Life History Plasticity and Gregarious Cocooning Behavior of the Wild Silkmoth Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) on a Novel Host Plant, Cinnamon, in Thailand
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
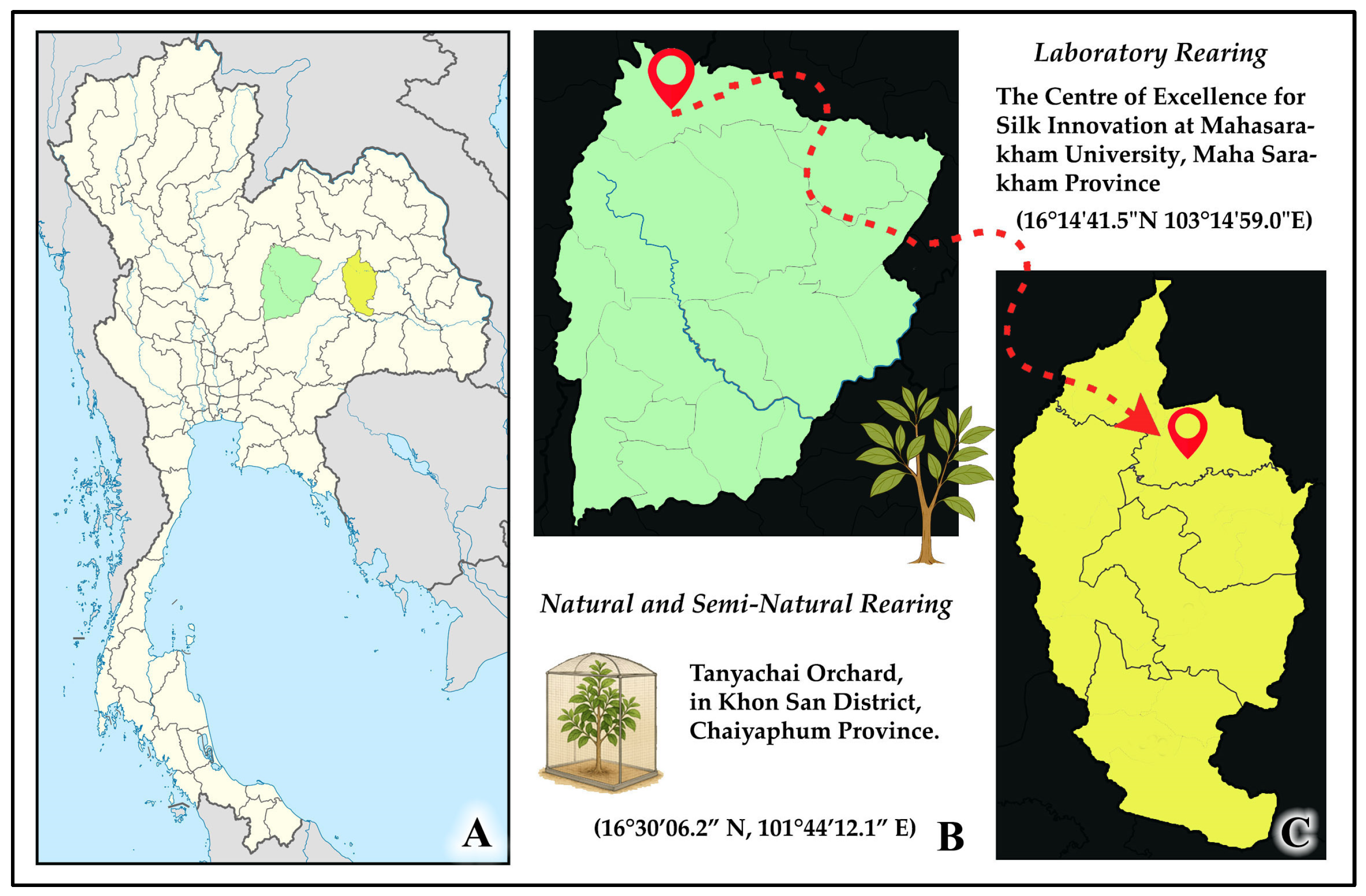
2.1. Study Sites and Host Plant Species
2.2. Insect Material and Rearing Conditions
2.2.1. Field Population Monitoring
2.2.2. Semi-Natural Rearing on Cinnamon
2.2.3. Laboratory Rearing
2.2.4. Opportunistic Collection from Hog Plum Trees
2.3. Data Collection Protocols
2.3.1. Life History and Behavioral Documentation
2.3.2. Oviposition Pattern Analysis
2.3.3. Gregarious Cocooning Behavior Assessment
2.3.4. Morphometric Cocoon Analysis
2.4. Statistical Analysis
3. Results
3.1. Life Cycle Duration Under Different Rearing Conditions
3.2. Stage-Specific Analysis
3.3. Morphological Characteristics and Developmental Stages
3.4. Behavioral Observations
3.4.1. Density-Dependent Oviposition Plasticity
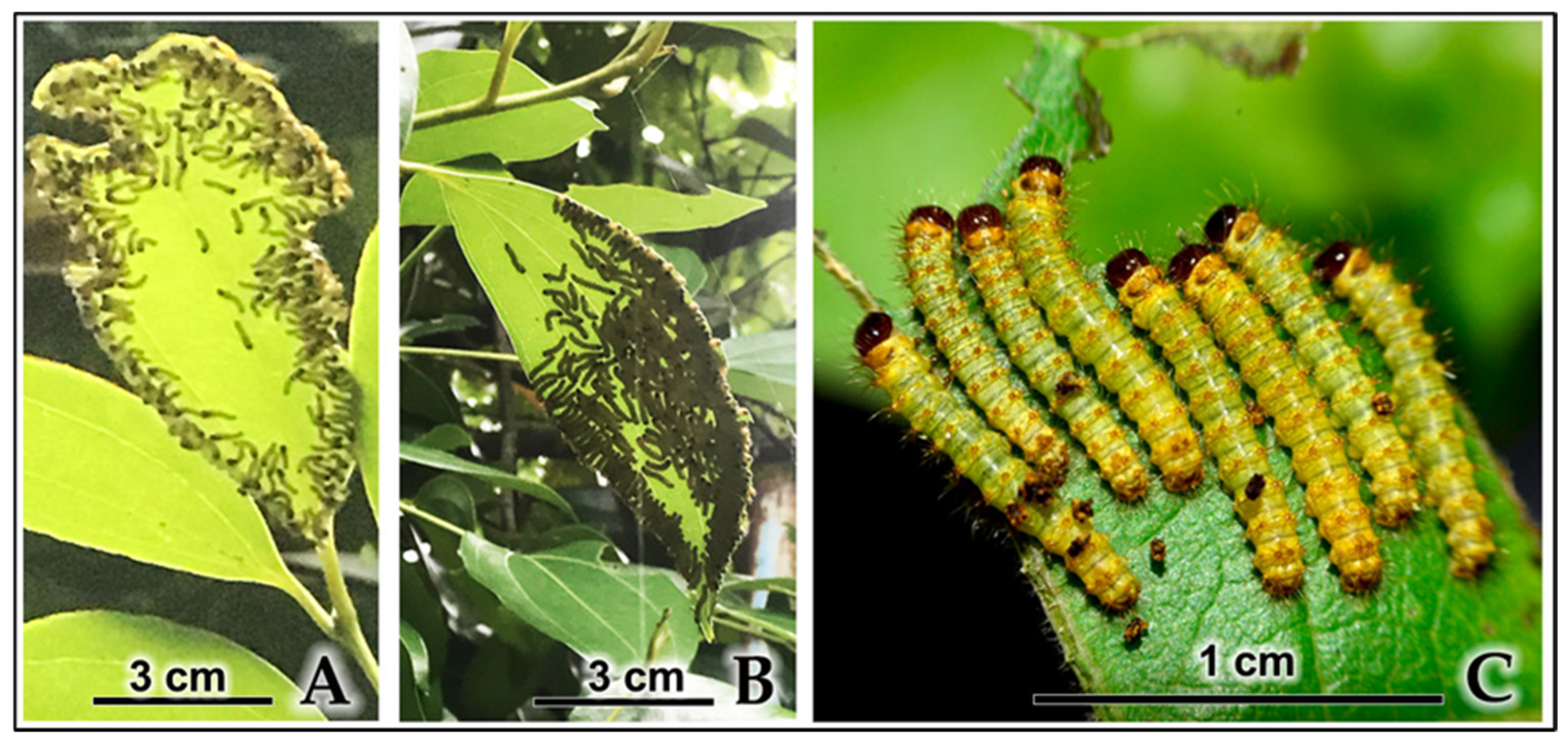
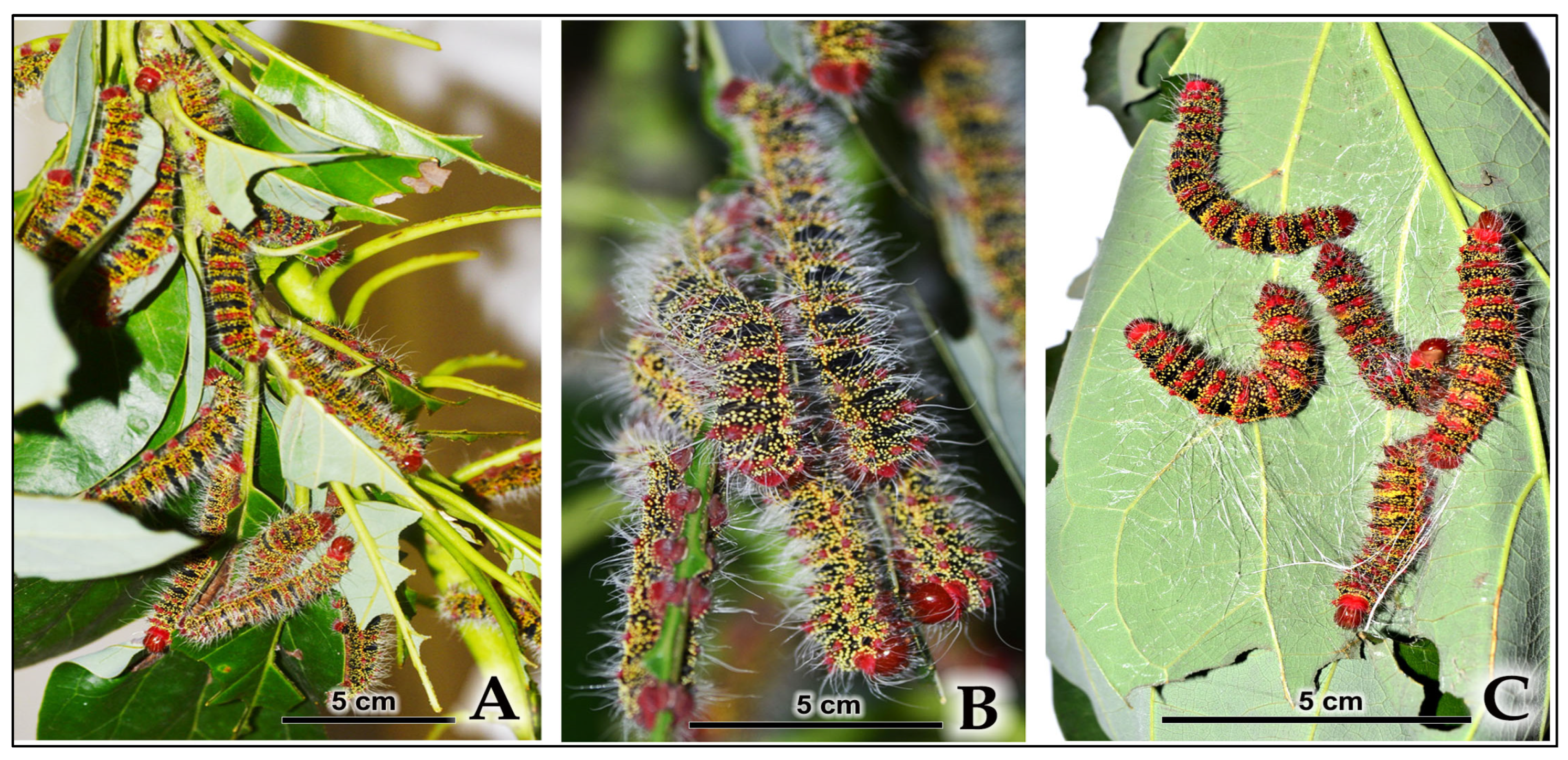
3.4.2. Larval Aggregation and Gregarious Cocooning Behavior
3.4.3. Quantitative Analysis of Gregarious Cocooning Behavior
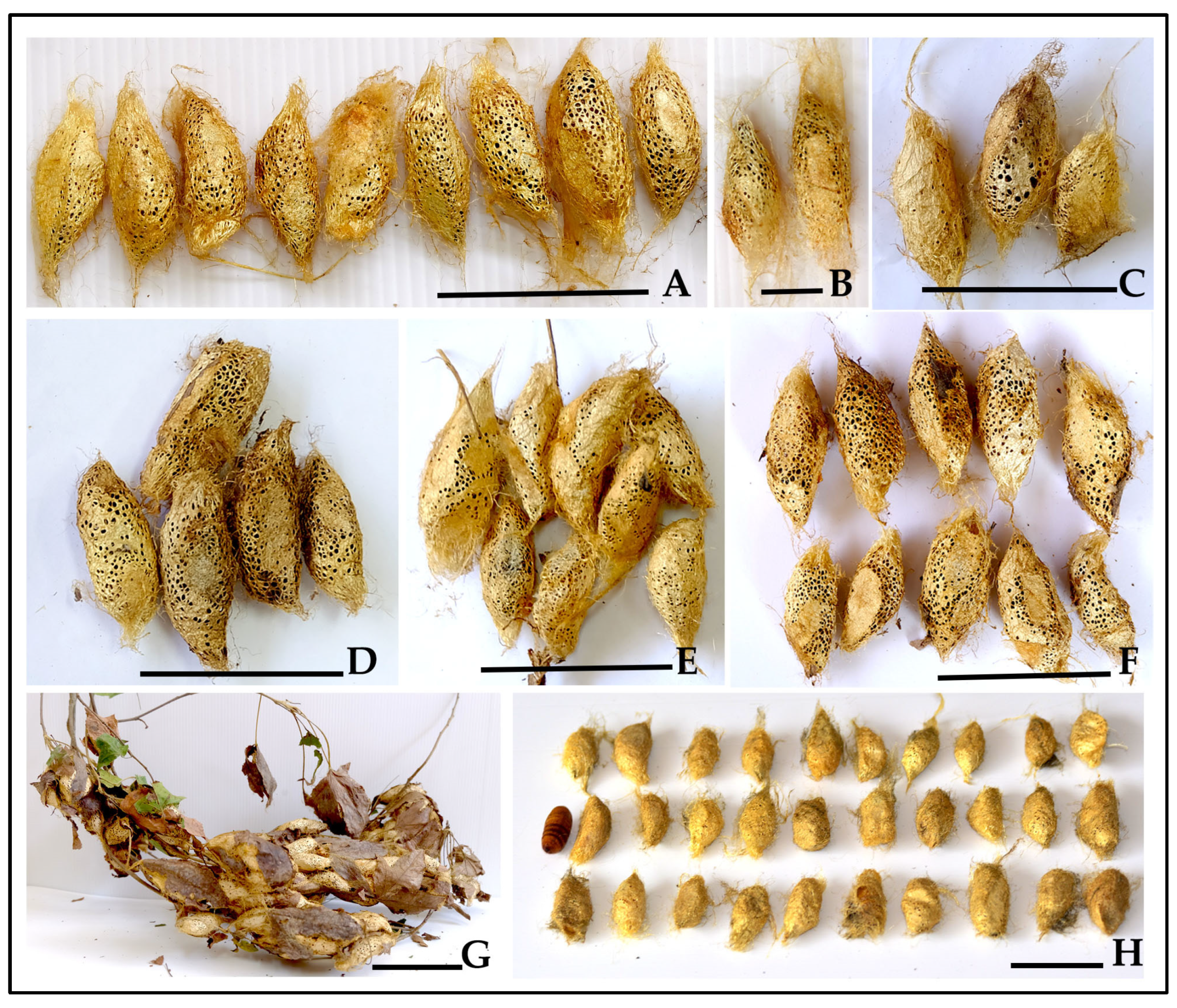
3.5. Cocoon Quality and Commercial Potential
3.5.1. Rearing Environment Effects on Cocoon Characteristics
3.5.2. Mass Production Potential from Opportunistic Hog Plum Collection
4. Discussion
4.1. Developmental Plasticity and Environmental Adaptation
4.2. Novel Host Adaptation and Dual-Host Strategy
4.3. Social Behavior and Collective Decision-Making
4.3.1. Implications of Density-Dependent Oviposition Plasticity
4.3.2. Active Gregarious Behavior Throughout Development
4.3.3. Ontogenetic Social Shifts and Cooperative Mechanisms
4.3.4. Gregarious Cocooning Behavior
4.4. Commercial Silk Production and Integrated Pest Management
4.4.1. Silk Quality and Host-Mediated Effects
4.4.2. Pest-to-Product Transformation Strategy
4.5. Evolutionary Significance and Ecological Implications
4.6. Study Limitations and Future Research Directions
4.6.1. Methodological Constraints
4.6.2. Priority Research Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hridya, H.; Guha, L.; Mazumdar, M.; Sarkar, B.N.; Vijayakumar, S.; Borpuzari, P. Probing the potentiality of the defoliator Cricula trifenestrata Helfer silk: A revisit. Bull. Natl. Res. Cent. 2021, 45, 215. [Google Scholar] [CrossRef] [PubMed]
- Narkdang, T.; Sirimungkararat, S.; Phochan, D.; Ta-aun, M. Value Added for Rearing Wild Silkmoth Cricula trifenestrata [การสร้างมูลค่าเพิ่มให้กับการเพาะเลี้ยงไหมป่า Cricula trifenestrata]; Research Report; Khon Kaen University: Khon Kaen, Thailand, 2011. (In Thai) [Google Scholar] [CrossRef]
- Hampson, G.F. The Fauna of British India, Ceylon and Burma: Moths; Taylor & Francis: London, UK, 1892; Volume I, pp. 28–29. Available online: https://www.biodiversitylibrary.org/item/180068#page/5/mode/1up (accessed on 15 December 2024).
- Tikader, A.; Vijayan, K.; Saratchandra, B. Cricula trifenestrata (Helfer) (Lepidoptera: Saturniidae)—A silk-producing wild insect in India. Trop. Lepid. Res. 2014, 24, 22–29. [Google Scholar]
- Akai, H. A successful example of wild silk development from Cricula trifenestrata in Indonesia. Int. J. Wild Silkmoth Silk 2000, 5, 91–97. [Google Scholar]
- Sirimungkararat, S.; Seksirirat, W.; Pitirit, T.; Apinakpong, K.; Nathongkam, A. Value-added creation of processed insect food menus of avocado hairy caterpillar (Cricula trifenestrata H.). Int. J. Wild Silkmoth Silk 2010, 14, 31–38. [Google Scholar]
- Magnussen, K.; Butiman, C.; Promma, S.; Wisrisit, J.; Thunchailertthakul, T.; Sumida, M. First report of Cricula silkmoth, Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) on cinnamon tree, Cinnamomum sp. (Lauraceae) from Chaiyaphum Province, Northeastern Thailand: Morphological characteristics, host plants, and parasitic fly. Int. J. Wild Silkmoth Silk 2023, 24, 11–20. [Google Scholar]
- Magnussen, K.; Sumida, M.; Kangrang, A.; Vollrath, F.; Katisart, T.; Butiman, C. External morphology, defensive adaptations, aposematic coloration, and sexual dimorphism of the fifth instar larva of Cricula silkmoth, Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) from Thailand. Insects 2025, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Ahad, M.A.; Rono, M.M.A.; Tithi, D.A. Life history traits of Cricula trifenestrata (Lepidoptera: Saturniidae) feeding on Mangifera indica. J. Agrofor. Environ. 2008, 2, 1–6. [Google Scholar]
- Yadav, S.; Kumar, A. New record of wild silk caterpillar Cricula trifenestrata Helfer on large cardamom and notes on its biology. Uttar Pradesh J. Zool. 2003, 23, 67–69. [Google Scholar]
- Scriber, J.M.; Slansky, F., Jr. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Battisti, A.; Larsson, S.; Roques, A. Processionary moths and associated urtication risk: Global change-driven effects. Annu. Rev. Entomol. 2017, 62, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Underwood, D.L.A. Maintenance of sociality in a communal caterpillar, Eucheira socialis westwoodi (Lepidoptera: Pieridae). Evol. Ecol. Res. 2011, 13, 625–635. [Google Scholar]
- Hamilton, W.D. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Gamberale, G.; Tullberg, B.S. Aposematism and gregariousness: The combined effect of group size and coloration on signal repellence. Proc. R. Soc. B 1998, 265, 889–894. [Google Scholar] [CrossRef]
- Wang, L.; Cornell, S.J.; Speed, M.P.; Arbuckle, K. Coevolution of group-living and aposematism in caterpillars: Warning colouration may facilitate the evolution from group-living to solitary habits. BMC Ecol. Evol. 2021, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Deml, R.; Dettner, K. Chemical defence of emperor moths and tussock moths (Lepidoptera: Saturniidae, Lymantriidae). Entomol. Gen. 1997, 21, 225–251. [Google Scholar] [CrossRef]
- Denno, R.F.; Benrey, B. Aggregation facilitates larval growth in the neotropical nymphalid butterfly Chlosyne janais. Ecol. Entomol. 1997, 22, 133–141. [Google Scholar] [CrossRef]
- Hamilton, W.D. The genetical evolution of social behaviour. I. J. Theor. Biol. 1964, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, S.I.; Pierotti, R. Distinctions among reciprocal altruism, kin selection, and cooperation and a model for the initial evolution of beneficent behavior. Ethol. Sociobiol. 1988, 9, 189–209. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A. Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 2007, 20, 415–432. [Google Scholar] [CrossRef] [PubMed]
- De Nardin, J.; Araújo, A.M. Kin recognition in immatures of Heliconius erato phyllis (Lepidoptera; Nymphalidae). J. Ethol. 2011, 29, 499–503. [Google Scholar] [CrossRef]









| Developmental Stage | Field | Semi-Natural | Laboratory | F-Statistic | p-Value |
|---|---|---|---|---|---|
| Egg | 9.50 ± 0.53 a | 9.40 ± 0.52 a | 11.00 ± 0.67 b | 24.371 | <0.001 |
| 1st-Instar Larva | 3.30 ± 0.48 | 3.60 ± 0.52 | 3.60 ± 0.52 | 1.174 | 0.324 |
| 2nd-Instar Larva | 4.50 ± 0.53 | 4.60 ± 0.52 | 4.60 ± 0.52 | 0.123 | 0.885 |
| 3rd-Instar Larva | 5.80 ± 0.79 | 6.20 ± 0.63 | 6.50 ± 0.71 | 2.431 | 0.107 |
| 4th-Instar Larva | 6.90 ± 0.88 | 6.90 ± 0.88 | 7.00 ± 0.82 | 0.045 | 0.956 |
| 5th-Instar Larva | 9.50 ± 0.53 | 9.40 ± 0.52 | 9.70 ± 0.48 | 0.9 | 0.418 |
| Pupa | 11.10 ± 0.88 a | 11.20 ± 1.03 a | 13.70 ± 1.42 b | 16.934 | <0.001 |
| Adult | 4.70 ± 0.48 ab | 4.60 ± 0.52 a | 5.20 ± 0.42 b | 4.574 | 0.019 |
| Total Duration | 56.30 ± 1.83 a | 56.90 ± 1.60 a | 62.30 ± 3.68 b | 16.838 | <0.001 |
| Life Stage | Approx. Length/Wingspan (cm) | Distinguishing Features |
|---|---|---|
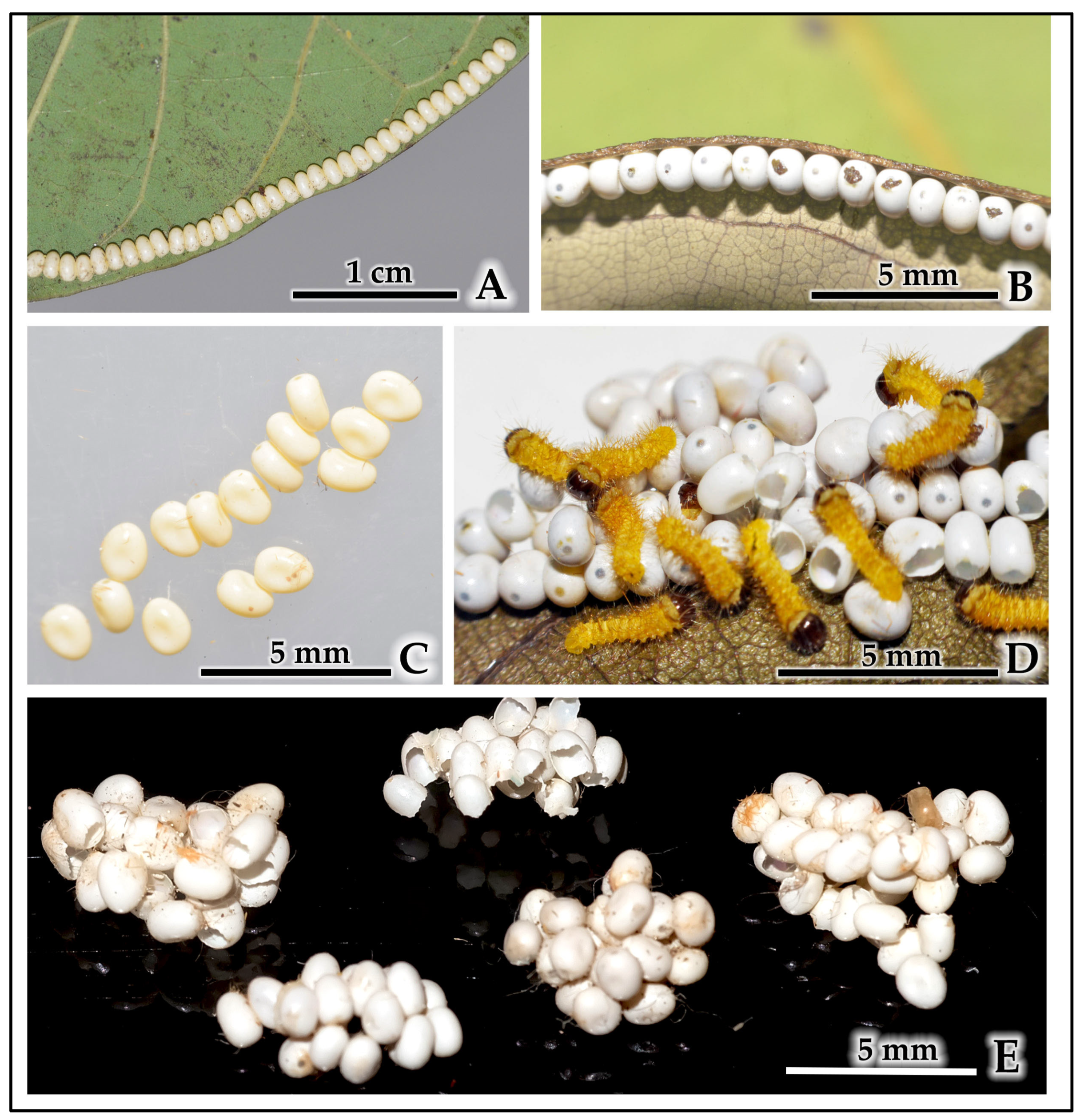
| Egg | 0.12 ± 0.02 | Ivory-white; oval and dorsoventrally flattened, resembling beads on a garland; laid in 1–3 neat parallel rows on the leaf edge. |
| 1st-Instar Larva | 0.58 ± 0.12 | Cream-yellow body, later turning yellowish-red with dark transverse bands; prominent glossy dark head and dark-margined prothoracic plate; body covered in sparse, fine setae arising from light-brown scoli. |
| 2nd-Instar Larva | 0.65 ± 0.10 | Body color a combination of red, yellow, and black; glossy brown head; golden-brown legs; clothed in a tuft of long white and short black hairs arising from brown scoli bearing golden spines. |
| 3rd-Instar Larva | 2.5 ± 0.11 | Reddish-yellow body with yellow-black stripes; deep-red head capsule and prothoracic shield; legs are brick red; dense growth of long white setae arising from scoli that are encircled by a yellow band. |
| 4th-Instar Larva | 5.5 ± 0.1 | Black body with vivid red-orange transverse bands; red head and anal claspers; prominent, long white setae become more conspicuous; body dots turn yellow. |
| 5th-Instar Larva | 7.5 ± 0.10 | Black body with crimson-red transverse bands and glowing yellow dots; crimson-red head; covered with long whitish hairs projecting from pink tubercles (scoli); the yellow dots are luminescent under normal light and exhibit fluorescence under UV light. |
| Pupa | 4.5 ± 0.13 | Dark-brown in color; obtect, with a hard cuticle; female distinguished by a fine longitudinal line on the ventral side of the 8th abdominal segment, which is absent in the male. |
| Adult Male | Wingspan 6.5 ± 0.2 | ♂: Quadripectinate antennae; light golden-brown scales; forewing with one transparent fenestra and one small dark spot. |
| Adult Female | Wingspan 7.0 ± 0.02 | ♀: Bipectinate antennae (less feathery than male); dark brown and orange scales; forewing with three transparent fenestrae. |
| Environment | Pattern Type | Sample Size | Frequency (%) | Examples (Eggs) | Substrate Types |
|---|---|---|---|---|---|
| Field | Linear (1–3 rows) | 5 batches | 100 | 34, 135, 169, 180, 260 | Host leaves only |
| Laboratory | Small clusters | 7/15 clusters | 46.7 | 1, 2, 2, 3, 5, 6, 7 | Mixed substrates * |
| Laboratory | Medium clusters | 6/15 clusters | 40 | 11, 14, 16, 25, 26, 30 | Mixed substrates * |
| Laboratory | Large clusters | 2/15 clusters | 13.3 | 60, 98 | Mixed substrates * |
| Cluster Size | Field | Semi-Field | Laboratory | Total (n) | Frequency (%) |
|---|---|---|---|---|---|
| Solitary (1) | 3 | 2 | 2 | 7 | 14.9 |
| Small (2–10) | 12 | 5 | 6 | 23 | 48.9 |
| Medium (11–30) | 7 | 0 | 2 | 9 | 19.1 |
| Large (31–100+) | 8 | 0 | 0 | 8 | 17 |
| Total | 30 | 7 | 10 | 47 | 100 |
| Cocoon Trait | Field | Semi-Natural | Lab | Hog Plum | F-Statistic (Welch’s) | p-Value |
|---|---|---|---|---|---|---|
| Female | ||||||
| Fresh cocoon weight (g) | 1.724 ± 0.151 | 1.618 ± 0.178 | 1.604 ± 0.167 | 1.564 ± 0.103 | 1.102 | 0.399 |
| Weight of pupa (g) | 1.637 ± 0.139 | 1.522 ± 0.151 | 1.525 ± 0.131 | 1.466 ± 0.099 | 1.462 | 0.29 |
| Cocoon shell weight (g) | 0.066 ± 0.009 ab | 0.058 ± 0.018 a | 0.081 ± 0.017 b | 0.083 ± 0.006 b | 5.23 | 0.027 |
| Cocoon shell ratio (%) | 3.92 ± 0.51 a | 3.56 ± 0.67 a | 5.02 ± 0.72 b | 5.30 ± 0.30 b | 13.17 | 0.002 |
| Male | ||||||
| Fresh cocoon weight (g) | 0.963 ± 0.227 | 0.941 ± 0.305 | 1.028 ± 0.192 | 1.009 ± 0.081 | 0.136 | 0.935 |
| Weight of pupa (g) | 0.917 ± 0.225 | 0.883 ± 0.297 | 0.944 ± 0.185 | 0.922 ± 0.078 | 0.045 | 0.986 |
| Cocoon shell weight (g) | 0.045 ± 0.017 | 0.046 ± 0.024 | 0.070 ± 0.011 | 0.059 ± 0.019 | 2.828 | 0.102 |
| Cocoon shell ratio (%) | 4.59 ± 1.03 | 4.96 ± 2.59 | 6.82 ± 0.78 | 5.85 ± 1.97 | 4.495 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnussen, K.; Sumida, M.; Promma, S.; Kangrang, A.; Vollrath, F.; Thunchailertthakul, T.; Butiman, C. Life History Plasticity and Gregarious Cocooning Behavior of the Wild Silkmoth Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) on a Novel Host Plant, Cinnamon, in Thailand. Insects 2025, 16, 914. https://doi.org/10.3390/insects16090914
Magnussen K, Sumida M, Promma S, Kangrang A, Vollrath F, Thunchailertthakul T, Butiman C. Life History Plasticity and Gregarious Cocooning Behavior of the Wild Silkmoth Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) on a Novel Host Plant, Cinnamon, in Thailand. Insects. 2025; 16(9):914. https://doi.org/10.3390/insects16090914
Chicago/Turabian StyleMagnussen, Kanitsara, Motoyuki Sumida, Suwat Promma, Anongrit Kangrang, Fritz Vollrath, Thanupong Thunchailertthakul, and Chirapha Butiman. 2025. "Life History Plasticity and Gregarious Cocooning Behavior of the Wild Silkmoth Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) on a Novel Host Plant, Cinnamon, in Thailand" Insects 16, no. 9: 914. https://doi.org/10.3390/insects16090914
APA StyleMagnussen, K., Sumida, M., Promma, S., Kangrang, A., Vollrath, F., Thunchailertthakul, T., & Butiman, C. (2025). Life History Plasticity and Gregarious Cocooning Behavior of the Wild Silkmoth Cricula trifenestrata Helfer (Lepidoptera: Saturniidae) on a Novel Host Plant, Cinnamon, in Thailand. Insects, 16(9), 914. https://doi.org/10.3390/insects16090914








