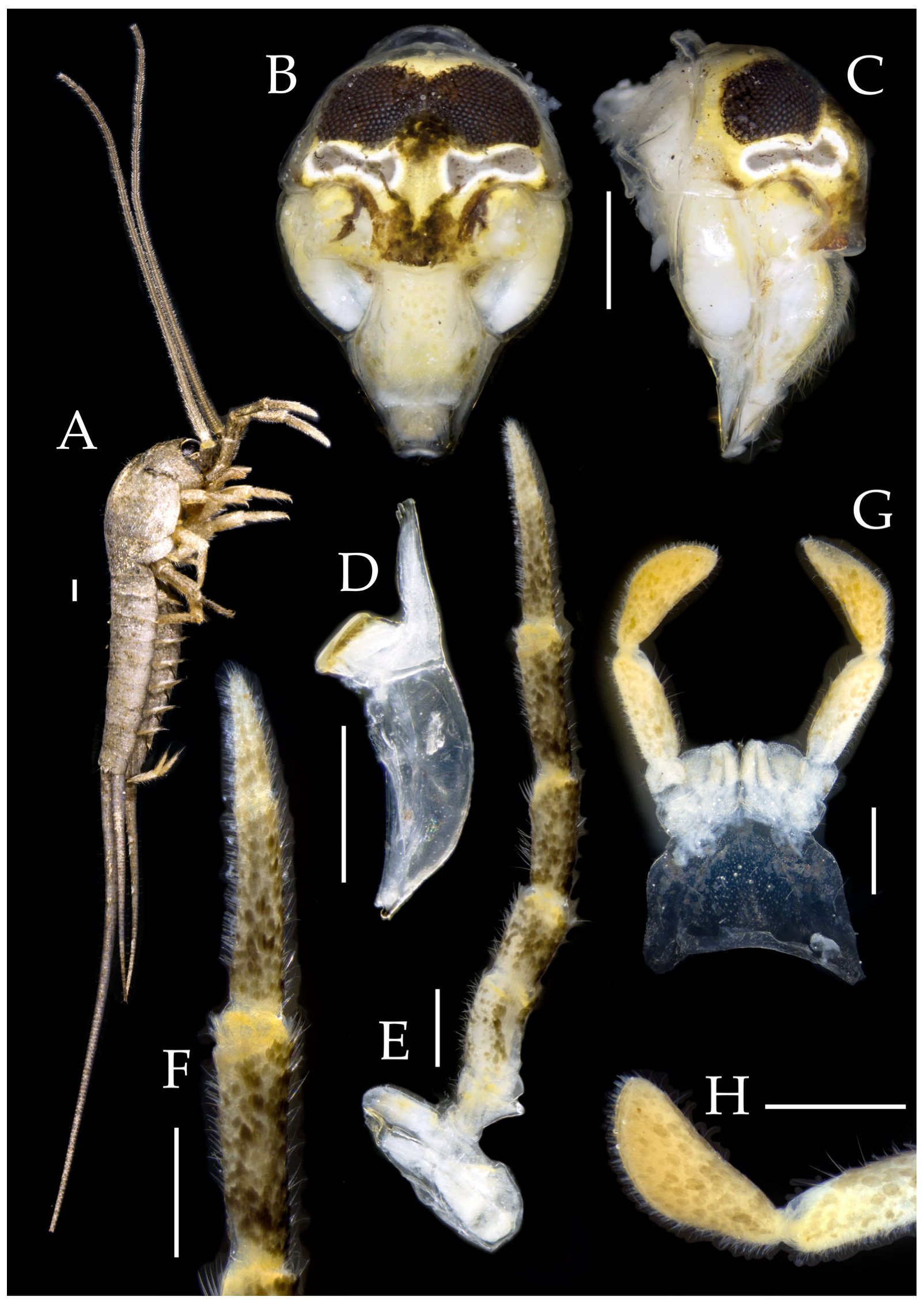
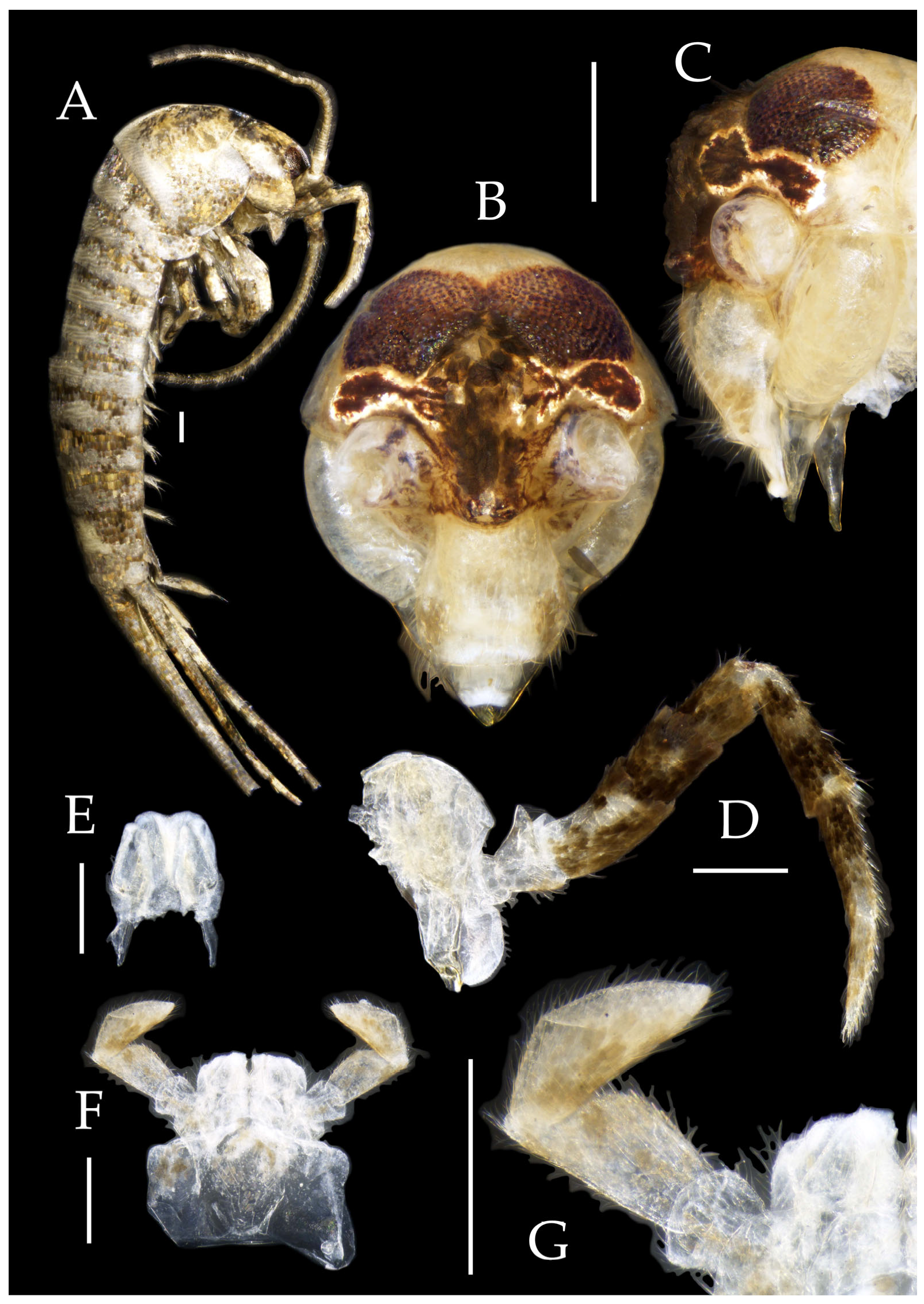
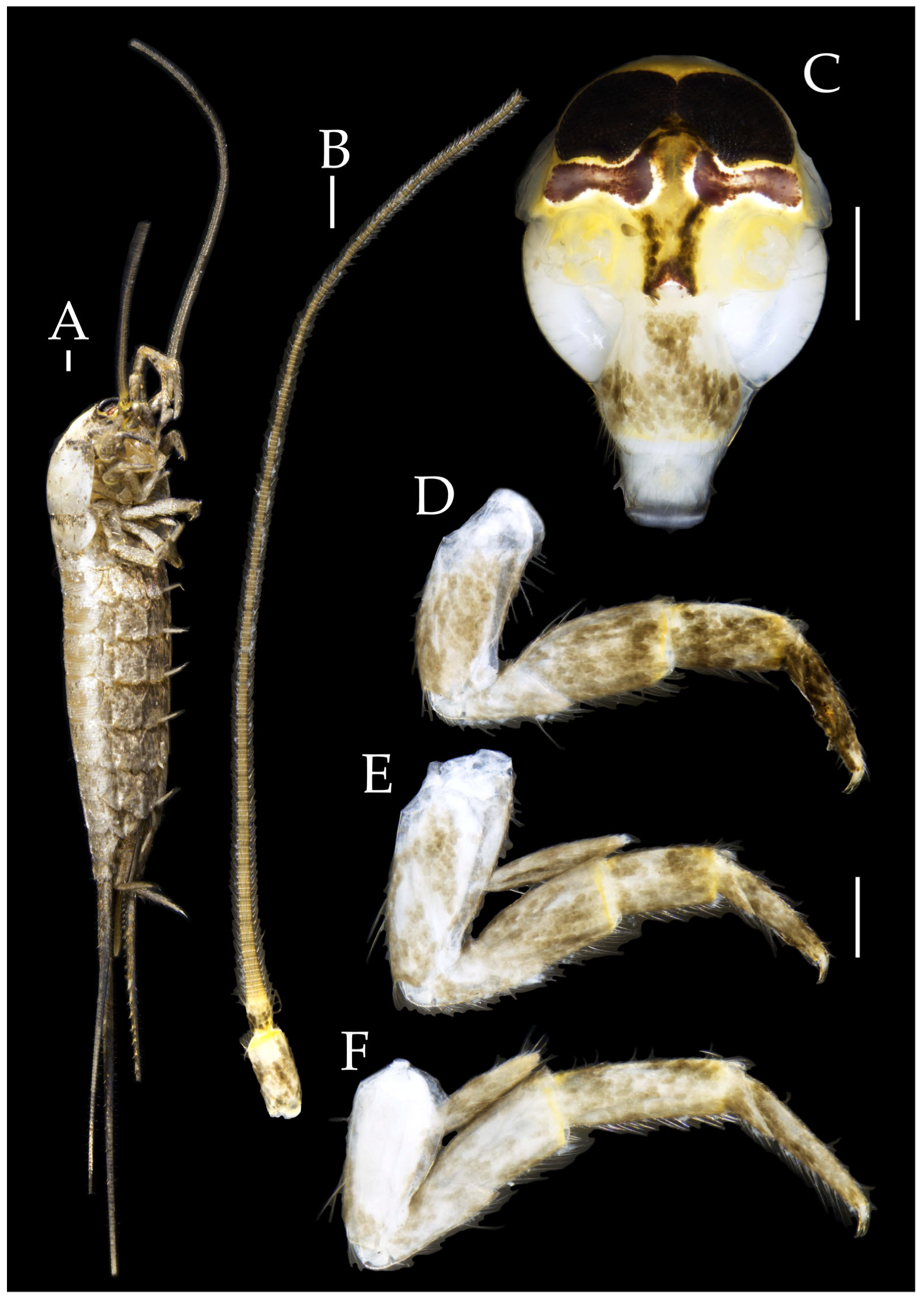
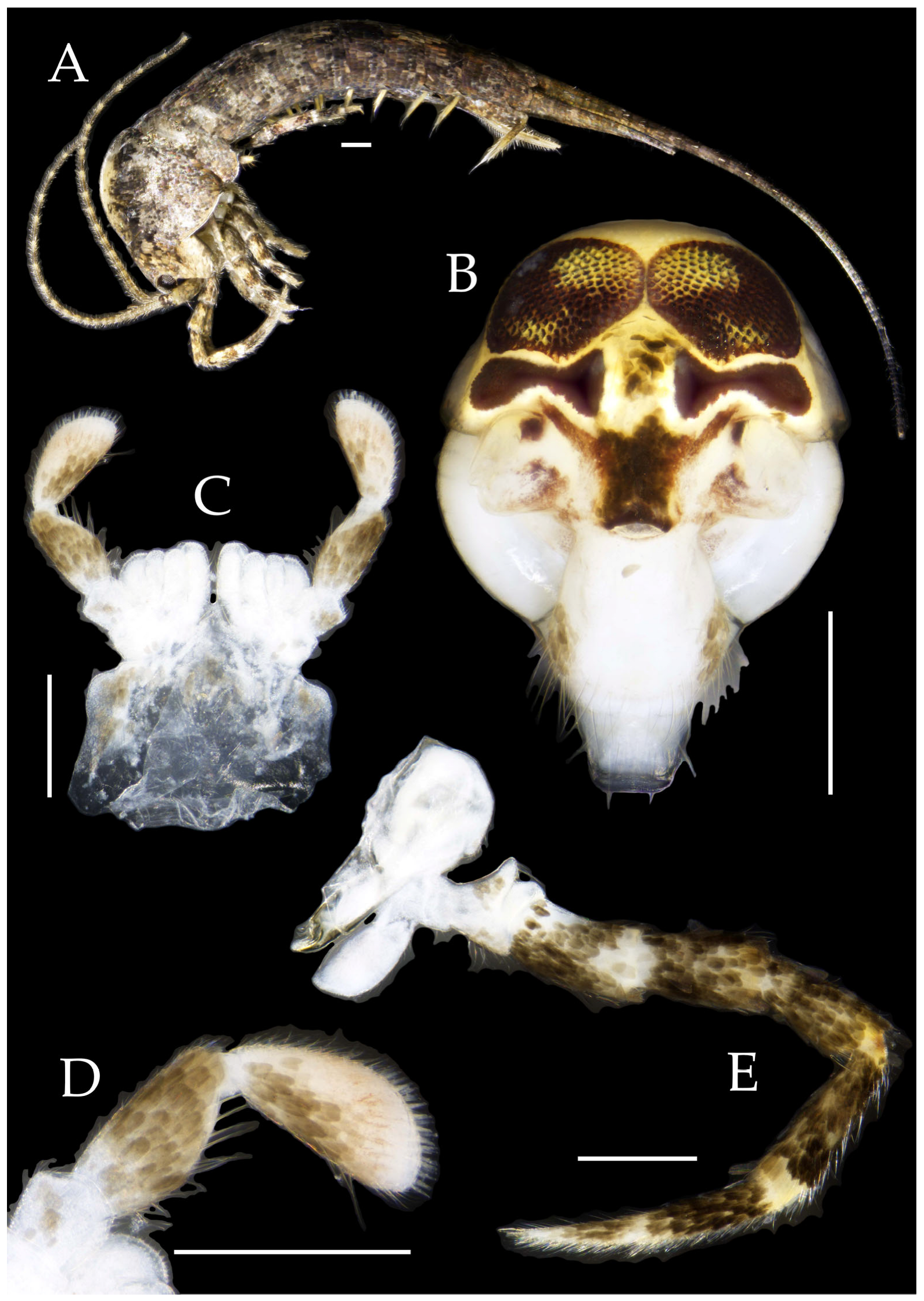
Figure 2.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view. (B) Antenna. (C) Terminal chain of flagellum. (D) Head, frontal view. (E) Ditto, lateral view. (F,G) Compound eyes and paired ocelli. The white elongated rectangles as the scale bars. Scale bars: 500 μm.
Figure 2.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view. (B) Antenna. (C) Terminal chain of flagellum. (D) Head, frontal view. (E) Ditto, lateral view. (F,G) Compound eyes and paired ocelli. The white elongated rectangles as the scale bars. Scale bars: 500 μm.
Antennae scaled except on flagellum; flagellum extremely long, with dark annulations and well-developed annulate setae; terminal chains divided into 21–22 segments. Scape length-to-width ratio 2.2–2.33 (
Figure 2B).
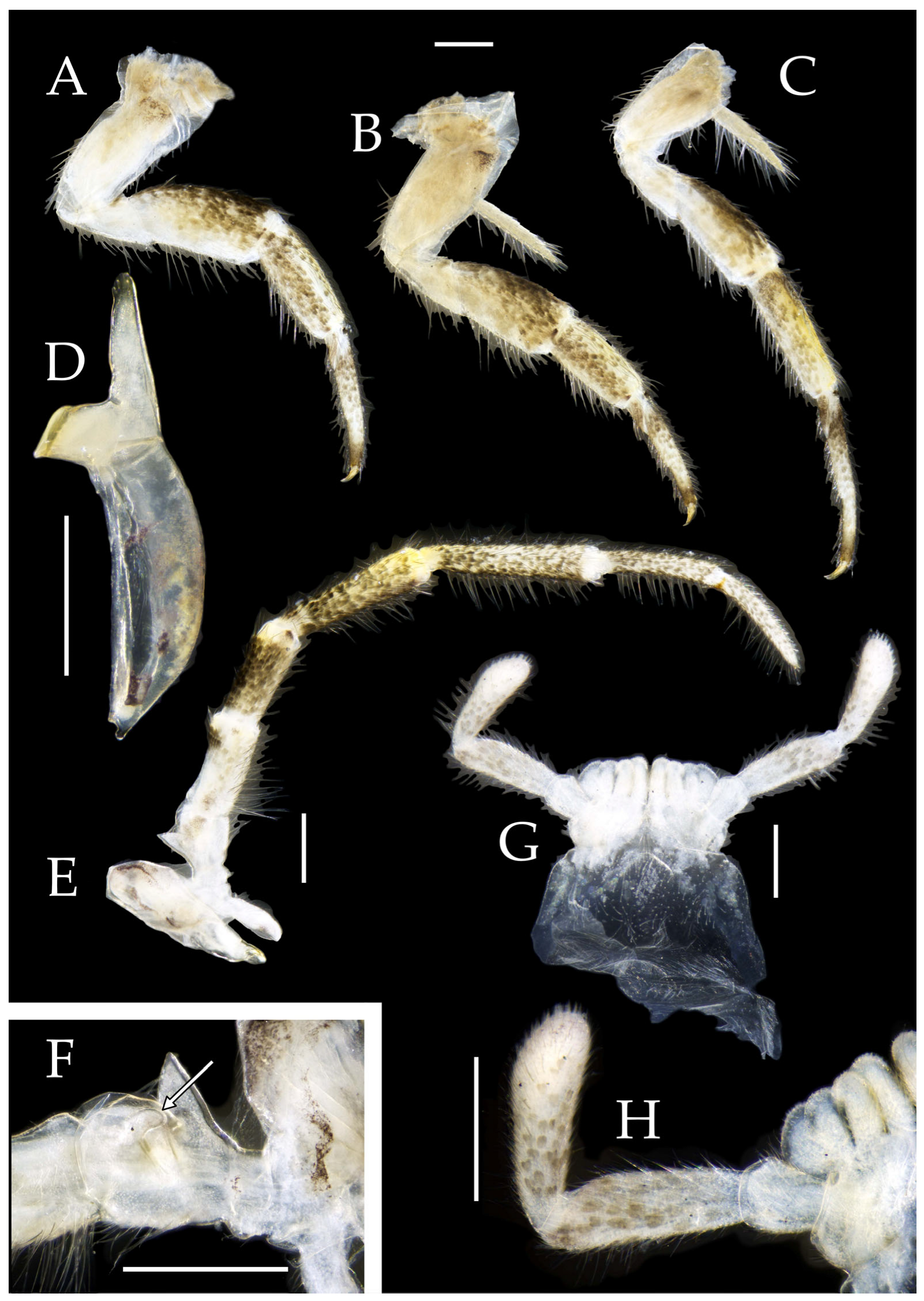
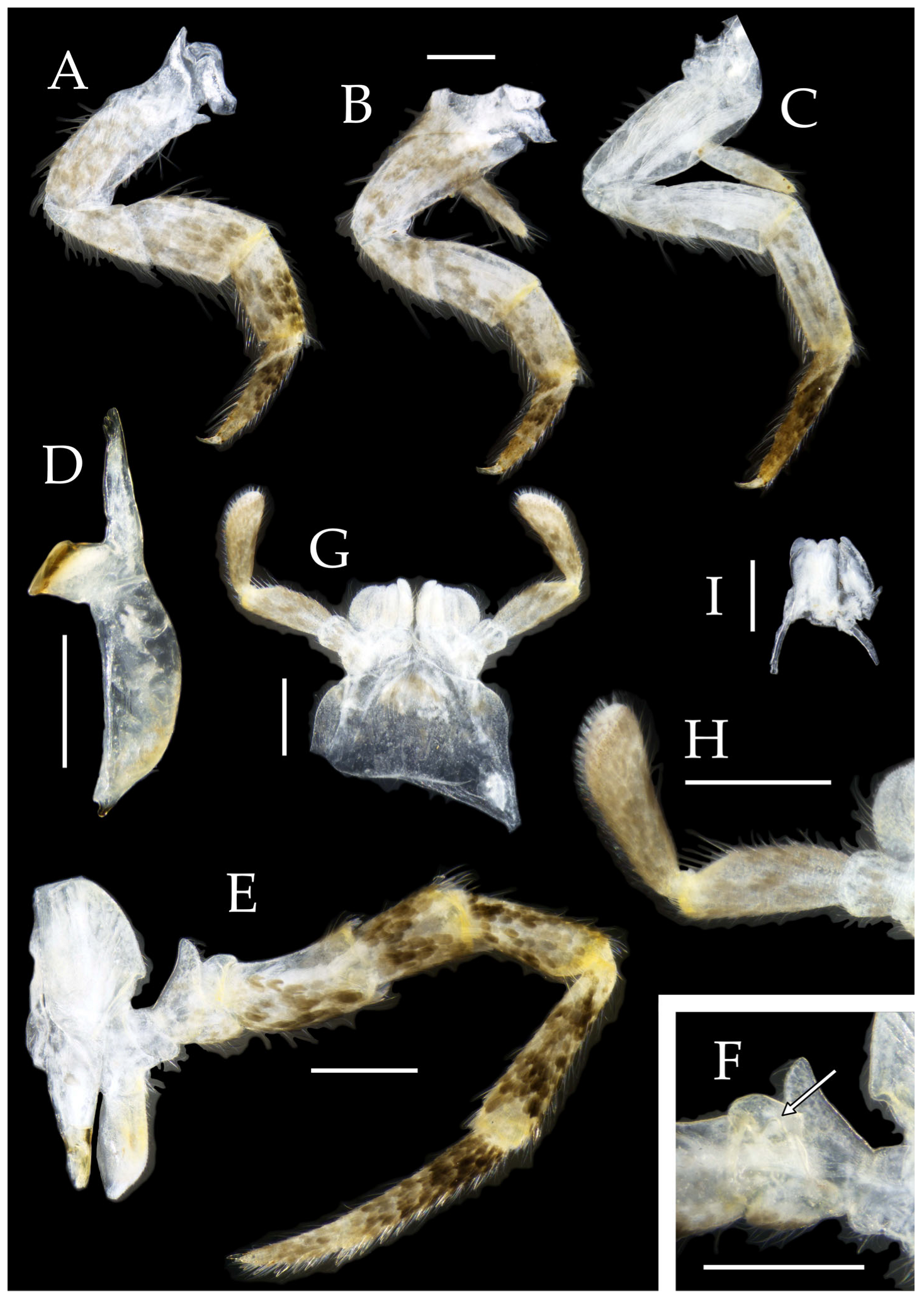
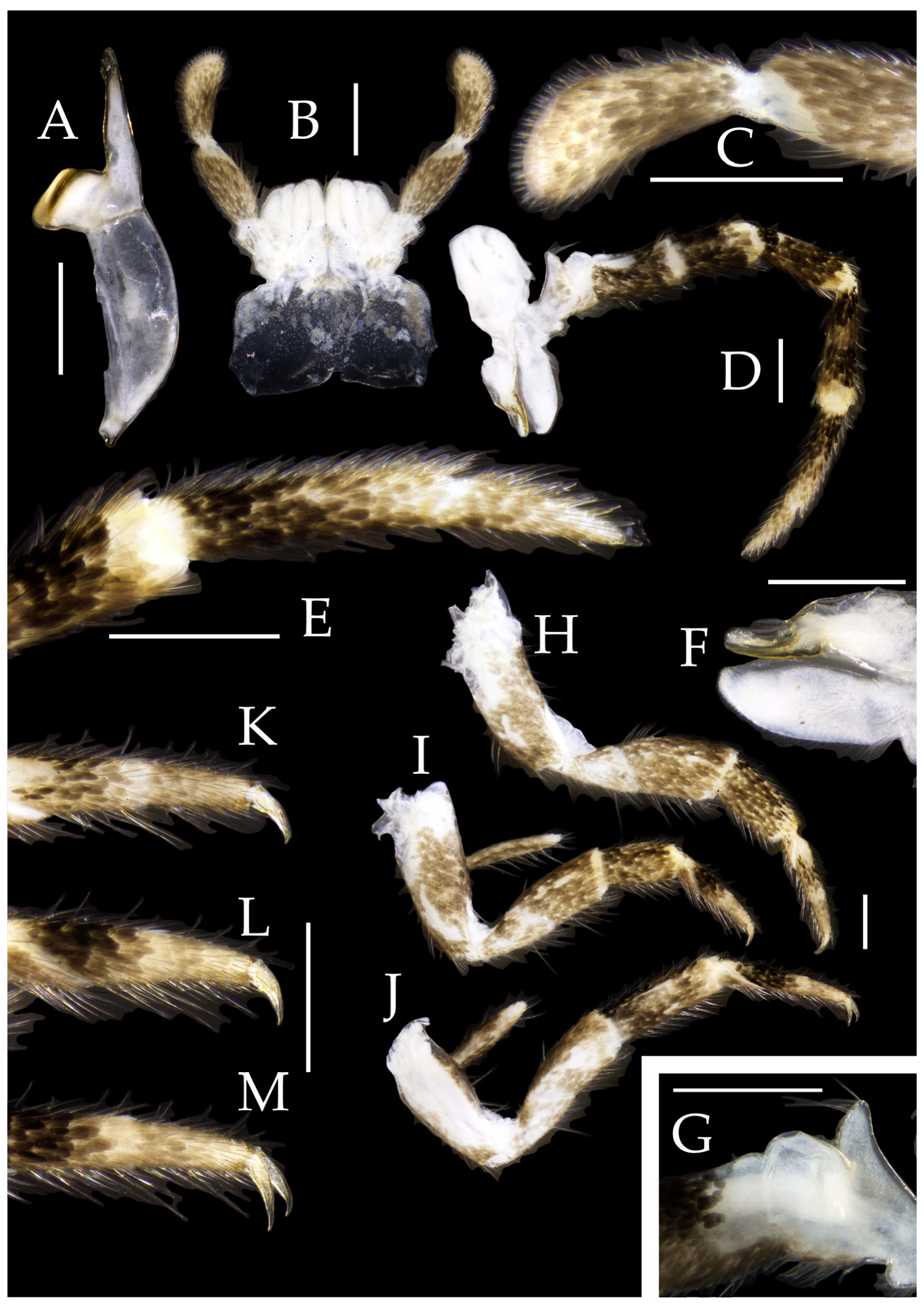
Mandibles typical, apex with four distal teeth (
Figure 3D).
Maxilla basal segment with one processus basalis and one inner protuberance (
Figure 3F). Male maxillary palp articles III–VII with well-developed long brush-like setae ventrally; female with long brush-like setae only on article 2. Ultimate article of maxillary palp subequal in length to penultimate article (
Figure 3E and
Figure 5D,E). Maxillary palp articles V–VII bearing transparent spines dorsally (
Figure 3E and
Figure 5E); numbers given in
Table 2.
Labial palp third article not modified, clavate, apex bearing sensory cone setae; numbers given in
Table 2. Male labial palp II and III bearing long hair-like setae; female lacking these (
Figure 3G,H and
Figure 5F,G).
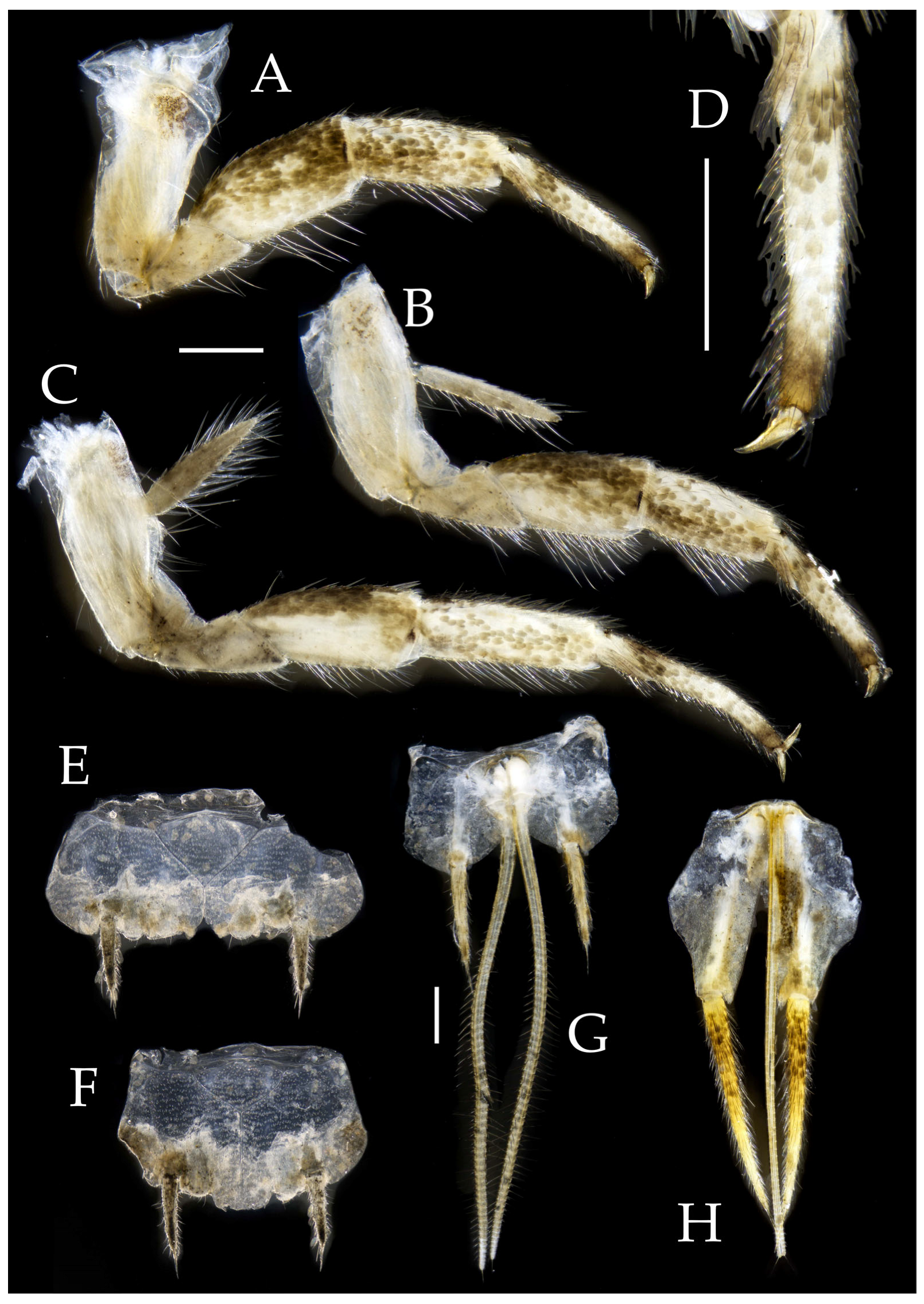
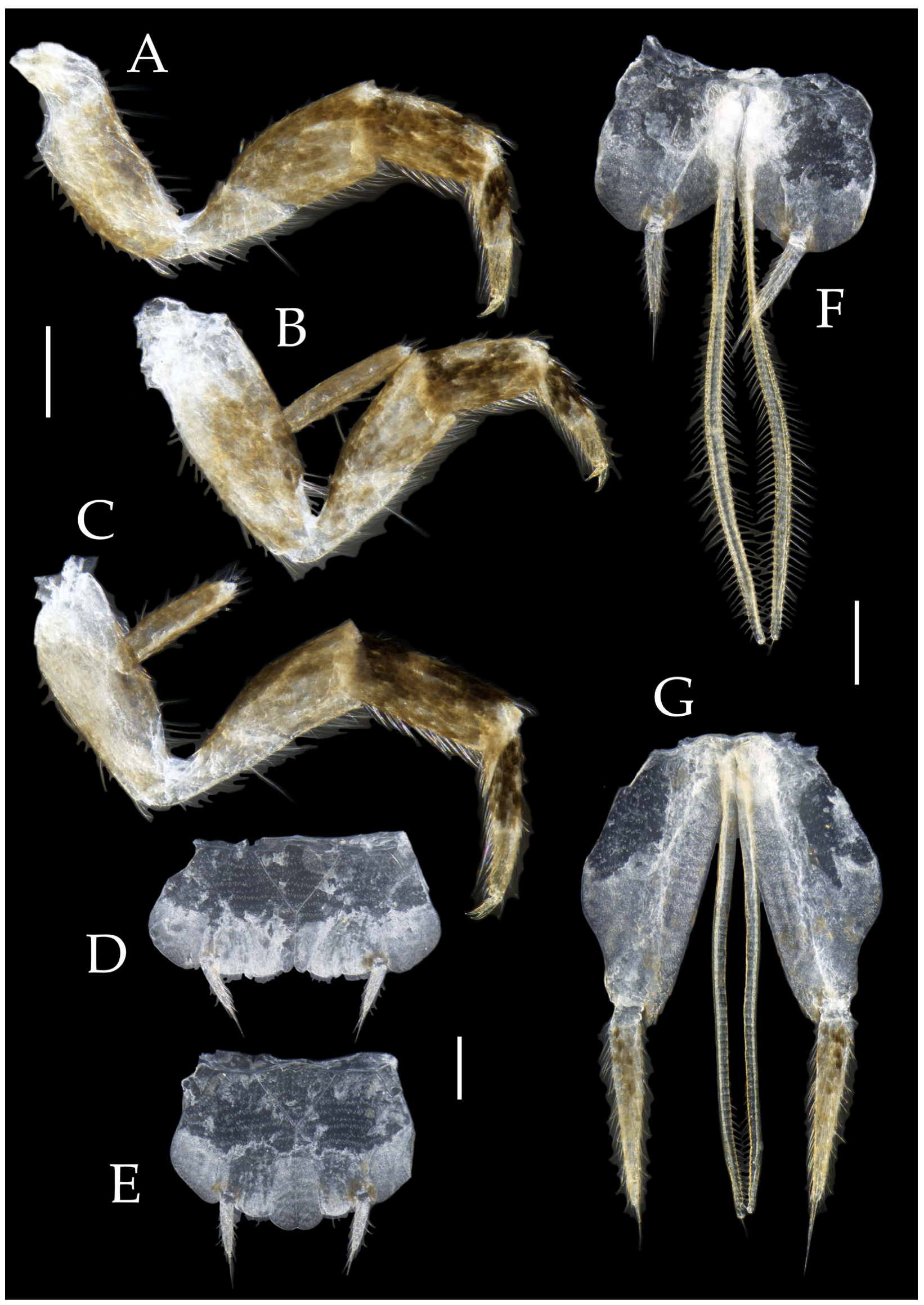
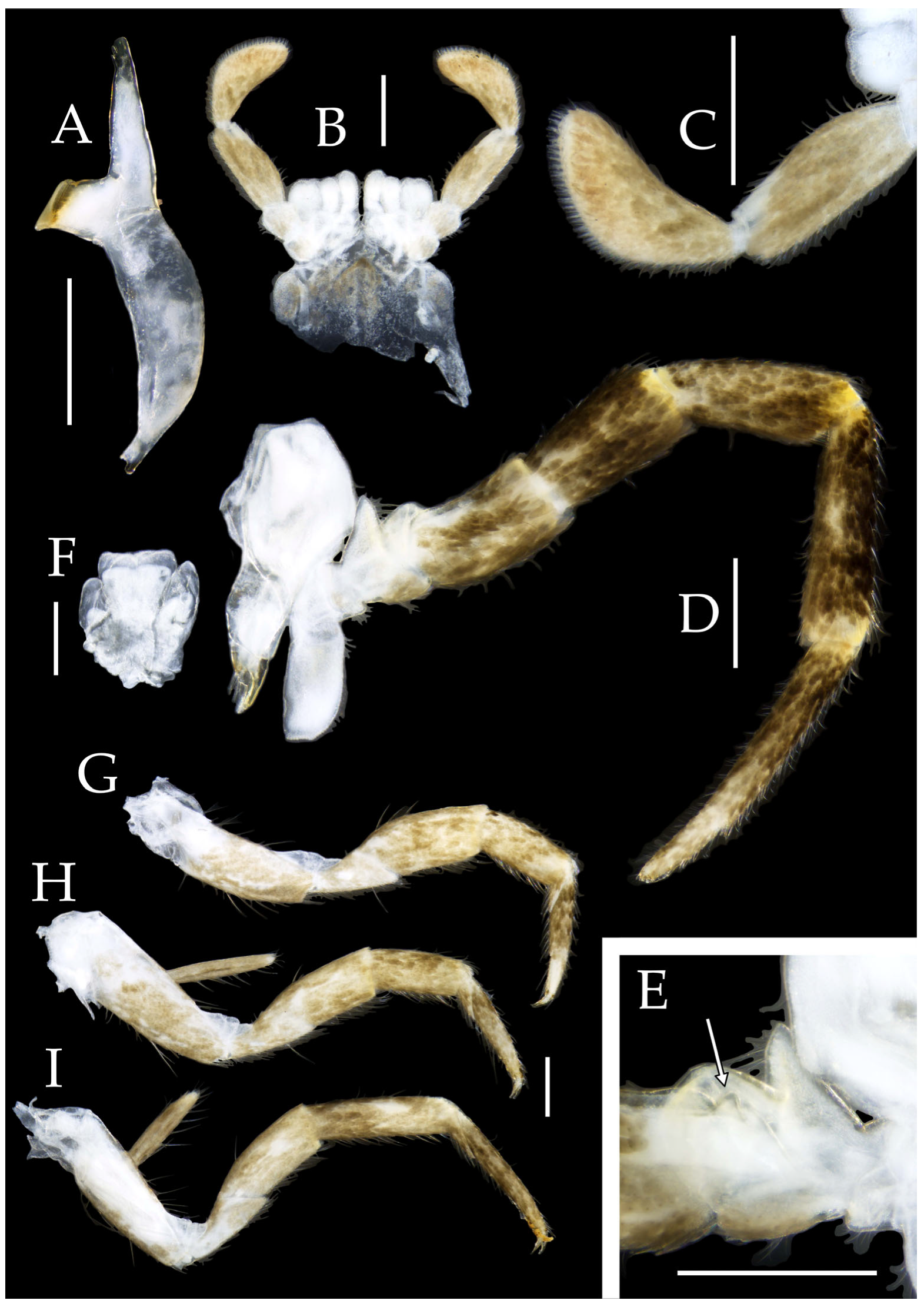
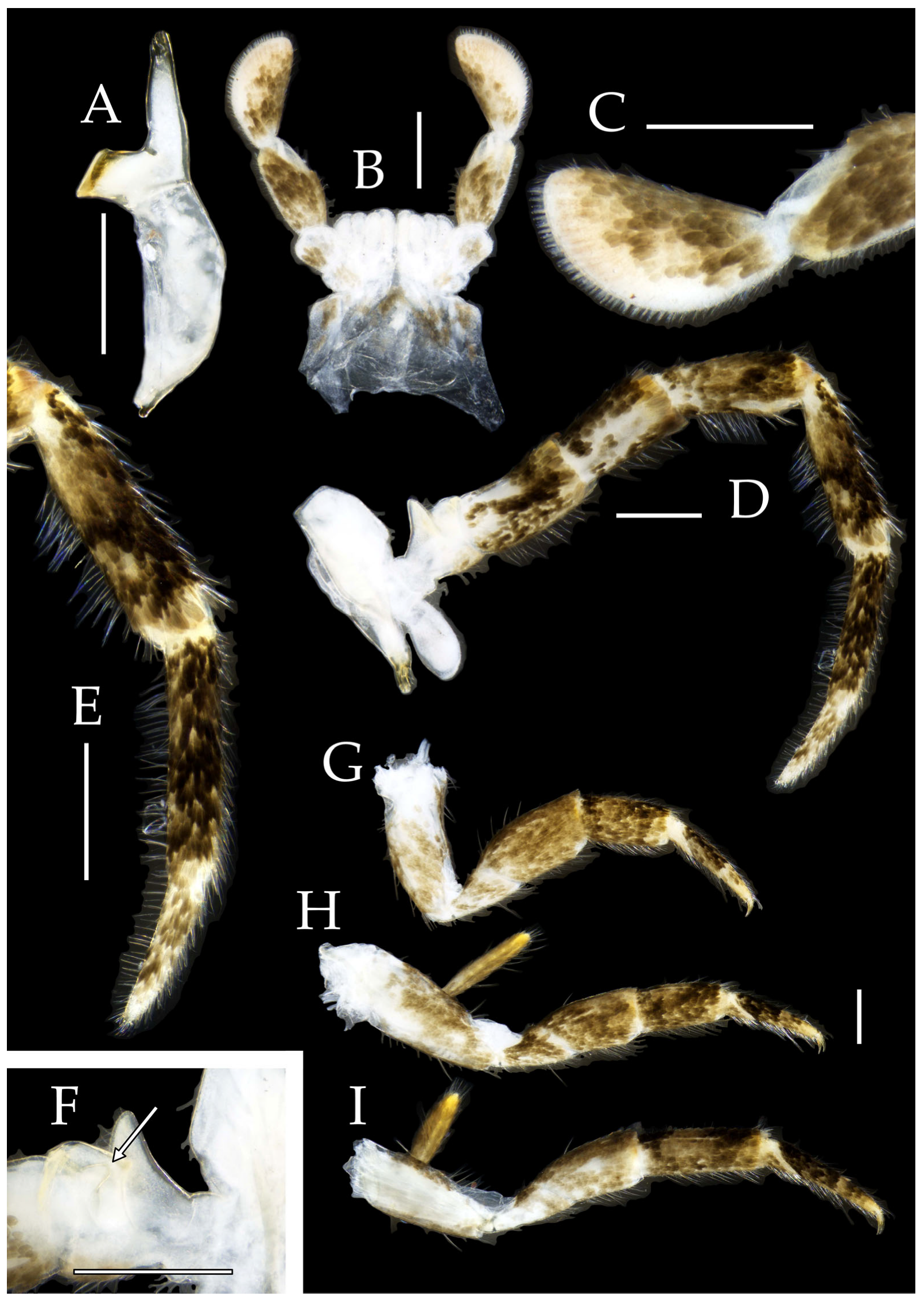
Figure 3.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–C) Foreleg, midleg, and hindleg. (D) Mandible. (E) Maxilla. (F) Maxillary inner protuberance, as indicates by the arrow. (G) Labium. (H) Labial palp. Scale bars: 500 μm.
Figure 3.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–C) Foreleg, midleg, and hindleg. (D) Mandible. (E) Maxilla. (F) Maxillary inner protuberance, as indicates by the arrow. (G) Labium. (H) Labial palp. Scale bars: 500 μm.
Thorax. Thorax normal. Foreleg femur not expanded, without sensory field; mid- and hindlegs with coxal styli. Coxae, trochanters, femora, and tibiae with long bristles ventrally; tarsi and hind tibiae bearing transparent needle-shaped setae ventrally; numbers given in
Table 3. Tibiae bearing black setae dorsally. Pretarsal claw structure normal (
Figure 3A–C and
Figure 6A–D).
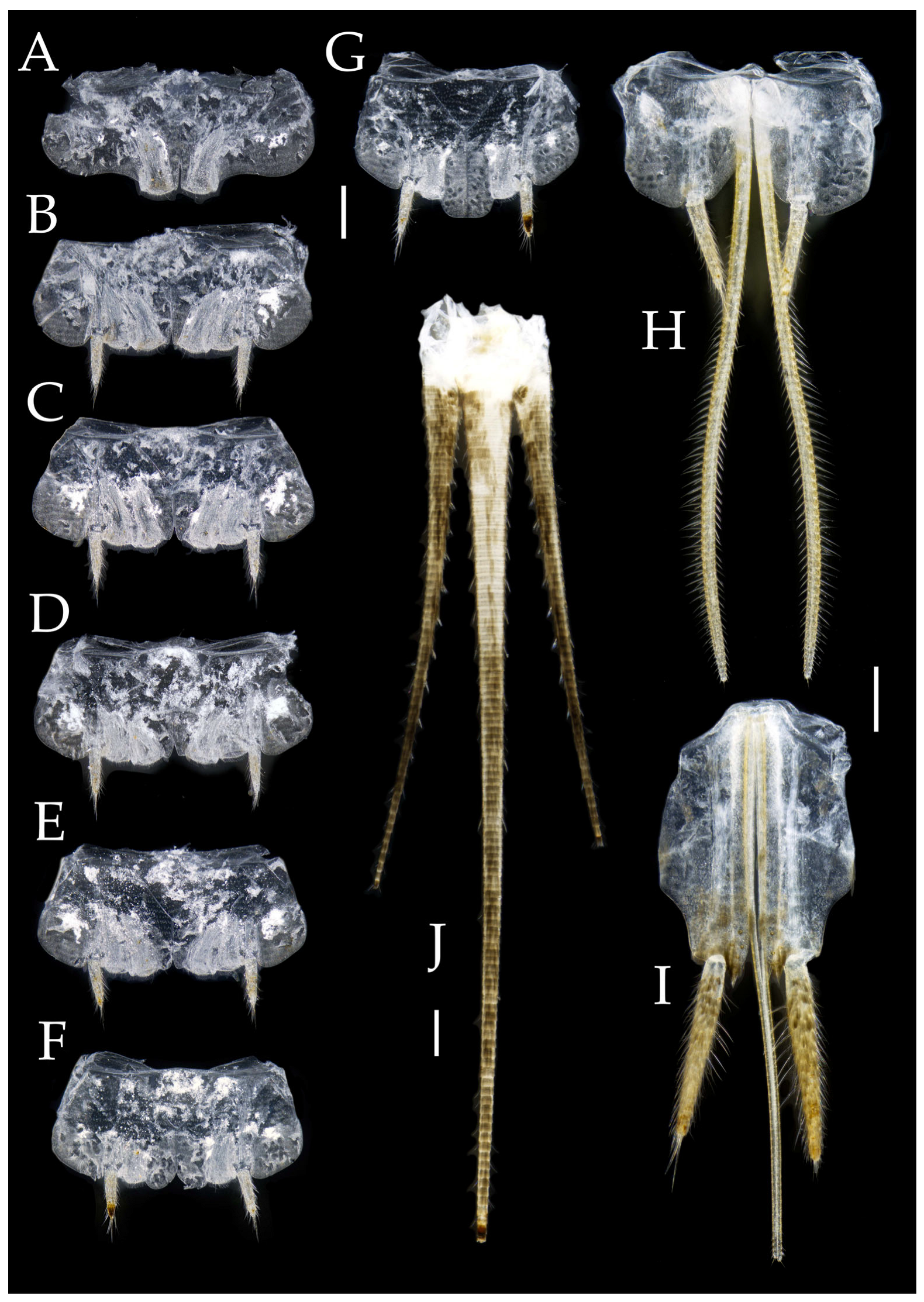
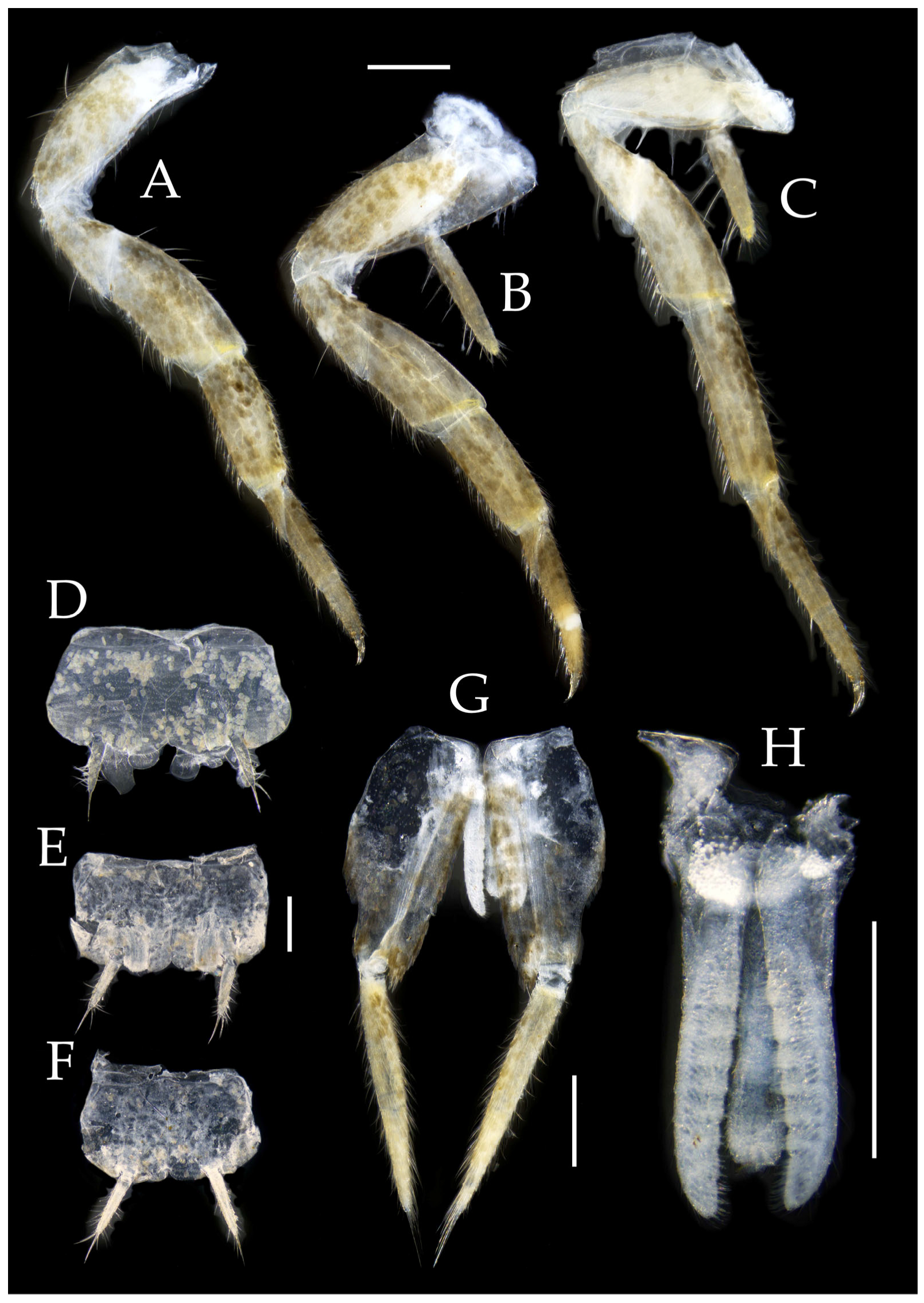
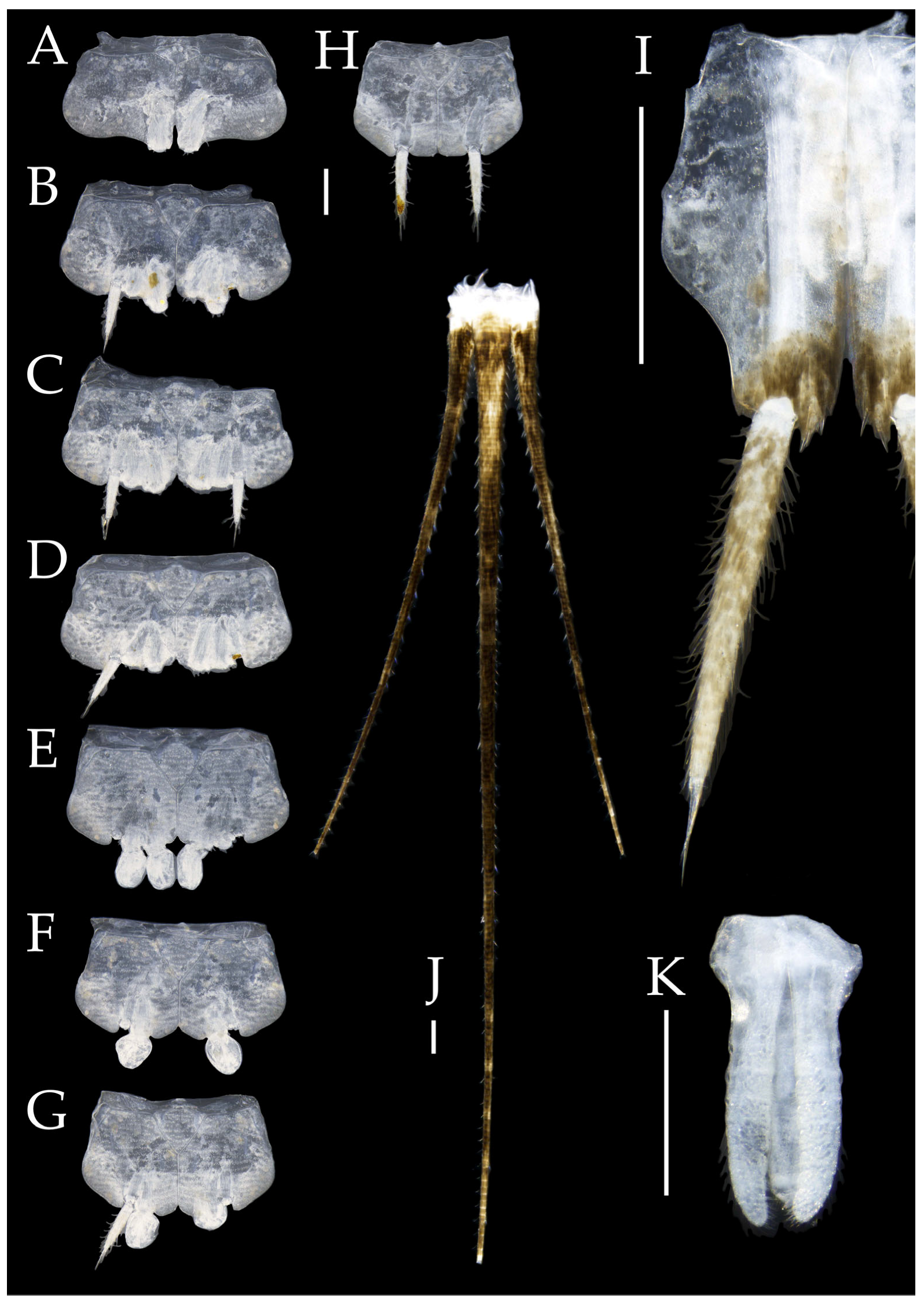
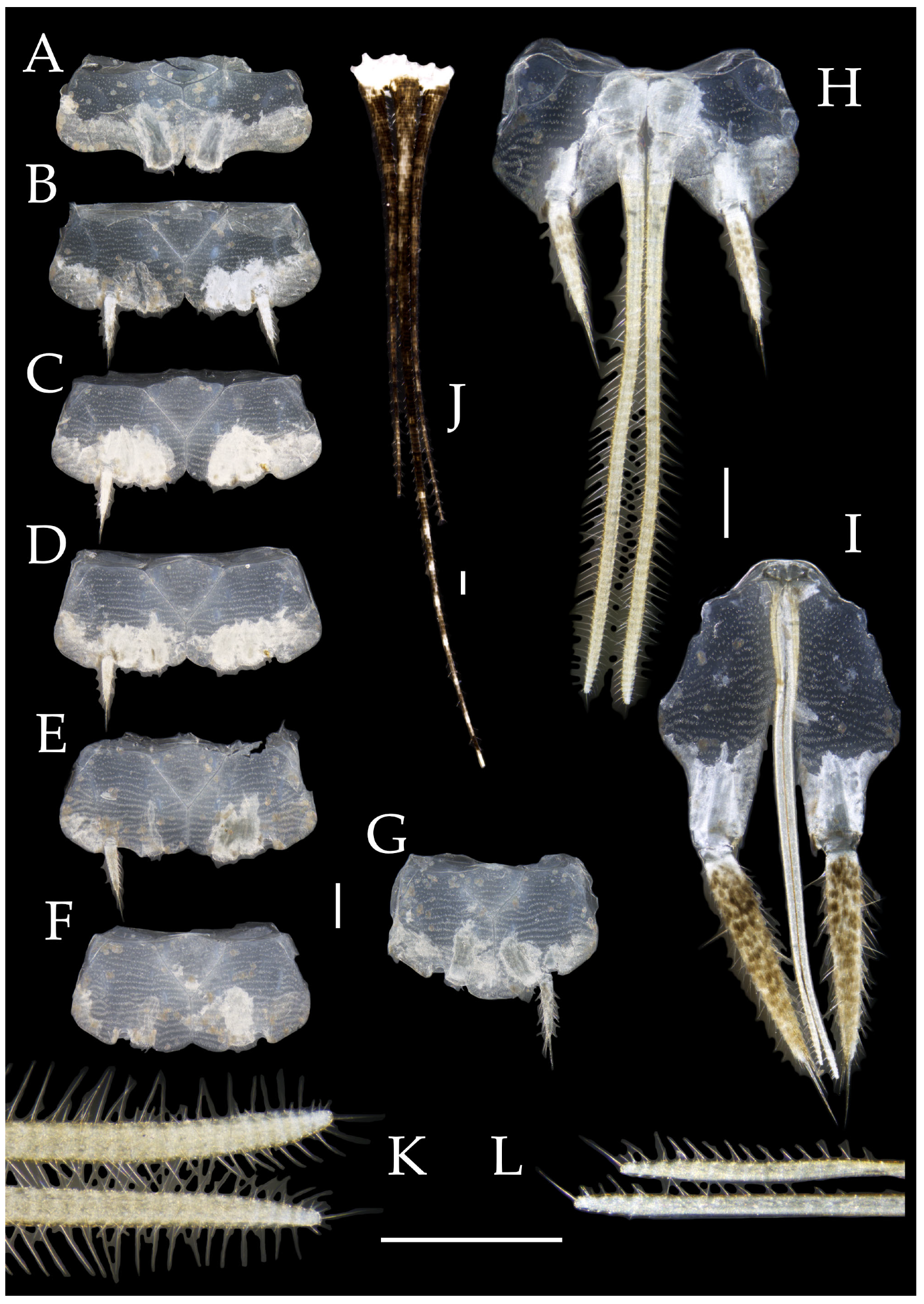
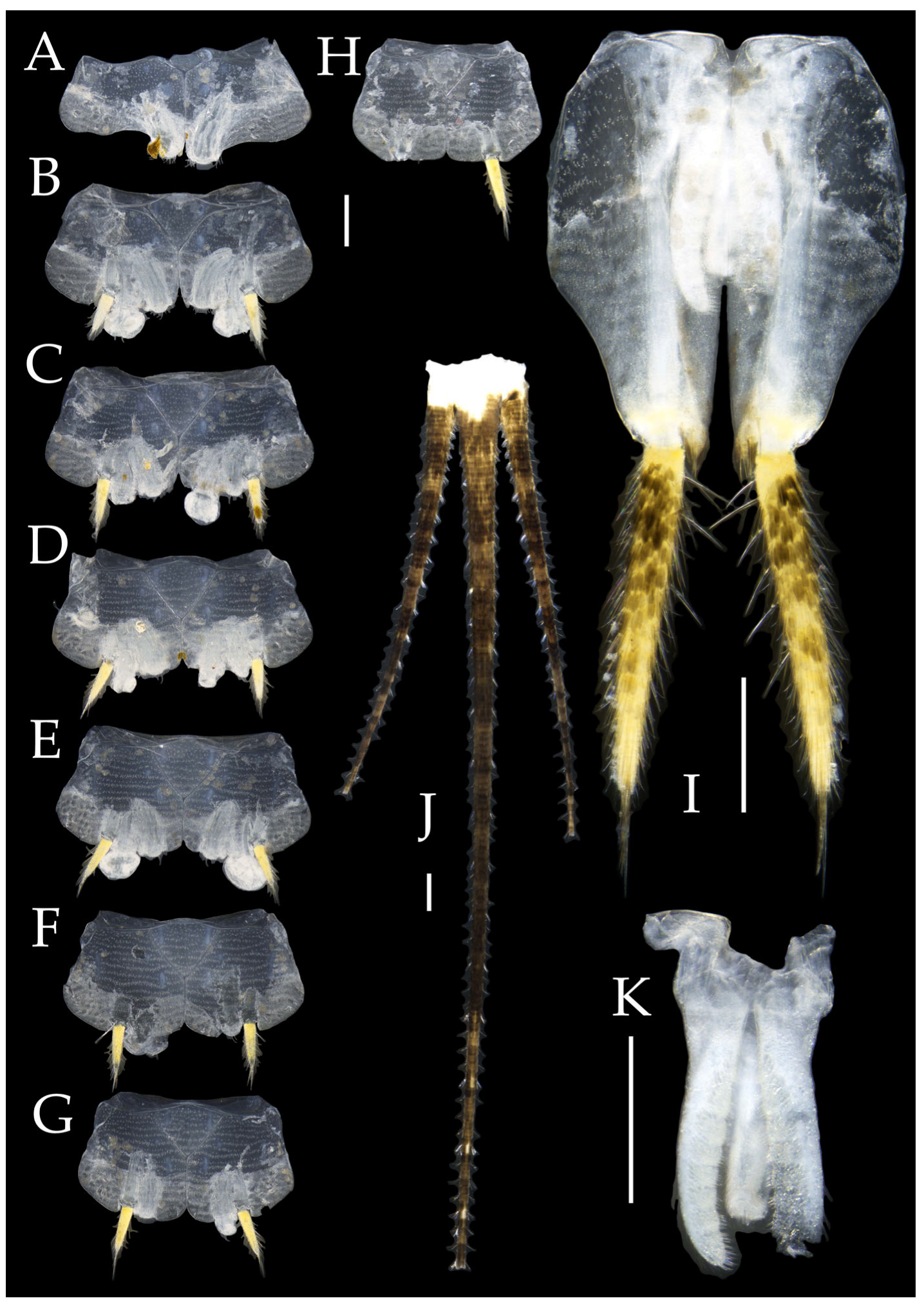
Figure 4.
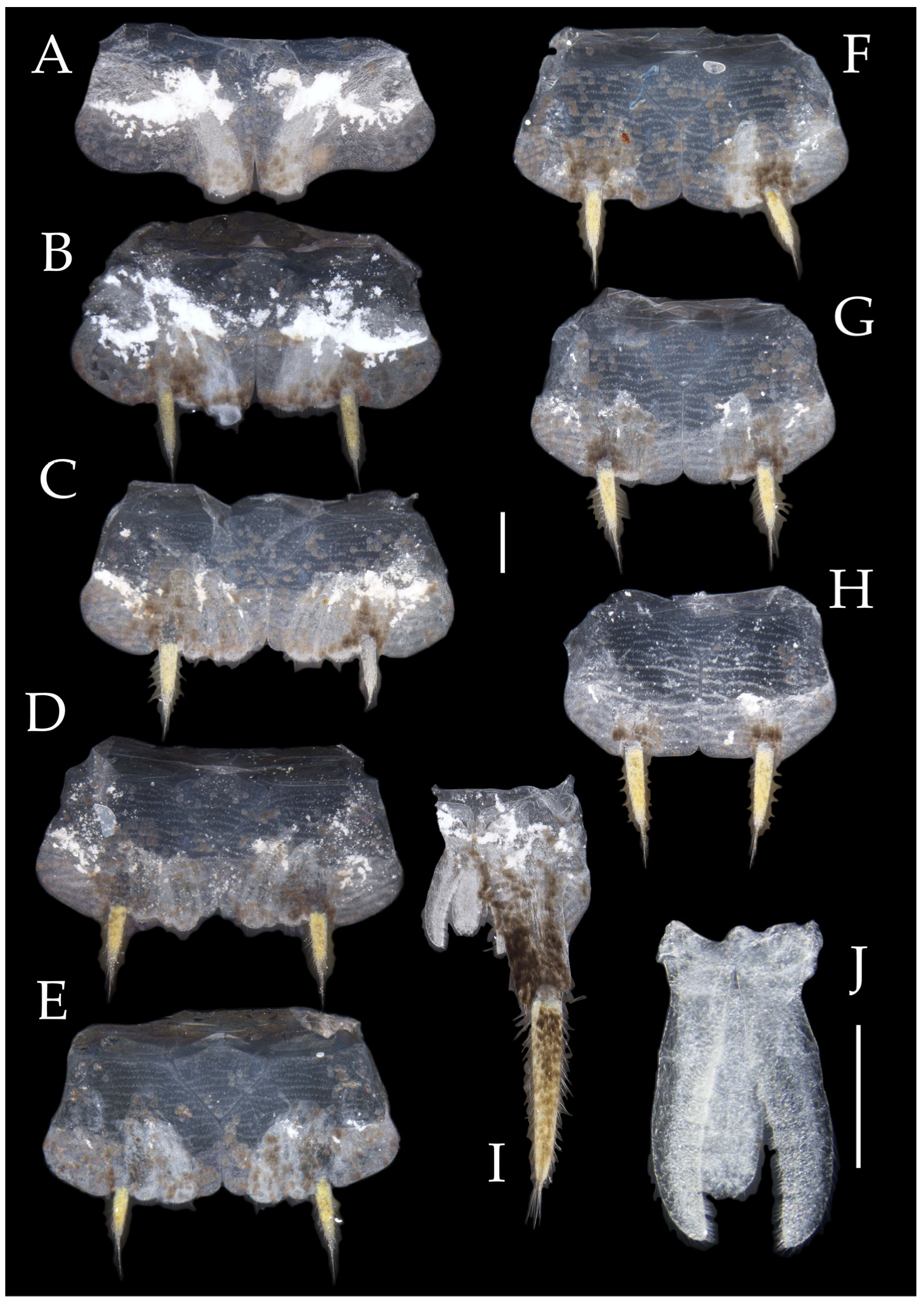
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Penis and parameres. (K) Caudal filament and cerci. Scale bars: 500 μm.
Figure 4.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Penis and parameres. (K) Caudal filament and cerci. Scale bars: 500 μm.
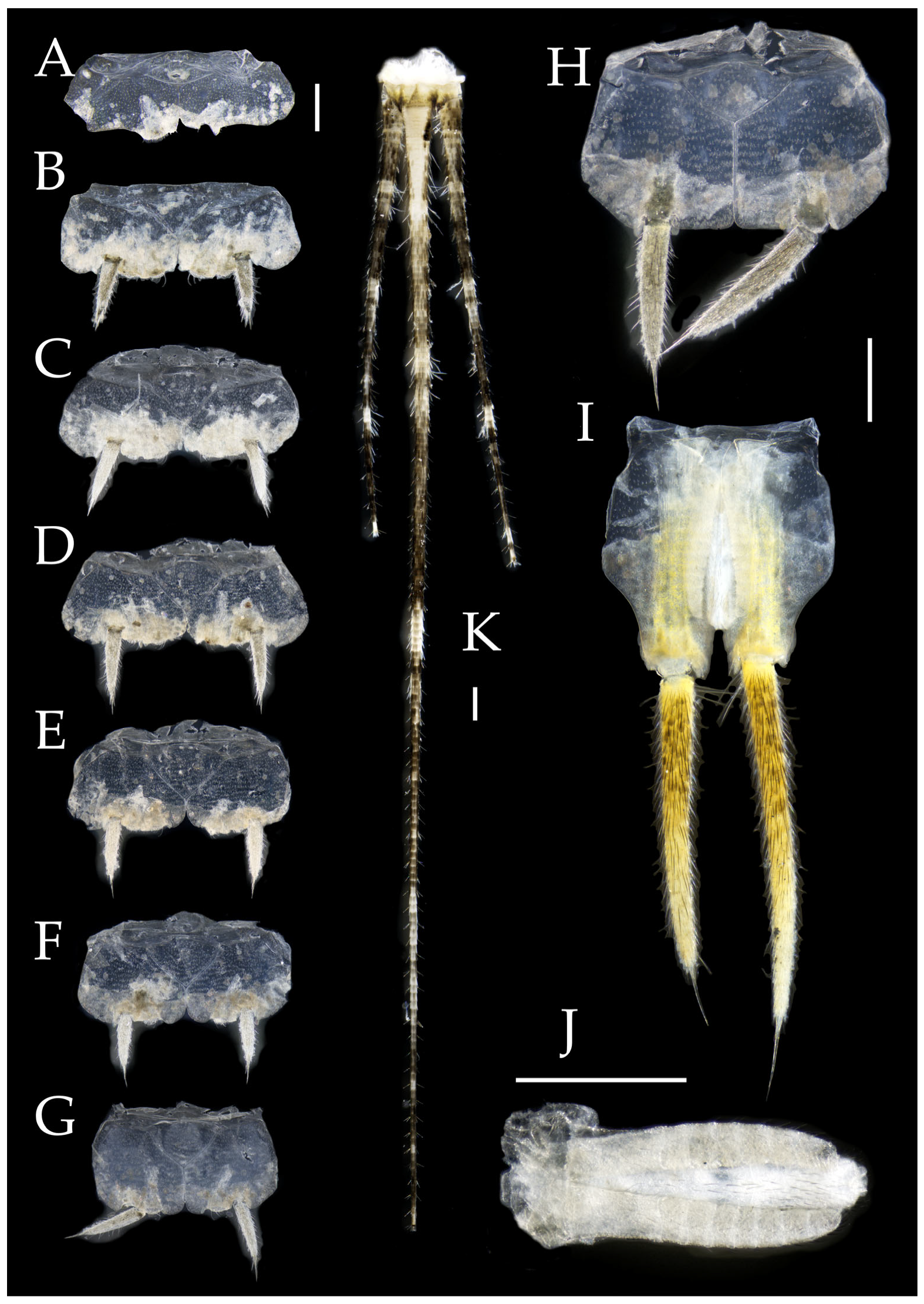
Abdomen. Abdominal coxites I, VI, and VII with one pair of retractile vesicles; coxites II–V with two pairs of retractile vesicles (
Figure 4A–G and
Figure 6E,F). Female coxite VII with inner lobes fused and extending posteriorly (
Figure 6F). Angles of posterior angles of abdominal sternites given in
Table 4. Ratios of styli length to coxites length on abdominal segment V, styli length to supporting spines length, and basal width to length of sternites given in
Table 5. Coxite IX with 2–4+2–4 transparent macrochaetae internally (
Figure 4I and
Figure 6H). Caudal filament laterally and cerci internally bearing deciduous long setae and supporting spines (
Figure 4K).
Figure 5.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Head, frontal view. (C) Ditto, lateral view. (D) Maxilla. (E) Maxillary palp articles V–VII. (F) Labium. (G) Labium palp. Scale bars: 500 μm.
Figure 5.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Head, frontal view. (C) Ditto, lateral view. (D) Maxilla. (E) Maxillary palp articles V–VII. (F) Labium. (G) Labium palp. Scale bars: 500 μm.
Coxite VIII without parameres. Coxite IX with penis and parameres (
Figure 4I,J). Penis bearing black setae, opening apically, surpassing parameres; parameres 1+7 segmented (
Figure 4J). Ovipositor of primary type, surpassing styli (including supporting spines) of coxite IX (
Figure 6G,H). Number of segments of anterior gonapophyses and posterior gonapophyses given in
Table 6.
Figure 6.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., paratype, female. (A–C) Foreleg, midleg, and hindleg. (D) Foreleg tarsus. (E–H) Abdominal sternites and coxites V, VII–IX. Scale bars: 500 μm.
Figure 6.
Pedetontus (Verhoeffilis) elegans Shen, Yang, Ji & Zhang sp. n., paratype, female. (A–C) Foreleg, midleg, and hindleg. (D) Foreleg tarsus. (E–H) Abdominal sternites and coxites V, VII–IX. Scale bars: 500 μm.
Differential diagnosis. Pedetontus (Verhoeffilis) elegans sp. n. is most striking due to its extremely large compound eyes and the well-developed ultimate article of the maxillary palp—subequal in length to the penultimate article. P. (V.) formosanus Silvestri, 1943, which also has large eyes and slender maxillary palps; they are morphologically the closest species, but the two can be distinguished by the shape of the lateral ocelli and the length of the article IV of maxillary palp. When viewed laterally from the front, the lateral ocelli of P. (V.) elegans sp. n. are constricted medially, presenting a dumbbell shape, whereas those of P. (V.) formosanus are almost clavate. The maxillary palp IV of P. (V.) elegans sp. n. is subequal in length to palp V, while that of P. (V.) formosanus is distinctly shorter than the palp V. P. (V.) diversicornis Silvestri, 1943, is also similar to P. (V.) elegans sp. n. Key distinguishing characters are antennae and penis length: the former has antennae shorter than the body and a penis subequal in length to the parameres, while the latter has antennae much longer than the body and a penis longer than the parameres.
Etymology. The specific epithet “elegans” (Latin adjective meaning “slender” or “graceful”) refers to the species’ slender antennae, elegant markings, and fascinating green eyes. The Chinese name for P. (V.) elegans sp. n. is 秀丽跳蛃.
Distribution. Zhejiang Province, China.
Zoobank: urn:lsid:zoobank.org:act:7B7D8D89-1579-40FA-A1EB-04C3B6DEB877
Type Specimens and Type Locality. Holotype, 1♀ (in 70% ethanol), paratypes: 1♂6♀ (in 70% ethanol), Huangyao Town, Hezhou City, Guangxi Zhuang Autonomous Region, China (24°14′ N 111°13 E), 18.XII.2024, collected by Jia-Yong Zhang. All type specimens deposited in the Animal Herbarium, Zhejiang Normal University, Jinhua, China.
Figure 7.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–C) Dorsal, lateral, and anterolateral view.
Figure 7.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–C) Dorsal, lateral, and anterolateral view.
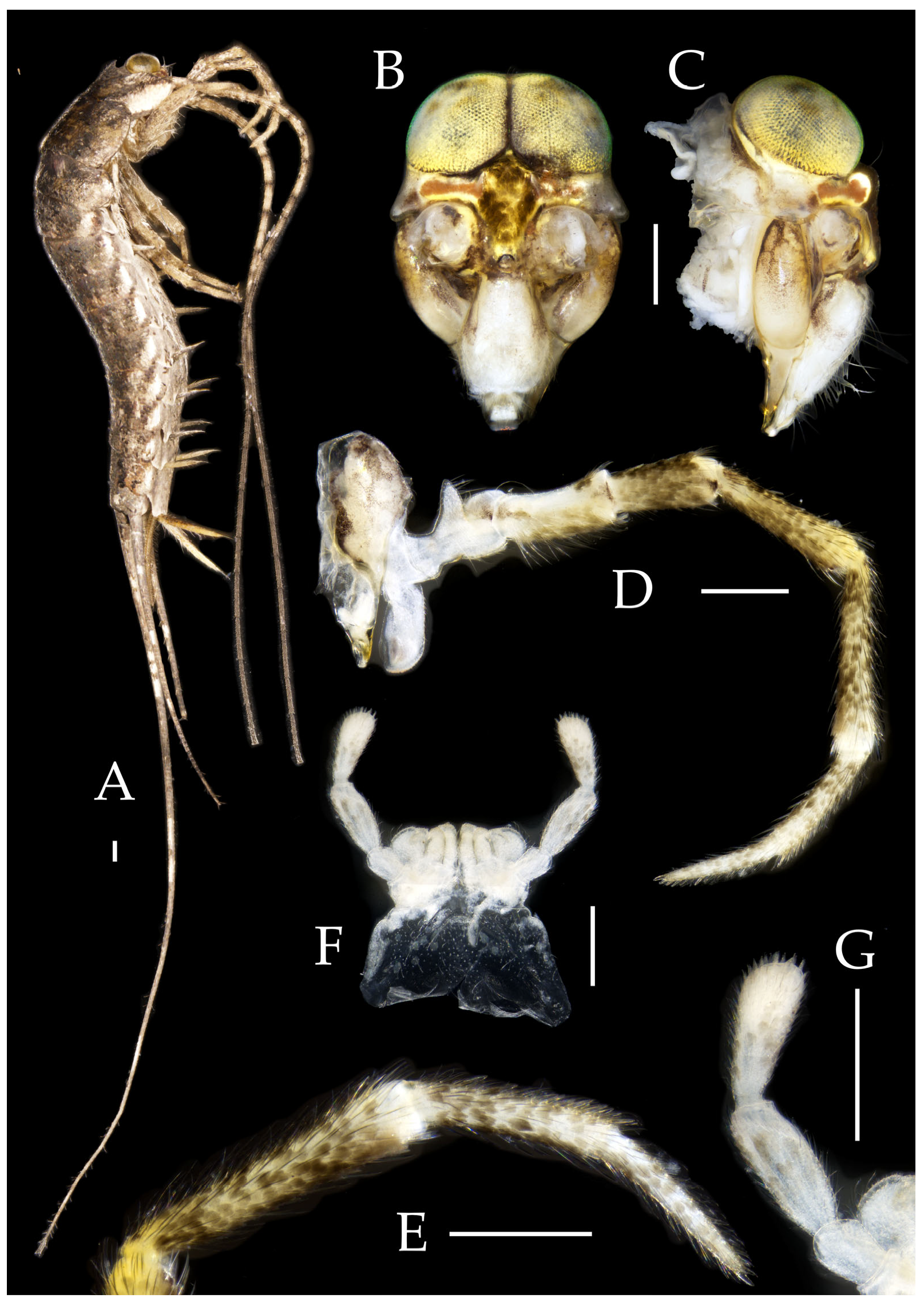
Description. Male body length 7.9 mm, female 7.6–8.9 mm. Body covered with grayish-green scales; posterior part of mesonotum with a dark arched marking; tergites of subsequent segments bearing variously sized paired black spots. Caudal filament and cerci with white annulations. Antennae slightly longer than body length; caudal filament nearly 1.5 times body length; cerci longer than half of body length (
Figure 7A,B).
Head. Compound eyes small, smooth, dark-colored, with indistinct yellow markings internally (
Figure 7C,
Figure 8C–F and
Figure 11B,C); ratios of eye length to width and contact line length to eye length given in
Table 1. Paired ocelli subinferior to compound eyes, black (fading to dark gray when preserved in 70% ethanol), with white borders, shoe-shaped. Ratios of ocellus length to width and distance between inner margins of ocelli to combined width of eyes given in
Table 1. Vertex without markings. Frons smooth, with long transparent bristles, covered with scales. Genae without setae. Clypeus and labrum with transparent bristles (
Figure 8C–E and
Figure 11B,C).
Antennae scaled except on flagellum; flagellum without annulations, bearing well-developed annulate setae; terminal chain divided into 10–13 segments. Scape length-to-width ratio 1.73–1.84 (
Figure 8B).
Mandibles typical, apex with four distal teeth (
Figure 9D).
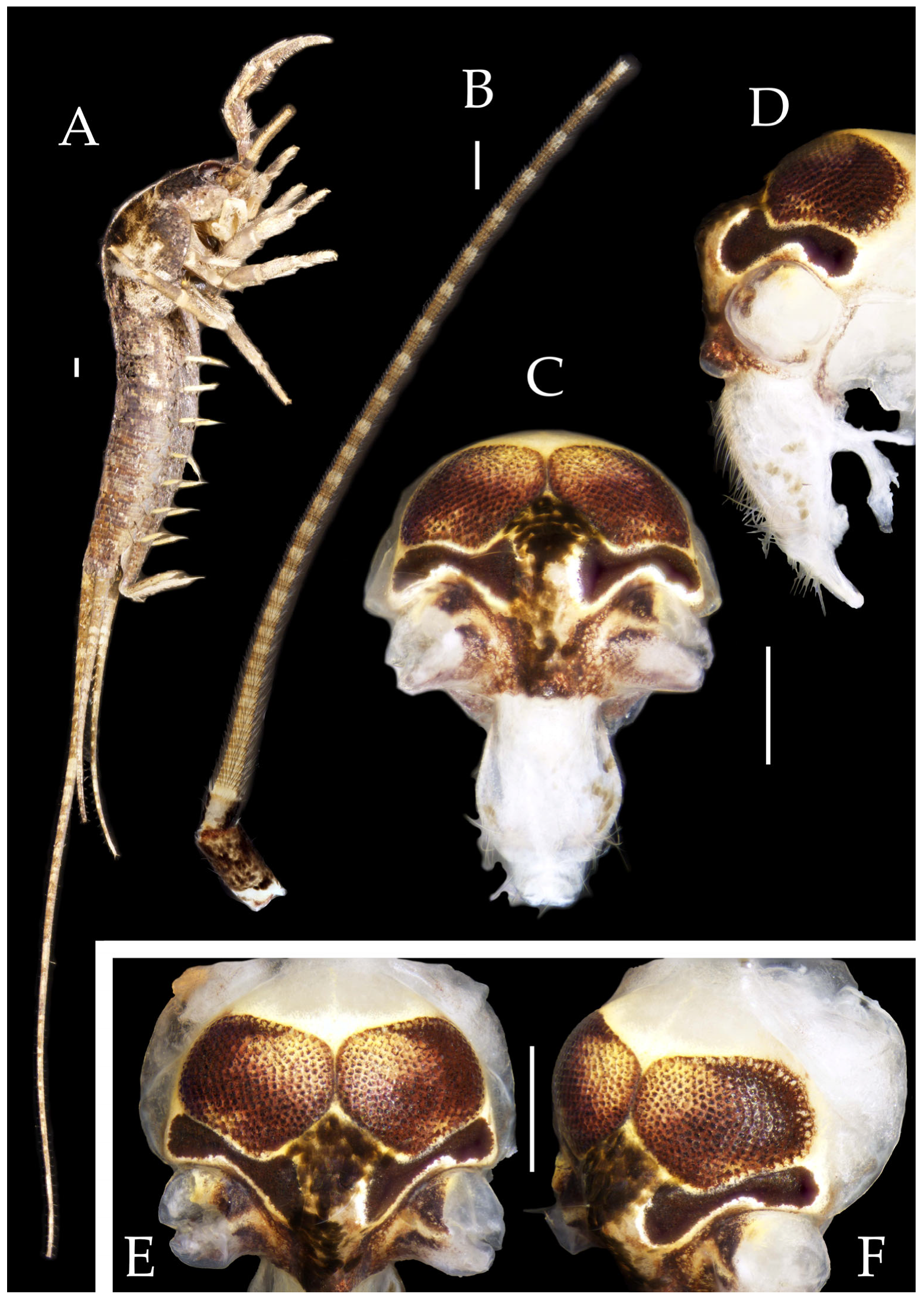
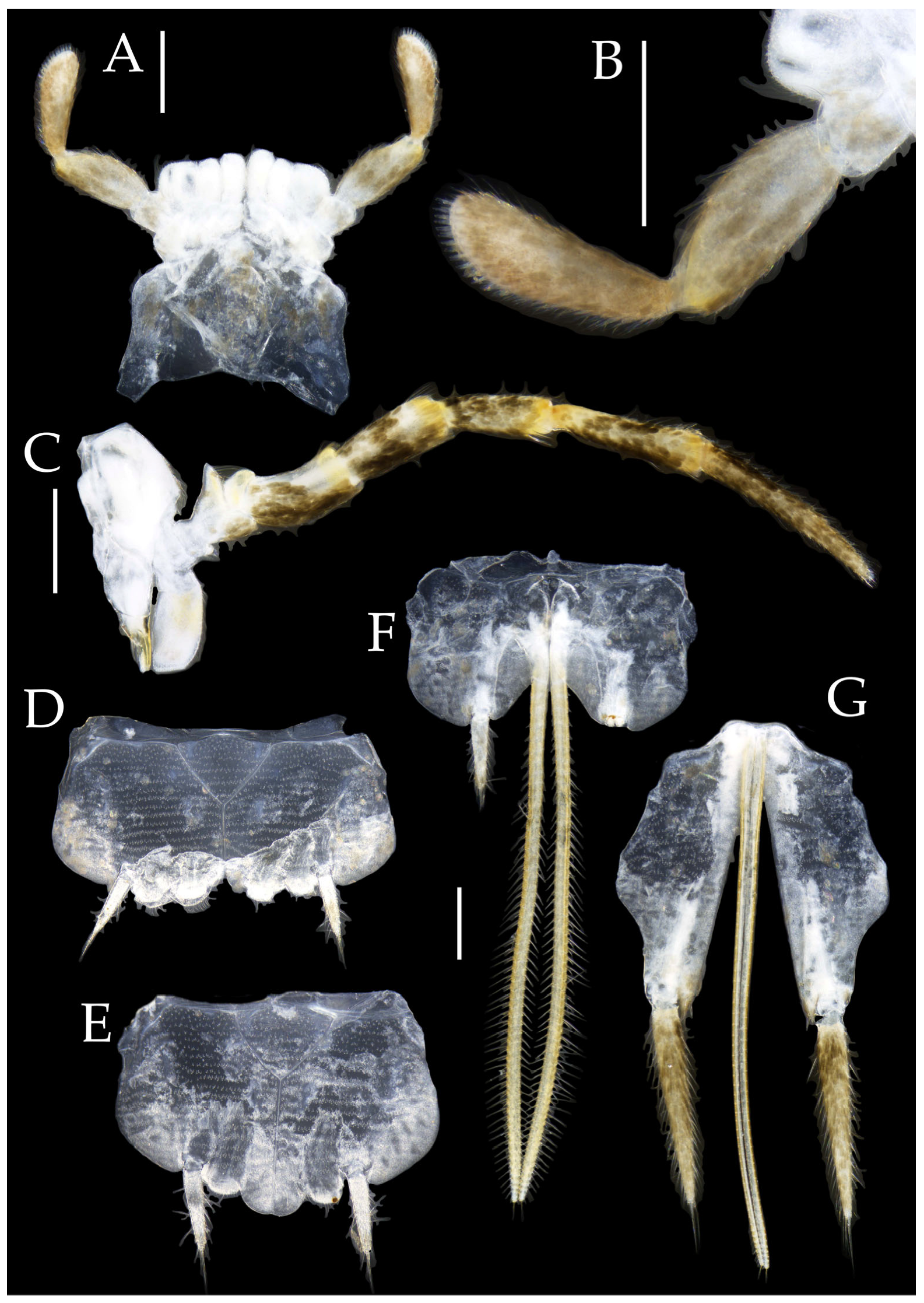
Figure 8.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. Scale bars: 500 μm.
Figure 8.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. Scale bars: 500 μm.
Maxillary palp I with one processus basalis and one inner protuberance (
Figure 9E,F and
Figure 11E). Male maxillary palp articles II–VII with well-developed short brush-like setae ventrally; female without. Ratio of ultimate article to penultimate article length 0.57–0.62 (
Figure 9E and
Figure 11E,F). Dorsal surface of maxillary palp articles V–VII with transparent spines (
Figure 9E and
Figure 11E,F); numbers given in
Table 2.
Male labial palp yellow, third segment modified, laterally expanded, apex with sensory cones; numbers given in
Table 2; ultimate article of female less swollen, clavate. Labial palp without long hair-like setae (
Figure 9G,H and
Figure 11G,H).
Hypopharynx structure typical (
Figure 9I).
Thorax. Thorax normal. Foreleg femur not expanded, without sensory field; mid- and hind legs with coxal styli. Ventral surface of legs with long bristles, without needle-shaped setae. Pretarsal claw structure normal (
Figure 9A–C and
Figure 12A–C).
Figure 9.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–C) Foreleg, midleg and hindleg. (D) Mandible. (E) Maxilla. (F) Maxillary inner protuberance, as indicates by the arrow. (G) Labium. (H) Labial palp. (I) Hypopharynx. Scale bars: 500 μm.
Figure 9.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–C) Foreleg, midleg and hindleg. (D) Mandible. (E) Maxilla. (F) Maxillary inner protuberance, as indicates by the arrow. (G) Labium. (H) Labial palp. (I) Hypopharynx. Scale bars: 500 μm.
Abdomen. Abdominal coxites I, VI, and VII with one pair of retractile vesicles; coxites II–V with two pairs of retractile vesicles (
Figure 10A–G and
Figure 12D,E); inner lobes of female coxite VII fused and posteriorly extended (
Figure 10G). Ratios of styli length to coxites length on abdominal segment V, styli length to supporting spines length, and basal width to length of sternites given in
Table 5. Coxite IX medially with 4–8+4–8 transparent macrochaetae (
Figure 10I and
Figure 12G). Caudal filament laterally and cerci medially with deciduous long hairs and supporting spines (
Figure 10J).
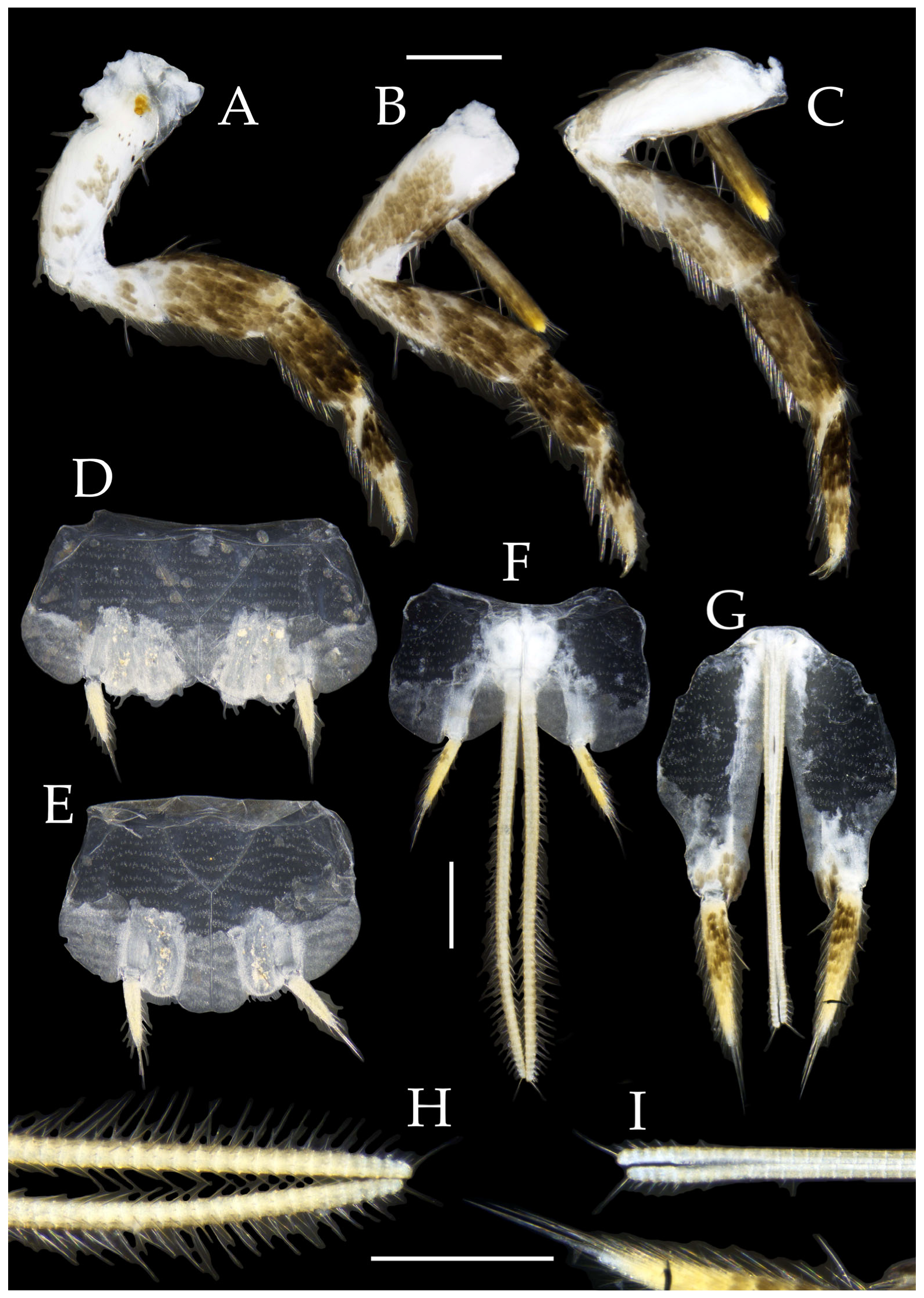
Figure 10.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. Scale bars: 500 μm.
Figure 10.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. Scale bars: 500 μm.
Coxite VIII without parameres, coxite IX with penis and parameres (
Figure 12F,G). Penis opening apically, slightly shorter than parameres; parameres 1+8 segmented (
Figure 10H). Ovipositor of primary type, surpassing styli (including supporting spines) of coxite IX (
Figure 10H,I). Number of segments of anterior gonapophyses and posterior gonapophyses given in
Table 6.
Figure 11.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., paratype, male. (A) Habitus, lateral view. (B) Head, frontal view. (C) Ditto, lateral view. (D) Mandible. (E) Maxilla. (F) Maxillary palp articles V–VII. (G) Labium. (H) Labium palp. Scale bars: 500 μm.
Figure 11.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., paratype, male. (A) Habitus, lateral view. (B) Head, frontal view. (C) Ditto, lateral view. (D) Mandible. (E) Maxilla. (F) Maxillary palp articles V–VII. (G) Labium. (H) Labium palp. Scale bars: 500 μm.
Figure 12.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., paratype, male. (A–C) Foreleg, midleg, and hindleg. (D–G) Abdominal sternites and coxites V, VII–IX. (H) Penis and parameres. Scale bars: 500 μm.
Figure 12.
Pedetontus (Verhoeffilis) hezhouensis Shen, Yang, Ji & Zhang sp. n., paratype, male. (A–C) Foreleg, midleg, and hindleg. (D–G) Abdominal sternites and coxites V, VII–IX. (H) Penis and parameres. Scale bars: 500 μm.
Differential diagnosis. Pedetontus (Verhoeffilis) hezhouensis sp. n. is morphologically most similar to P. (V.) sauterii Silvestri, 1943, from Taiwan, China. They differ primarily in the length of the contact line of the eyes: in the former, the ratio of this structure’s length to the length of the eye is <0.4, while in the latter, it is >0.4.
Etymology. The species is named after its type locality, Hezhou City. The Chinese name for P. (Verhoeffilis) hezhouensis sp. n. is 贺州跳蛃.
Distribution. Hezhou City, Guangxi Zhuang Autonomous Region, China.
Zoobank: urn:lsid:zoobank.org:act:4C21B2F2-5BD0-4D46-BD78-7A78A941DCA0
Type Specimens and Type Locality. Holotype, 1♂ (in 70% ethanol), paratypes: 15♂2♀ (in 70% ethanol), China, Guangxi Zhuang Autonomous Region, Jinxiu County, Dayao Mountain (24°6′4.88″ N, 110°9′37.69″ E, 947 m), 1.V.2023, collected by Wen-Yong Feng. All type specimens deposited in the Animal Herbarium, Zhejiang Normal University, Jinhua, China.
Figure 13.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n. (A–C) Lateral, dorsal, and anterior view.
Figure 13.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n. (A–C) Lateral, dorsal, and anterior view.
Description. Male body length 11.2 mm, female 9.7 mm. Body covered with dark brown scales; mesonotum with an arched dark marking; subsequent nota bearing paired dark patches; abdominal nota patterned with three longitudinal pale diamond-shaped markings. Antennae equal to or slightly longer than body length; caudal filament nearly 1.5 times body length; cerci about half as long as body length (
Figure 13A,B).
Figure 14.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. Scale bars: 500 μm.
Figure 14.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. Scale bars: 500 μm.
Head. Compound eyes smooth, deep reddish-brown, with indistinct yellow blotches (
Figure 14C–F and
Figure 17B,C); ratios of eye length to width and contact line length to eye length given in
Table 1. Ocelli dark brown with white borders, dumbbell-shaped. Ratios of ocellus length to width and distance between inner margins of ocelli to combined width of eyes given in
Table 1. Vertex without markings. Frons convex, with long transparent bristles, scaled. Genae without setae. Clypeus and labrum with transparent bristles (
Figure 14C–F and
Figure 17B,C).
Figure 15.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–C) Foreleg, midleg, and hindleg. (D) Mandible. (E) Maxilla. (F) Maxillary palp article VI and VII. (G) Maxillary inner protuberance, as indicates by the arrow. (H) Labium. Scale bars: 500 μm.
Figure 15.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–C) Foreleg, midleg, and hindleg. (D) Mandible. (E) Maxilla. (F) Maxillary palp article VI and VII. (G) Maxillary inner protuberance, as indicates by the arrow. (H) Labium. Scale bars: 500 μm.
Antennae scaled except on flagellum; flagellum with pale annulations, bearing well-developed annulate setae; terminal chain divided into 8–10 segments. Scape length-to-width ratio 1.86–1.91 (
Figure 14B).
Mandibles typical, apex with four distal teeth (
Figure 15D).
Maxillary palp I with one processus basalis and one inner protuberance (
Figure 15E,G and
Figure 17D). Male maxillary palp articles II–VII with well-developed short brush-like setae ventrally; female without. Ratio of ultimate article to penultimate article length 0.69–0.85. Dorsal surface of maxillary palp articles V–VII with transparent spines (
Figure 15E,F and
Figure 17D); numbers given in
Table 2.
Labial palp third segment modified, extremely laterally expanded, apex with sensory cones; numbers given in
Table 2; ultimate article of female less swollen, apex without sensory cones. Labial palp without long hair-like setae (
Figure 15H and
Figure 17F,G).
Thorax. Thorax normal. Foreleg femur not expanded, without sensory field; mid- and hind legs with coxal styli, apex of coxal styli yellow. Ventral surface of legs with long bristles, without needle-shaped setae. Pretarsal claw structure normal (
Figure 15A–C and
Figure 18A–C).
Figure 16.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Penis and parameres. Scale bars: 500 μm.
Figure 16.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Penis and parameres. Scale bars: 500 μm.
Abdomen. Abdominal coxites I, VI, and VII with one pair of retractile vesicles; coxites II–V with two pairs of retractile vesicles; coxites I–IX each with a pair of yellow styli (
Figure 16A–I and
Figure 18D–G); inner lobes of female coxite VII fused and posteriorly extended (
Figure 18E). Ratios of styli length to coxites length on abdominal segment V, styli length to supporting spines length, and basal width to length of sternites given in
Table 5. Coxite IX medially with 3–4+3–4 transparent macrochaetae (
Figure 16I and
Figure 18G). Caudal filament laterally and cerci medially with deciduous long hairs and supporting spines (
Figure 13B and
Figure 14A).
Figure 17.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Head, frontal view. (C) Ditto, lateral view. (D) Maxilla. (E) Hypophraynx. (F) Labium. (G) Labium palp. Scale bars: 500 μm.
Figure 17.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Head, frontal view. (C) Ditto, lateral view. (D) Maxilla. (E) Hypophraynx. (F) Labium. (G) Labium palp. Scale bars: 500 μm.
Coxite VIII without parameres. Coxite IX with penis and parameres (
Figure 16I,J). Penis opening apically, slightly shorter than parameres; parameres 1+7 segmented (
Figure 16J). Ovipositor of primary type, surpassing styli (including supporting spines) of coxite IX (
Figure 18G). Number of segments of anterior gonapophyses and posterior gonapophyses given in
Table 6.
Figure 18.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A–C) Foreleg, midleg, and hindleg. (D–G) Abdominal sternites and coxites V, VII–IX. Scale bars: 500 μm.
Figure 18.
Pedetontus (Verhoeffilis) jinxiuensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A–C) Foreleg, midleg, and hindleg. (D–G) Abdominal sternites and coxites V, VII–IX. Scale bars: 500 μm.
Differential diagnosis. Pedetontus (Verhoeffilis) jinxiuensis sp. n. is morphologically very similar to P. (V.) xanthospilus sp. n. from Zhaoqing, Guangdong Province, but can be distinguished by the form of the labial palps: the former has a highly modified ultimate article (width significantly greater than length), with the anterolateral part of article I in males also laterally expanded. Additionally, the apex of the female ultimate article lacks sensory cones—an extremely rare feature. In contrast, although the labial palps of P. (V.) xanthospilus sp. n. are also swollen, they are far less modified than in P. (V.) jinxiuensis sp. n.; article I lacks specialization, and both sexes possess sensory cones at the apex of the ultimate article.
Etymology. The species is named after its type locality, Jinxiu County. The Chinese name for P. (V.) jinxiuensis is 金秀跳蛃.
Distribution. Jinxiu County, Guangxi Zhuang Autonomous Region, China.
Zoobank: urn:lsid:zoobank.org:act:4D83A441-4098-4384-B529-75E5B3D303A4
Type Specimens and Type Locality. Holotype, 1♂ (in 70% ethanol), paratypes: 4♂ 6♀ (in 70% ethanol), collected from Jiangnan District, Nanning City, Guangxi Zhuang Autonomous Region, China, 18 II.2025, by Jia-Yong Zhang. All type specimens deposited in the Animal Herbarium, Zhejiang Normal University, Jinhua, China.
Figure 19.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n. (A–C) Dorsal, anterolateral and lateral view.
Figure 19.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n. (A–C) Dorsal, anterolateral and lateral view.
Description. Male body length 10.6 mm, female 11.6 mm. Body covered with grayish-green scales. Antennae slightly shorter than body length; caudal filament slightly longer than body length; cerci about three-quarters as long as body length (
Figure 19A,C).
Figure 20.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. Scale bars: 500 μm.
Figure 20.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. Scale bars: 500 μm.
Head. Compound eyes smooth, black, without markings (
Figure 19B,
Figure 20C–F and
Figure 23C); ratios of eye length to width and contact line length to eye length given in
Table 1. Ocelli dark brown with white borders, dumbbell-shaped. Ratios of ocellus length to width and distance between inner margins of ocelli to combined width of eyes given in
Table 1. Vertex without markings. Frons convex, with long transparent bristles, scaled. Genae without bristles. Clypeus and labrum with transparent bristles (
Figure 20C–F and
Figure 23C).
Figure 21.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Mandible (B) Labium. (C) Labial palp (D) Maxilla. (E) Maxillary inner protuberance, as indicates by the arrow. (F) Hypopharynx. (G–I) Foreleg, midleg, and hindleg. Scale bars: 500 μm.
Figure 21.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Mandible (B) Labium. (C) Labial palp (D) Maxilla. (E) Maxillary inner protuberance, as indicates by the arrow. (F) Hypopharynx. (G–I) Foreleg, midleg, and hindleg. Scale bars: 500 μm.
Antennae scaled except on flagellum; flagellum almost without dark annulations, bearing well-developed annulate setae; terminal chain divided into 14 segments. Scape length-to-width ratio 1.97–2.11 (
Figure 20B and
Figure 23B).
Mandibles typical, apex with four distal teeth (
Figure 21A).
Maxillary palp I with one processus basalis and one inner protuberance (
Figure 21D,E and
Figure 24C). Maxillary palp articles without short brush-like setae ventrally. Ratio of ultimate article to penultimate article length 0.45–0.52. Dorsal surface of maxillary palp articles V–VII with transparent spines (
Figure 21D and
Figure 24C); numbers given in
Table 2.
Male labial palp third segment modified, laterally expanded, apex with sensory cones; numbers given in
Table 2; ultimate article of female almost unswollen, clavate. Labial palp without long hair-like setae (
Figure 21B,C and
Figure 24A,B).
Thorax. Thorax normal. Foreleg without sensory field or coxal styli; mid- and hind legs with coxal styli. Ventral surface of legs with long bristles, without needle-shaped setae. Pretarsal claw structure normal (
Figure 21G–I and
Figure 23D–F).
Figure 22.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. (K) Penis and parameres. Scale bars: 500 μm.
Figure 22.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. (K) Penis and parameres. Scale bars: 500 μm.
Abdomen. Abdominal coxites I, VI, and VII with one pair of retractile vesicles; coxites II–V with two pairs of retractile vesicles (
Figure 22A–G and
Figure 24D,E); inner lobes of female coxite VII fused and posteriorly extended (
Figure 24E). Ratios of styli length to coxites length on abdominal segment V, styli length to supporting spines length, and basal width to length of sternites given in
Table 5. Coxite IX medially with 6+6 transparent macrochaetae (
Figure 22I and
Figure 24G). Caudal filament laterally and cerci medially with deciduous long hairs and supporting spines (
Figure 22J).
Figure 23.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D–F) Foreleg, midleg, and hindleg. Scale bars: 500 μm.
Figure 23.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D–F) Foreleg, midleg, and hindleg. Scale bars: 500 μm.
Coxite VIII without parameres. Coxite IX with penis and parameres (
Figure 22H,I). Penis opening apically, slightly shorter than parameres; parameres 1+7 segmented (
Figure 22K). Ovipositor of primary type, narrowly exceeding styli (including supporting spines) of coxite IX (
Figure 24F,G). Number of segments of anterior gonapophyses and posterior gonapophyses given in
Table 6.
Figure 24.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Labium. (B) Labial palp. (C) Maxilla. (D–G) Abdominal sternites and coxites V, VII–IX. Scale bars: 500 μm.
Figure 24.
Pedetontus (Verhoeffilis) nanningensis Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Labium. (B) Labial palp. (C) Maxilla. (D–G) Abdominal sternites and coxites V, VII–IX. Scale bars: 500 μm.
Differential diagnosis. Pedetontus (Verhoeffilis) nanningensis sp. n. is morphologically most similar to P. (V.) gershneri Allen, 1995, from Arkansas, USA. They can be distinguished by the maxillary palps: the former lacks brush-like setae ventrally on the male maxillary palps, whereas the latter possesses brush-like setae ventrally on articles V–VII. Additionally, the ultimate article of the maxillary palp in P. (V.) nanningensis sp. n. is only half the length of the penultimate article, while in P. (V.) gershneri it exceeds three-quarters the length of the penultimate article.
Etymology. The species is named after its type locality, Nanning City. The Chinese name for P. (V.) nanningensis sp. n. is 南宁跳蛃.
Distribution. Guangxi Zhuang Autonomous Region, China.
Zoobank: urn:lsid:zoobank.org:act:D809BC47-4BED-4B63-993D-31267071EE31
Type Specimens and Type Locality. Holotype, 1♀ (in 70% ethanol), paratype, 1♀ collected from Yangtai Mountain, Shenzhen City, Guangdong Province, China (22°39′57″ N 113°58′29″ E, 129 m), 3.IV.2023, by Yong-Ying Ruan All type specimens deposited in the Animal Herbarium, Zhejiang Normal University, Jinhua, China.
Figure 25.
Pedetontus (Verhoeffilis) shenzhenensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. (G) Excrement. Scale bars: 500 μm.
Figure 25.
Pedetontus (Verhoeffilis) shenzhenensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A) Habitus, lateral view. (B) Antenna. (C) Head, frontal view. (D) Ditto, lateral view. (E,F) Compound eyes and paired ocelli. (G) Excrement. Scale bars: 500 μm.
Description. Female 11.2 mm. Body covered with brown scales (in 70% ethanol). Antennae longer than body length; caudal filament approximately 1.5 times body length; cerci slightly shorter than body length (
Figure 25A).
Head. Compound eyes smooth, yellowish-green with maroon borders (
Figure 25C–F); ratios of eye length to width and contact line length to eye length given in
Table 1. Ocelli dark brown with white borders, dumbbell-shaped. Ratios of ocellus length to width and distance between inner margins of ocelli to combined width of eyes given in
Table 1. Vertex without markings, bearing minute black setae arranged longitudinally. Frons convex, with long transparent bristles and minute black setae, scaled. Genae with black setae near compound eyes. Clypeus and labrum with transparent setae (
Figure 25C–F).
Figure 26.
Pedetontus (Verhoeffilis) shenzhenensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A) Mandible. (B) Labium. (C) Labial palp. (D) Maxilla. (E) Maxillary palp article VI and VII. (F) Lacinia and galea. (G) Maxillary inner protuberance. (G) Labium. (H–J) Foreleg, midleg, and hindleg. (K–M) Foreclaw, midclaw, and hindclaw. Scale bars: 500 μm.
Figure 26.
Pedetontus (Verhoeffilis) shenzhenensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A) Mandible. (B) Labium. (C) Labial palp. (D) Maxilla. (E) Maxillary palp article VI and VII. (F) Lacinia and galea. (G) Maxillary inner protuberance. (G) Labium. (H–J) Foreleg, midleg, and hindleg. (K–M) Foreclaw, midclaw, and hindclaw. Scale bars: 500 μm.
Antennae scaled except on flagellum; flagellum with distinct pale annulations, bearing well-developed annulate setae; terminal chain divided into 10 segments. Scape length-to-width ratio 1.90 (
Figure 25B).
Mandibles typical, apex with four distal teeth (
Figure 26A).
Maxillary palp I with one processus basalis and one inner protuberance (
Figure 26D,G). Maxillary palp articles without short brush-like setae ventrally. Ratio of ultimate article to penultimate article length 0.62. Dorsal surface of maxillary palp articles V–VII with transparent spines (
Figure 26D,E); numbers given in
Table 2.
Labial palp third segment slightly swollen apically, with sensory cones; numbers given in
Table 2. Labial palp without long hair-like setae (
Figure 26B,C).
Figure 27.
Pedetontus (Verhoeffilis) shenzhenensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. (K) Apex of anterior gonapophyses. (L) Apex of posterior gonapophyses. Scale bars: 500 μm.
Figure 27.
Pedetontus (Verhoeffilis) shenzhenensis Shen, Yang, Ji & Zhang sp. n., holotype, female. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. (K) Apex of anterior gonapophyses. (L) Apex of posterior gonapophyses. Scale bars: 500 μm.
Thorax. Thorax normal. Foreleg without sensory field or coxal styli; mid- and hind legs with coxal styli. Ventral surface of legs with long bristles, without needle-shaped setae. Pretarsal claw structure normal (
Figure 26H–M).
Abdomen. Abdominal coxites I, VI, and VII with one pair of retractile vesicles; coxites II–V with two pairs of retractile vesicles (
Figure 27A–G); inner lobes of female coxite VII fused and posteriorly extended (
Figure 27G). Ratios of styli length to coxites length on abdominal segment V, styli length to supporting spines length, and basal width to length of sternites given in
Table 5. Coxite IX medially with 4–5+4–5 transparent macrochaetae (
Figure 27I). Caudal filament laterally and cerci medially with deciduous long hairs and supporting spines (
Figure 27J).
Ovipositor of primary type, slightly shorter than styli (including supporting spines) of coxite IX (
Figure 27H,I). Number of segments of anterior gonapophyses and posterior gonapophyses given in
Table 6.
Differential Diagnosis. Pedetontus (Verhoeffilis) shenzhenensis sp. n. is morphologically closest to P. (V.) bianchii Silvestri, 1936, from Hong Kong. The abdominal segment IX provides a key distinction: in P. (V.) shenzhengensis sp. n., the styli (including supporting spines) of coxite IX are longer than coxite IX itself, while the posterior gonapophyses are slightly shorter than these styli. Conversely, in P. (V.) bianchii, the styli (including supporting spines) of coxite IX are shorter than coxite IX, and the posterior gonapophyses exceed their length. Additionally, the scape of P. (V.) shenzhenensis sp. n. is slender than that of P. (V.) bianchii, with length-to-width ratios of 1.90 and 1.42, respectively.
Etymology. The species is named after its type locality, Shenzhen City. The Chinese name for P. (V.) shenzhenensis sp. n. is 深圳跳蛃.
Distribution. Guangdong Province, China.
Zoobank: urn:lsid:zoobank.org:act:8C07BFC0-2BB3-4053-A2AB-23B08DA13180
Type Specimens and Type Locality. Holotype, 1♂ (in 70% ethanol), paratypes: 11♂7♀ (in 70% ethanol), China, Guangdong Province, Zhaoqing City, Duanzhou District, Beiling Mountain Forest Park (23°09′ N 112°30′ E), from rocks along a stream, 20.XI.2024–3.I.2025, collected by Han-sheng Ou. All type specimens deposited in the Animal Herbarium, Zhejiang Normal University, Jinhua, China.
Figure 28.
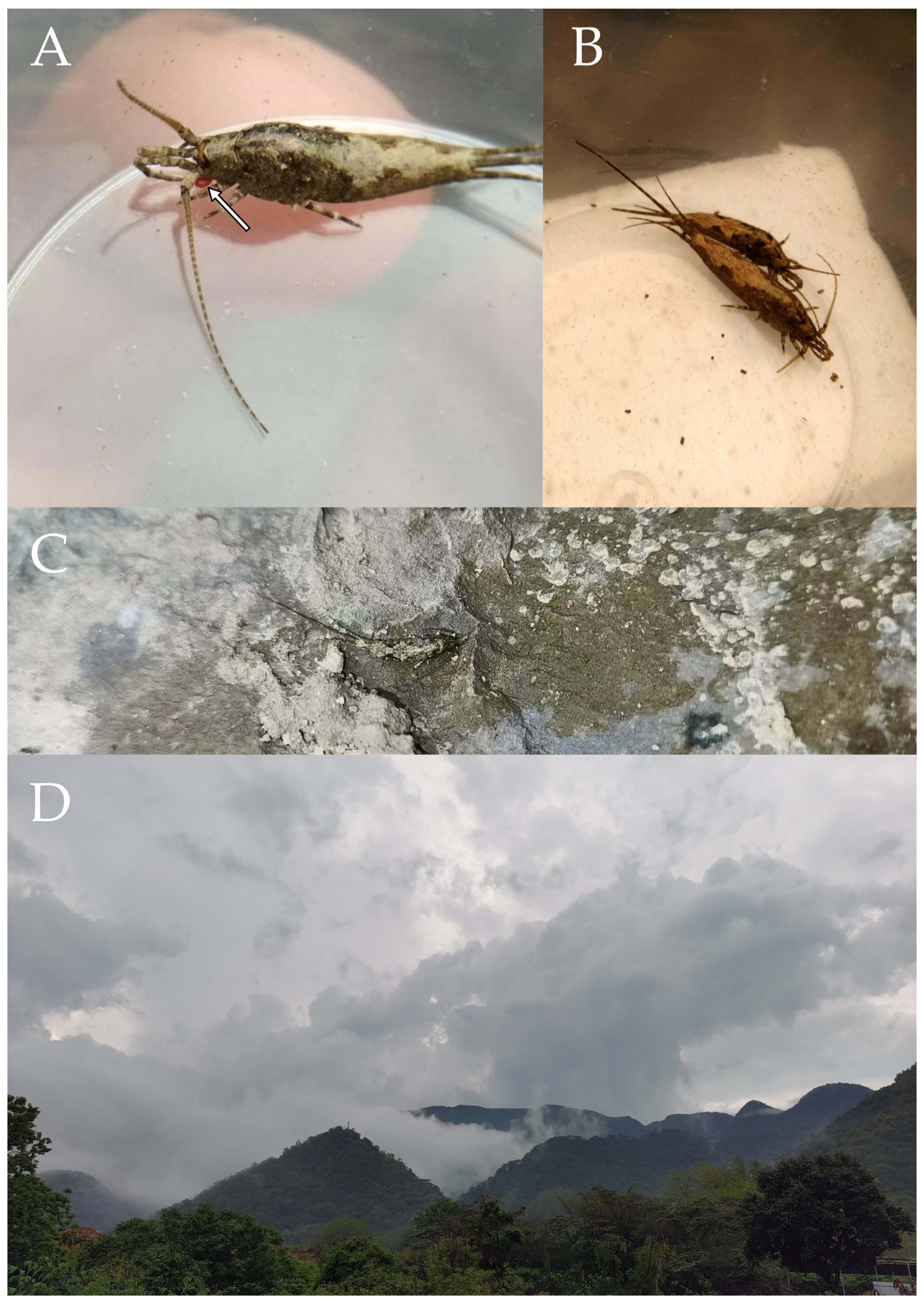
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n. (A) The captured P. (V.) xanthospilus sp. n., arrow indicates a mite. (B) Ditto. (C) In situ. (D) Habitat.
Figure 28.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n. (A) The captured P. (V.) xanthospilus sp. n., arrow indicates a mite. (B) Ditto. (C) In situ. (D) Habitat.
Description. Male body length 10.2 mm, female 9.2 mm. Body covered with dark brown scales; nota with a pale longitudinal stripe extending from the pronotum to the base of the caudal filament, terminating in two large and one small diamond-shaped markings. Antennae slightly shorter than body length; caudal filament slightly longer than body length; cerci about half as long as body length (
Figure 28A–C,
Figure 29A and
Figure 32A).
Figure 29.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view, arrow indicates a mite attached to the maxilla. (B) Antenna. (C) Terminal chain of flagellum. (D) Head, frontal view. (E) Ditto, lateral view. (F,G) Compound eyes and paired ocelli. Scale bars: 500 μm.
Figure 29.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Habitus, lateral view, arrow indicates a mite attached to the maxilla. (B) Antenna. (C) Terminal chain of flagellum. (D) Head, frontal view. (E) Ditto, lateral view. (F,G) Compound eyes and paired ocelli. Scale bars: 500 μm.
Head. Compound eyes smooth, dark brown, with a large bright yellow blotch internally and a smaller one inferiorly (
Figure 29D–G and
Figure 32B); ratios of eye length to width and contact line length to eye length given in
Table 1. Ocelli dark brown, dumbbell-shaped. Ratios of ocellus length to width and distance between inner margins of ocelli to combined width of eyes given in
Table 1. Vertex without markings or setae. Frons convex, with long transparent bristles, scaled. Genae without markings or setae. Clypeus and labrum with transparent setae (
Figure 29D–G and
Figure 32B).
Figure 30.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Mandible. (B) Labium. (C) Labial palp. (D) Maxilla. (E) Maxillary palp articles V–VII. (F) Maxillary inner protuberance, as indicates by the arrow. (G–I) Foreleg, midleg, and hindleg. Scale bars: 500 μm.
Figure 30.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., holotype, male. (A) Mandible. (B) Labium. (C) Labial palp. (D) Maxilla. (E) Maxillary palp articles V–VII. (F) Maxillary inner protuberance, as indicates by the arrow. (G–I) Foreleg, midleg, and hindleg. Scale bars: 500 μm.
Antennae scaled except on flagellum; flagellum with distinct pale annulations, bearing well-developed annulate setae; terminal chain divided into 11–12 segments. Scape length-to-width ratio 1.74–1.91 (
Figure 29B).
Mandibles typical, apex with four distal teeth (
Figure 30A).
Maxillary palp I with one processus basalis and one inner protuberance (
Figure 30D,F and
Figure 32E). Maxillary palp articles II–VII with brush-like setae ventrally, setae on articles IV–V longer than others. Ratio of ultimate article to penultimate article length 0.45–0.52. Dorsal surface of maxillary palp articles V–VII with transparent spines (
Figure 30D and
Figure 32E); numbers given in
Table 2.
Male labial palp third segment modified, laterally expanded, apex with sensory cones; numbers given in
Table 2; ultimate article of female almost unswollen, clavate. Labial palp without long hair-like setae (
Figure 30B,C and
Figure 32D).
Thorax. Thorax normal. Foreleg without sensory field or coxal styli; mid- and hind legs with coxal styli, apex of coxal styli bright yellow. Ventral surface of legs with long bristles, without needle-shaped setae. Pretarsal claw structure normal (
Figure 30G–I and
Figure 33A–C).
Figure 31.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. (K) Penis and paramere. Scale bars: 500 μm.
Figure 31.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., holotype, male. (A–I) Abdominal sternites and coxites I–IX. (J) Caudal filament and cerci. (K) Penis and paramere. Scale bars: 500 μm.
Abdomen. Abdominal coxites I, VI, and VII with one pair of retractile vesicles; coxites II–V with two pairs of retractile vesicles; coxites I–IX each with a pair of yellow styli (
Figure 31A–I and
Figure 33D–G); inner lobes of female coxite VII fused and posteriorly extended (
Figure 33E). Ratios of styli length to coxites length on abdominal segment V, styli length to supporting spines length, and basal width to length of sternites given in
Table 5. Coxite IX medially with 4–5+4–5 transparent macrochaetae (
Figure 31I and
Figure 33G). Caudal filament laterally and cerci medially with deciduous long hairs and supporting spines (
Figure 31J).
Figure 32.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Head, frontal view. (C) Labium. (D) Labial palp. (E) Maxilla. Scale bars: 500 μm.
Figure 32.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., paratype, female. (A) Habitus, lateral view. (B) Head, frontal view. (C) Labium. (D) Labial palp. (E) Maxilla. Scale bars: 500 μm.
Coxite VIII without parameres. Coxite IX with penis and parameres (
Figure 31I,K). Penis opening apically, shorter than parameres; parameres 1+7 segmented (
Figure 31K). Ovipositor of primary type, not exceeding styli (including supporting spines) of coxite IX (
Figure 33F,G). Number of segments of anterior gonapophyses and posterior gonapophyses given in
Table 6.
Figure 33.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., paratype, female. (A–C) Foreleg, midleg, and hindleg. (D–G) Abdominal sternites and coxites V, VII–IX. (H) Apex of anterior gonapophyses. (I) Apex of posterior gonapophyses. Scale bars: 500 μm.
Figure 33.
Pedetontus (Verhoeffilis) xanthospilus Shen, Yang, Ji & Zhang sp. n., paratype, female. (A–C) Foreleg, midleg, and hindleg. (D–G) Abdominal sternites and coxites V, VII–IX. (H) Apex of anterior gonapophyses. (I) Apex of posterior gonapophyses. Scale bars: 500 μm.
Differential Diagnosis. See corresponding section of Pedetontus (Verhoeffilis) jinxiuensis sp. n.
Etymology. The specific epithet “xanthospilus” is a Latinized adjective derived from Greek xanthos (ξανθός), meaning “yellow”, and Greek spilos (σπίλος), meaning “blotch” or “stain”. It refers to the species’ conspicuous yellow blotches on the compound eyes, a defining diagnostic trait that distinguishes this taxon from congeners. The Chinese name for P. (V.) xanthospilus sp. n. is 黄斑跳蛃.
Distribution. Guangdong Province, China.