Olive Pomace and Melon Bio-Byproducts from the Agribusiness: A Promising Combination for the Sustainable Production of Animal Protein from BSF Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
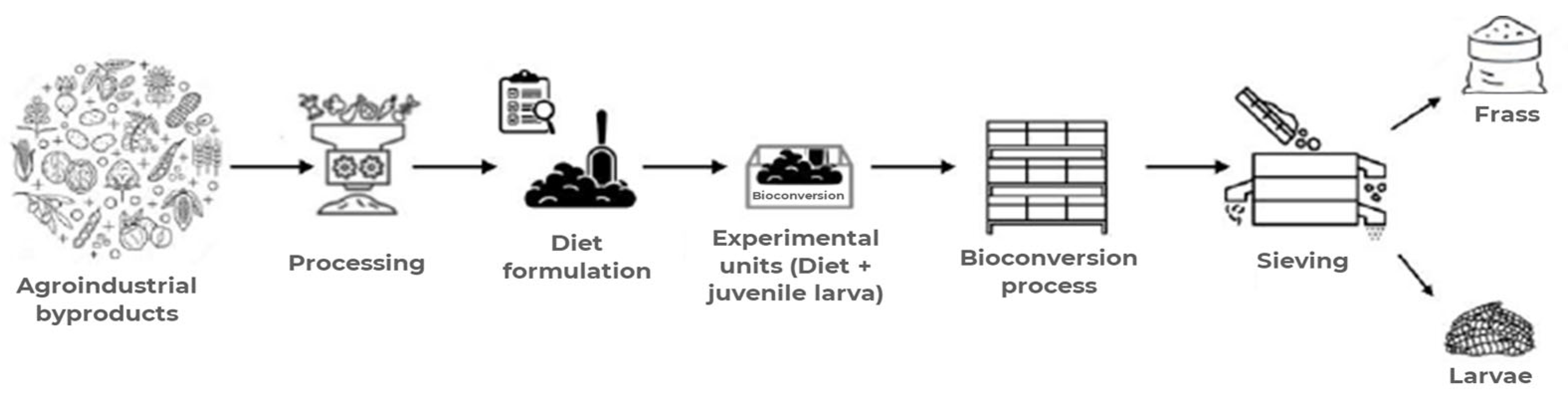
2.1. Bio-Byproducts Production and Chemical Composition
2.2. Diet Formulation
2.3. Bioconversion
3. Results
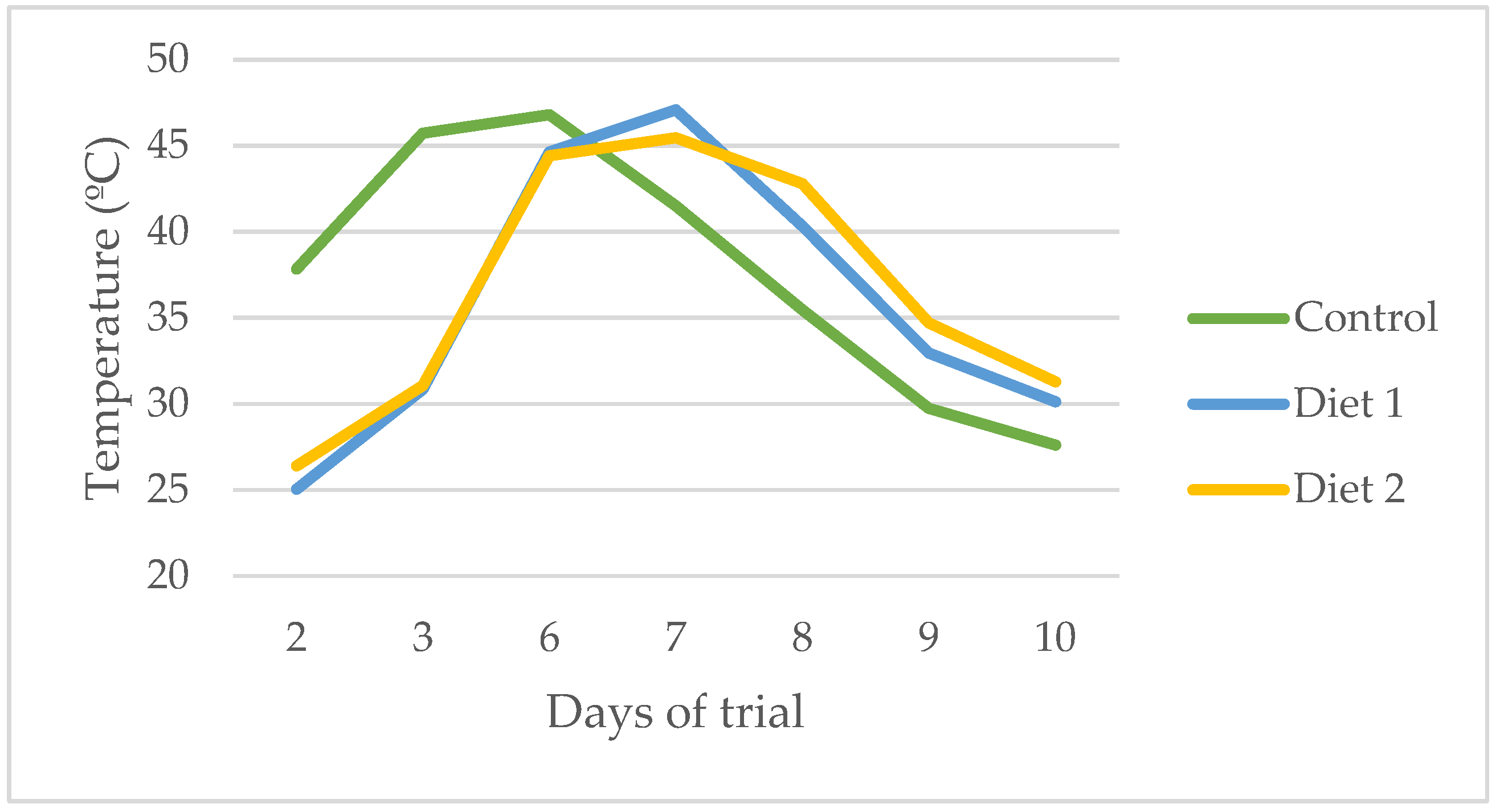
Larval Performance Parameters, Substrate Consumption, and Reduction Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EU | European Union |
| BSF | Black soldier fly |
| BSFL | Black soldier fly larvae |
| IO | Ingredient Odyssey |
| FCR | Feed conversion ratio |
References
- Jenkins, W.; Tucker, M.E.; Grim, J. Routledge Handbook of Religion and Ecology; Taylor & Francis: London, UK, 2016; pp. 1–439. [Google Scholar] [CrossRef]
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef]
- de Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; LeBlanc, A.d.M.d.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Islam, R.; Kamal, M.; Kabir, R.; Hasan, M.; Haque, A.R.; Hasan, S.M.K. Phenolic compounds and antioxidants activity of banana peel extracts: Testing and optimization of enzyme-assisted conditions. Meas. Food 2023, 10, 100085. [Google Scholar] [CrossRef]
- Neifar, M.; Jaouani, A.; Ayari, A.; Abid, O.; Ben Salem, H.; Boudabous, A.; Najar, T.; Ghorbel, R.E. Improving the nutritive value of Olive Cake by solid state cultivation of the medicinal mushroom Fomes fomentarius. Chemosphere 2013, 91, 110–114. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Kurtoğlu, S.; Uzundumlu, A.S.; Gövez, E. Olive Oil Production Forecasts for a Macro Perspective during 2024–2027. Appl. Fruit Sci. 2024, 66, 1089–1100. [Google Scholar] [CrossRef]
- Borja, R.; Raposo, F.; Rincón, B. Treatment technologies of liquid and solid wastes from two-phase olive oil mills. Grasas Aceites 2006, 57, 32–46. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/TCL (accessed on 10 July 2025).
- Mohanty, S.; Saha, S.; Santra, G.H.; Kumari, A. Future Perspective of Solid Waste Management Strategy in India. In Handbook of Solid Waste Management; Springer: Singapore, 2021; pp. 1–36. [Google Scholar] [CrossRef]
- Han, Y.; Liu, J.; Xu, H. A comprehensive assessment of the performance of China’s provincial zero-waste cities and impact factor diagnosis. Environ. Impact Assess. Rev. 2022, 95, 106778. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Farooqi, M.Q.U.; Singh, P.; Fernando, I.; Nagdalian, A. Consumer behavior towards cultured meat: A review since 2014. Appetite 2022, 179, 106314. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Turan, V.; Juárez, M.F.-D.; Oberegger, S.; Insam, H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy 2020, 10, 1578. [Google Scholar] [CrossRef]
- Kinasih, I.; Suryani, Y.; Paujiah, E.; Ulfa, R.; Afiyati, S.; Adawiyah, Y.; Putra, R. Performance of Black Soldier Fly, Hermetia illucens, Larvae during valorization of organic wastes with changing quality. IOP Conf. Ser. Earth Environ. Sci. 2020, 593, 012040. [Google Scholar] [CrossRef]
- Nardiello, M.; Scieuzo, C.; Salvia, R.; Leong, S.Y.; Kutty, R.M. Characteristic of Fatty Acids Biotransform from Hermetia illucens Prepupae Fed with Various Organic Wastes Before Conversion to Methyl Ester Form. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 042004. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Taufek, N.M.; Zulkifli, N.F.N.M.; Nazri, H.A. Upcycling of Food Waste Generated from the Fresh Market by Utilising Black Soldier Fly Larvae: Influence on Growth, Bioconversion, and Nutritional Composition. J. Environ. Manag. 2024, 349, 119467. [Google Scholar] [CrossRef]
- Magee, K.; Halstead, J.; Small, R.; Young, I. Valorisation of organicwaste by-products using black soldier fly (Hermetia illucens) as a bio-convertor. Sustainability 2021, 13, 8345. [Google Scholar] [CrossRef]
- Arabzadeh, G.; Delisle-Houde, M.; Tweddell, R.J.; Deschamps, M.-H.; Dorais, M.; Lebeuf, Y.; Derome, N.; Vandenberg, G. Diet Composition Influences Growth Performance, Bioconversion of Black Soldier Fly Larvae: Agronomic Value and In Vitro Biofungicidal Activity of Derived Frass. Agronomy 2022, 12, 1765. [Google Scholar] [CrossRef]
- Dortmans, B.M.A.; Egger, J.; Diener, S.; Zurbrügg, C. Black Soldier Fly Biowaste Processing—A Step-by-Step Guide, 2nd ed.; Swiss Federal Institute of Aquatic Science and Technology (Eawag): Dübendorf, Switzerland, 2021. [Google Scholar]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. J. a Sustain. Circ. Econ. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Riekkinen, K.; Väkeväinen, K.; Korhonen, J. The Effect of Substrate on the Nutrient Content and Fatty Acid Composition of Edible Insects. Insects 2022, 13, 590. [Google Scholar] [CrossRef]
- Maltseva, T.; Rudoy, D.; Olshevskaya, A.; Odabashyan, M.; Shevchenko, V. Prospects for Using Hermetia illucens Larvae in the Diet of Farm Animals: A Review. Online J. Anim. Feed. Res. 2025, 15, 108–116. [Google Scholar] [CrossRef]
- Ramzy, R.R.; El-Dakar, M.A.; Wang, D.; Ji, H. Conversion Efficiency of Lignin-Rich Olive Pomace to Produce Nutrient-Rich Insect Biomass by Black Soldier Fly Larvae, Hermetia illucens. Waste Biomass-Valorization 2022, 13, 893–903. [Google Scholar] [CrossRef]
- Bonelli, M.; Bruno, D.; Caccia, S.; Sgambetterra, G.; Cappellozza, S.; Jucker, C.; Tettamanti, G.; Casartelli, M. Structural and functional characterization of Hermetia illucens larval midgut. Front. Physiol. 2019, 10, 204. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Pinho, M.; Domingues, M.R.; Lillebø, A.I.; Falabella, P. Bioconversion of olive oil pomace by black soldier fly increases eco-efficiency in solid waste stream reduction producing tailored value-added insect meals. PLoS ONE 2023, 18, e0287986. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Oddon, S.B.; Loiotine, Z.; Resconi, A.; Gasco, L. Wheat starch processing by-products as rearing substrate for black soldier fly: Does the rearing scale matter? Animal 2024, 18, 101238. [Google Scholar] [CrossRef]
- Rodríguez-González, E.; da Cunha-Borges, V.; Cantero-Bahillo, E.; Fornari, T.; García-Risco, M.R.; Martin, D. Black soldier fly (Hermetia illucens) larvae accumulate bioactive compounds that modulate antioxidant activity when reared with bioactive agrifood by-products. Food Res. Int. 2025, 219, 117013. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]


| Description | Availability | Dry Matter | Crude Protein | Ether Extract | Ash | Crude Fiber | Distance to IO |
|---|---|---|---|---|---|---|---|
| % | km | ||||||
| Corn cobbs | September/ October | 94.30 | 1.59 | 21.10 | 1.37 | 28.60 | 15.00 |
| Grape byproduct (stalk) | September/ October | 52.40 | 3.67 | 1.20 | 4.28 | 23.60 | 10.00 |
| Wheat bran | All year round | 87.94 | 15.65 | 4.40 | 3.20 | 8.60 | 40.00 |
| Carrots | All year round | 7.70 | 0.68 | 0.30 | 0.47 | <1.00 | 19.10 |
| Mixture of peppers | Mainly in summer | 7.30 | 1.14 | 0.30 | 0.41 | 1.40 | 19.10 |
| Green peas | Spring | 15.10 | 3.91 | 0.70 | 0.55 | 1.90 | 19.10 |
| Tomato | Mainly in summer | 6.10 | 1.10 | 0.40 | 0.35 | 1.20 | 19.10 |
| Green pepper | Mainly in summer | 19.80 | 3.72 | 3.10 | 1.23 | 7.40 | 19.10 |
| Mixture of peppers with leaves | Mainly in summer | 9.00 | 2.09 | 5.10 | 1.31 | 3.00 | 19.10 |
| Acorn | Winter | 92.20 | 4.98 | 9.00 | 1.82 | 3.00 | 50.00 |
| Watermelon | All year round | 5.40 | 0.78 | 0.30 | 0.31 | <1.00 | 19.80 |
| Yellow melon | All year round | 8.00 | 0.99 | 0.50 | 0.50 | 1.20 | 19.80 |
| Green melon | All year round | 5.40 | 0.71 | 0.30 | 0.43 | <1.00 | 19.80 |
| White melon | All year round | 7.20 | 0.65 | 0.30 | 0.57 | <1.00 | 19.10 |
| Zucchini | All year round | 3.60 | 1.43 | 0.30 | 0.34 | 1.00 | 19.10 |
| Broccoli | All year round | 10.30 | 3.95 | 0.50 | 1.05 | 1.40 | 19.10 |
| Olive pomace | From October | 23.00 | 2.28 | 3.00 | 1.22 | 10.30 | 10.00 |
| Diets | |||||
|---|---|---|---|---|---|
| Control | Diet 1 | Diet 2 | |||
| Ingredient Composition | Water | % | 66.00 | ||
| Gainesville | 34.00 | 11.60 | 7.80 | ||
| White melon | 72.60 | 21.60 | |||
| Olive pomace | 55.20 | ||||
| Wheat bran | 15.40 | ||||
| Nutrient Composition | Humidity | % | 70.00 | 70.00 | 70.00 |
| Crude protein | 4.27 | 4.27 | 4.27 | ||
| Crude fat | 1.01 | 1.25 | 2.05 | ||
| Crude fiber | 3.73 | 3.84 | 4.27 | ||
| Carbohydrates | 1.7 | 7.3 | 11.7 | ||
| Sugars | 5.98 | 7.77 | 4.07 | ||
| Ashes | 2.43 | 1.99 | 2.00 | ||
| Energy | Kcal | 118.06 | 339.57 | 174.88 | |
| Parameters | Control | Diet 1 | Diet 2 |
|---|---|---|---|
| Larval individual weight (mg) | 76.38 ± 9.20 | 81.95 ± 8.37 | 78.98 ± 4.26 |
| Larval biomass per box (kg) | 1.6 ± 0.03 | 1.82 ± 0.17 | 1.80 ± 0.11 |
| Frass per box (kg) | 2.41 ± 0.04 a | 2.33 ± 0.09 a | 2.87 ± 0.06 b |
| Number of larvae per box | 21169 ± 2259 | 22403 ± 3608 | 22743 ± 514 |
| Substrate reduction (%) | 82.17 ± 0.26 a | 82.77 ± 0.66 a | 78.72 ± 0.41 b |
| FCR | 8.42 ± 0.16 | 7.47 ± 0.75 | 7.53 ± 0.49 |
| Bioconversion ratio (%) | 11.88 ± 0.23 | 13.48 ± 1.29 | 13.31 ± 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligeiro, C.; Souza, C.S.e.; Fantatto, R.; Murta, D. Olive Pomace and Melon Bio-Byproducts from the Agribusiness: A Promising Combination for the Sustainable Production of Animal Protein from BSF Larvae. Insects 2025, 16, 889. https://doi.org/10.3390/insects16090889
Ligeiro C, Souza CSe, Fantatto R, Murta D. Olive Pomace and Melon Bio-Byproducts from the Agribusiness: A Promising Combination for the Sustainable Production of Animal Protein from BSF Larvae. Insects. 2025; 16(9):889. https://doi.org/10.3390/insects16090889
Chicago/Turabian StyleLigeiro, Carolina, Clarice Silva e Souza, Rafaela Fantatto, and Daniel Murta. 2025. "Olive Pomace and Melon Bio-Byproducts from the Agribusiness: A Promising Combination for the Sustainable Production of Animal Protein from BSF Larvae" Insects 16, no. 9: 889. https://doi.org/10.3390/insects16090889
APA StyleLigeiro, C., Souza, C. S. e., Fantatto, R., & Murta, D. (2025). Olive Pomace and Melon Bio-Byproducts from the Agribusiness: A Promising Combination for the Sustainable Production of Animal Protein from BSF Larvae. Insects, 16(9), 889. https://doi.org/10.3390/insects16090889








