Ultrastructural Morphology and Descriptive Analysis of Cuticular Sensilla in Adult Tomicus pilifer (Coleoptera: Curculionidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect
2.2. Molecular Identification
2.3. Scanning Electron Microscopy
2.4. Terminology and Statistical Analysis
3. Results
3.1. Antennal Morphology and Sensilla Types of T. pilifer
3.1.1. Sensilla Trichoidea (ST)
3.1.2. Sensilla Zigzag (SZ)
3.1.3. Sensilla Coeloconica (Sco)
3.1.4. Sensilla Chaetica (Sch)
3.1.5. Böhm Bristles (Bb)
3.1.6. Sensilla Basiconica (Sb)
3.2. The Mouthpart Structure and Sensilla Types of T. pilifer
3.2.1. Sensilla Basiconica (SB)
3.2.2. Sensilla Twig Basiconica (Stb)
3.2.3. Sensilla Coeloconica (Sco)
3.2.4. Sensilla Trichoidea (ST)
3.2.5. Sensilla Chaetica (Sch)
3.2.6. Sensilla Zigzag (SZ)
3.2.7. Sensilla Digitiformia (Sdi)
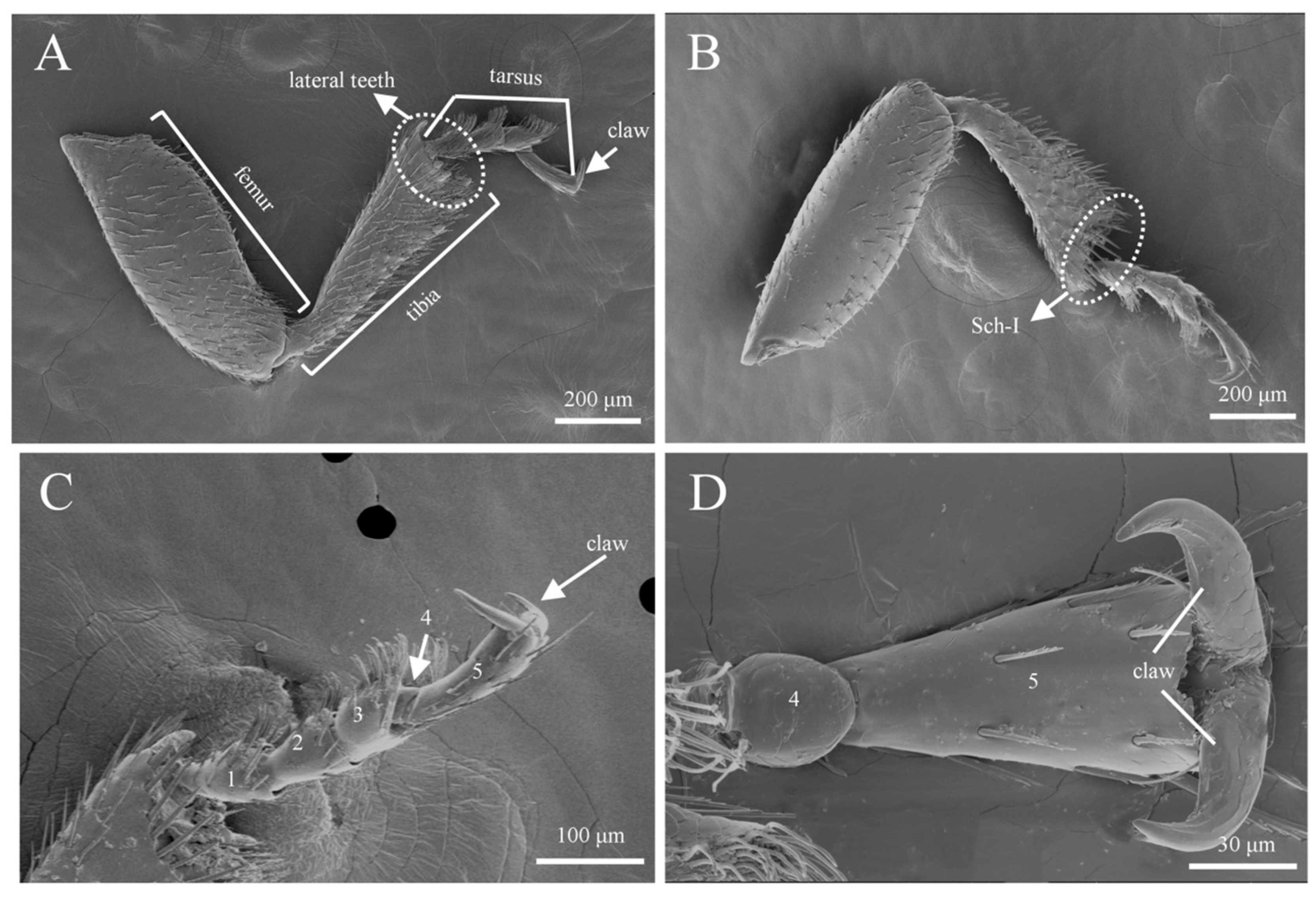
3.3. Sensilla Types and Morphology on Legs
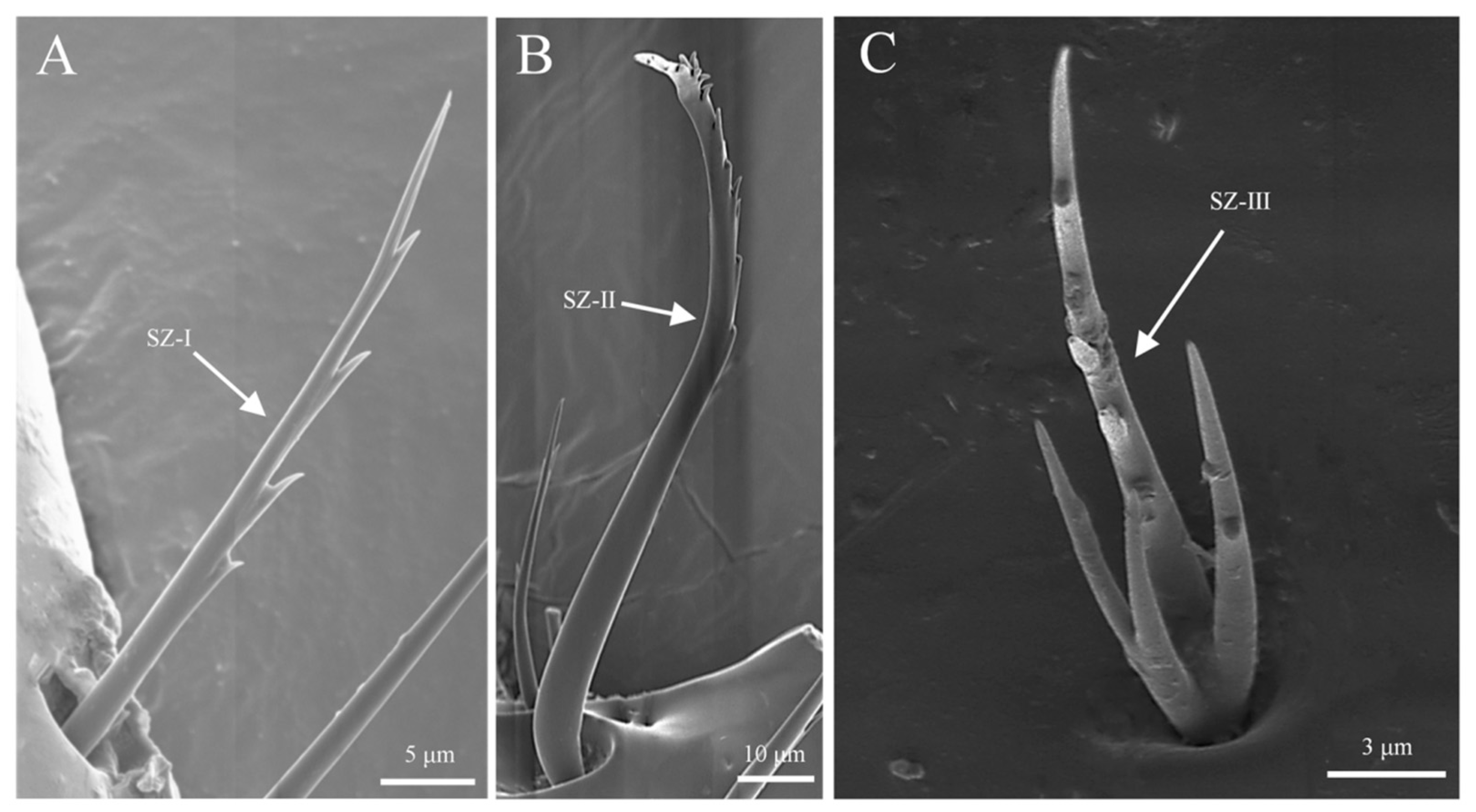
3.3.1. Sensilla Zigzag (SZ)
3.3.2. Sensilla Trichoidea (ST)
3.3.3. Sensilla Chaetica (Sch)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lieutier, F.; Langstrom, B.; Faccoli, M. “The Genus Tomicus”, in Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 371–426. [Google Scholar]
- Wang, H.; Liu, C.; Yue, F.; Yan, D.H.; Lu, Q. Identification of ophiostomatalean fungi associated with Tomicus pilifer infesting Pinus koraiensis in Northeastern China. Front. Microbiol. 2022, 2, 919302. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhao, B.; Wang, T.K.; Li, C.; Ma, L.; Li, G. Study on occurring regulation and control method of Tomicus pilifer. Jilin For. Sci. Technol. 2000, 29, 10–11. (In Chinese) [Google Scholar]
- Zhang, X.M.; Liu, Y.H.; Zhou, J.Q. Bionomics and control of Tomicus pilifer. Forest Pest. Dis. 2000, 5, 30–31. (In Chinese) [Google Scholar]
- Schneider, D. Insect Olfaction: Deciphering System for Chemical Messages. Science 1969, 163, 1031–1037. [Google Scholar] [CrossRef]
- Daly, P.J.; Ryan, M.F. Ultrastructure of antennal sensilla of Nebria brevicollis (Fab.) (Coleoptera: Carabidae). Int. J. Insect Morphol. Embryol. 1979, 8, 169–181. [Google Scholar] [CrossRef]
- Rebora, M.; Piersanti, S.; Gaino, E. The antennal sensilla of the adult of Libellula depressa (Odonata: Libellulidae). Arthropod Struct. Dev. 2008, 37, 504–510. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticle mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef]
- Chapman, R.F. Mechanoreception. Chemoreception. In The Insects, Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 610–652. [Google Scholar]
- Brożek, J.; Poprawa, I.; Wegierek, P.; Stroiński, A. Functional Morphology and Ultrastructure of the Peripheral Antennal Sensillar System of Graphosoma italicum (Müller, 1766) (Insecta: Hemiptera: Pentatomidae). Insects 2024, 15, 528. [Google Scholar] [CrossRef]
- Li, W.B.; Liu, N.Y.; Xu, Q.; Sun, M.; Fang, J.; Wang, S.Y. Ultrastructure structure of antennal sensilla of carabid beetle Carabus elysii Thomson, 1856 (Coleoptera: Carabidae). Entomol. Res. 2019, 49, 81–86. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, L.Z.; Hammond, A. Antennal morphology, structure and sensilla distribution in Miroplitis pallidipes (Hymenoptera: Braconidae). Micron 2007, 38, 684–693. [Google Scholar] [CrossRef]
- Ren, L.L.; Wu, Y.; Shi, J.; Zhang, L.; Luo, Y.Q. Antenna morphology and sensilla ultrastructure of Tetrigus lewisi Candèze (Coleoptera: Elateridae). Micron 2014, 60, 29–38. [Google Scholar] [CrossRef]
- Guo, J.; Du, Z.; Cui, G.; Wang, Z.; Wang, J.; Zhou, X. Ultrastructure Characteristics and Sexual Dimorphism of Antennal Sensilla in Tirathaba rufivena (Lepidoptera: Pyralidae). Insects 2022, 13, 797. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Wang, Q.; Yan, S. Comparative Morphology and Ultrastructure of Antennal Sensilla in Dendrolimus superans (Lepidoptera: Lasiocampidae) and Lymantria dispar (Lepidoptera: Lymantriidae). Insects 2024, 15, 655. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Keil, T.A.; Steinbrecht, R.A. Mechanosensitive and Olfactory Sensilla of Insects. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 477–516. [Google Scholar]
- Huang, X.; Xu, X.L.; Li, R.X.; Wang, S.; Tian, L.X. Ultrastructure and distribution of antennal sensilla of Bombus terrestris (Hymenoptera: Apidae). Zool. Anz. 2023, 302, 239–247. [Google Scholar] [CrossRef]
- Altner, H.; Routil, C.; Loftus, R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. 1981, 215, 289–308. [Google Scholar] [CrossRef]
- Zacharuk, R.Y.; Shields, V.D. Sensilla of Immature Insects. Annu. Rev. Entomol. 1991, 36, 331–354. [Google Scholar] [CrossRef]
- Keil, T.A. Sensilla on the maxillary palps of Helicoverpa armigera caterpillars: In search of the CO2-receptor. Tissue Cell 1996, 28, 703–717. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Ultrastructure and Function of Insect Chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Wilson, R.I.; Mainen, Z.F. Early events in olfactory processing. Annu. Rev. Neurosci. 2006, 29, 163–201. [Google Scholar] [CrossRef]
- Fleischer, J.; Krieger, J. Insect Pheromone Receptors-Key Elements in Sensing Intraspecific Chemical Signals. Front. Cell Neurosci. 2018, 12, 425. [Google Scholar] [CrossRef]
- Visser, J.H. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Vosshall, L.B. Olfaction in Drosophila. Curr. Opin. Neurobiol. 2000, 10, 498–503. [Google Scholar] [CrossRef]
- Guo, M.; Chen, Q.; Liu, Y.; Wang, G.; Han, Z. Chemoreception of mouthparts: Sensilla morphology and discovery of chemosensory genes in proboscis and labial palps of adult Helicoverpa armigera (Lepidoptera: Noctuidae). Front. Physiol. 2018, 9, 970. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, D.; Liu, F.F.; Yin, L.; Liu, T.X. Ultrastructure of the sensilla on antennae and mouthparts of larval and adult Plutella xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 2018, 17, 1409–1420. [Google Scholar] [CrossRef]
- Vega, F.E.; Bauchan, G.; Infante, F.; Davis, S. Mouthpart structure and elemental composition of the mandibles in the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). Ann. Entomol. Soc. Am. 2017, 110, 381–389. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, H.; Zhang, Y.; Zhang, X. Morphology and distribution of antennal, maxillary palp and labial palp sensilla of the adult bruchid beetles, Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Entomol. Res. 2018, 48, 466–479. [Google Scholar] [CrossRef]
- Lei, G.; Fu, Y.; Wu, W. Fine structure of mouthparts and forelegs of Euplatypus parallelus (Coleoptera: Curculionidae) with emphasis on the behavior of gallery excavation. Micron 2020, 130, 102815. [Google Scholar] [CrossRef]
- Nowińska, A.; Franielczyk-Pietyra, B.; Polhemus, D.A. The Leg Sensilla of Insects from Different Habitats—Comparison of Strictly Aquatic and Riparian Bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera). Insects 2023, 14, 441. [Google Scholar] [CrossRef]
- Brooks, G.T. Comprehensive insect physiology, biochemistry and pharmacology: Edited by G. A. Kerkut and L. I. Gilbert. Pergamon Press, Oxford. 1985. 13 Volumes. 8200 pp approx. £1700.00/$2750.00. ISBN 0 08 026850 1. Insect Biochem. 1985, 15, i–xiv. [Google Scholar] [CrossRef]
- Keil, T.A. Morphology and development of the peripheral olfactory organs. In Insect Olfaction; Hansson, B.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 5–47. [Google Scholar]
- Wang, P.Y.; Zhang, Z.; Kong, X.B.; Wang, H.B.; Zhang, S.F.; Gao, X.R.; Yuan, S.R. Antennal morphology and sensilla ultrastructure of three Tomicus species (Coleoptera: Curculionidae, Scolytinae). Microsc. Res. Tech. 2012, 75, 1672–1681. [Google Scholar] [CrossRef]
- Merivee, E.; Ploomi, A.; Rahi, M.; Luik, A.; Sammelselg, V. Antennal sensilla of the ground beetle Bembidion lampros Hbst (Coleoptera: Carabidae). Acta Zool. 2000, 81, 339–350. [Google Scholar] [CrossRef]
- Ploomi, A.; Merivee, E.; Rahi, M.; Bresciani, J. Antennal sensilla in ground beetles (Coleoptera: Carabidae). Agron. Res. 2003, 1, 221–228. [Google Scholar]
- Chen, H.B.; Zhang, Z.; Wang, H.B.; Kong, X.B. Antennal morphology and sensilla ultrastructure of Dendroctonus valens LeConte (Coleoptera: Curculionidae, Seolytinae), an invasive forest pest in China. Micron 2010, 41, 735–741. [Google Scholar] [CrossRef]
- Merivee, E.; Rahi, M.; Luik, A. Antennal sensilla of the click beetle, Melanotus villosus (Geoffroy) (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 1998, 28, 41–51. [Google Scholar] [CrossRef]
- Dickens, J.C.; Payne, T.L. Structure and function of the sensilla on the antennal club of the southern pine beetle, Dendroctonus frontalis (Zimmerman) (Coleoptera: Scolytidae). Int. J. Insect Morphol. Embryol. 1978, 7, 251–265. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Xu, F.; Yang, M.; Wang, X.; Wu, C. Ultrastructure of the Sensilla on the Antennae and Mouthparts of Bean Weevils, Megabruchidius dorsalis (Coleoptera: Bruchinae). Insects 2021, 12, 1112. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, S.F.; Liu, F.; Zhang, Z.; Xu, F.Y.; Yin, S.Y.; Kong, X.B. Sensilla on antennae and mouthparts of adult spruce bark beetle Ips typographus (Coleoptera: Curculionidae). Microsc. Res. Tech. 2021, 84, 1484–1497. [Google Scholar] [CrossRef]
- Peng, Q.Y.; Xie, M.H.; Pan, X.K.; Li, Y.; Gao, L.; Xu, F.; Wu, C.X.; Yang, M.F. Morphology and distribution of sensilla on antennae and mouthparts of the adult bruchid beetles, Bruchidius coreanus (Coleoptera: Bruchidae). Microsc. Res. Tech. 2024, 87, 922–932. [Google Scholar] [CrossRef]
- John, H.B. Antennal Morphology of Ips confuses (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1968, 61, 10–13. [Google Scholar]
- Shewale, M.K.; Nebesářová, J.; Grosse-Wilde, E.; Kalinová, B. Microscopic morphology and distribution of the antennal sensilla in the double-spined bark beetle, Ips duplicatus (Coleoptera: Curculionidae). Microsc. Res. Tech. 2023, 86, 1610–1625. [Google Scholar] [CrossRef]
- Chen, H.W.; Xie, D.; Jia, N.Y.; Yu, X.H.; Xu, J.J.; Chi, D.F.; Yu, J. Ultrastructure of the antennal sensilla of the red-haired pine bark beetle, Hylurgus ligniperda (Fabricius) (Coleoptera: Curculionidae). Plant Protection. 2023, 49, 236–246. (In Chinese) [Google Scholar]
- Lu, S.D.; Zhang, X.M.; Yang, B.; Zhao, N. Antennal Structure and Sensilla of Adult Tomicus yunnanensis Observed by Scanning Electron Microscope. J. Southwest. For. Univ. 2019, 39, 86–91. [Google Scholar]
- Zacharuk, R.Y. Antennae and Sensilla. Comparative Insect Physiology, Biochemistry and Pharmacology; Pergamon Press: Oxford, UK, 1985; pp. 1–69. [Google Scholar]
- Fernanda, L.; Francisco, A.T.; Arnulfo, A.; Gerardo, Z. Morphology of antennae of Dendroctonus vitei (Coleoptera: Curculionidae: Scolytinae), with special reference to sensilla clustered into pit craters. Can. Entomol. 2018, 150, 471–480. [Google Scholar]
- De, B.M.; Baker, T.C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef]
- Hallberg, E. Sensory Organs in Ips typographus (Insecta: Coleoptera) Fine structure of the sensilla of the maxillary and labial palps. Acta Zool. 1982, 63, 191–198. [Google Scholar] [CrossRef]
- Altner, H. Insect sensillum specificity and structure: An approach to a new typology. Olfaction Tast. 1977, 6, 295–303. [Google Scholar]
- Dai, H.G.; Honda, H. Sensilla on the antennal flagellum of the yellow spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1990, 25, 273–282. [Google Scholar] [CrossRef]
- Krishnan, A.; Prabhakar, S.; Sudarsan, S.; Sane, S.P. The neural mechanisms of antennal positioning in flying moths. J. Exp. Biol. 2012, 215, 3096–3105. [Google Scholar] [CrossRef]
- Orden, J.H.; Wood, D.L. The antennal receptors and olfactory response of Ips confusus (Coleoptera: Scolytidae) to male sex attractant in the Laboratory1. Ann. Entomol. Soc. Am. 1966, 59, 253–261. [Google Scholar] [CrossRef]
- Liu, G.Q.; Tian, M.Y. Scanning electron microscopic observation of Carabus prodigus antennae and their electroantennographic responses. J. South. China Agric. Univ. 2008, 29, 50–55. [Google Scholar]
- Urbanek, A.; Luszczek, D.; Kapusta, M. Antennal and mouthpart sensilla of Acanthoscelides obtectus say (Coleoptera: Bruchidae). Microsc. Res. Technol. 2016, 79, 1230–1235. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Zhu, H.; Yang, P.; Yang, B.; Li, Z. Fine Structure of the Mouthparts of Three Tomicus Beetles Co-Infecting Pinus yunnanensis in Southwestern China with Some Functional Comments. Insects 2023, 14, 933. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Whitehead, A.T. Ultrastructure of sensilla of the female mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Int. J. Insect Morphol. Embryol. 1981, 10, 19–28. [Google Scholar] [CrossRef]
- Shi, X.; Shen, J.C.; Zhang, S.F.; Liu, F.; Xu, F.Y.; Wang, G.L.; Zhang, Z.; Kong, X.B. Comparative analysis of the type and number of larval sensilla on the antennae and mouthparts of Ips typographus and Ips subelongatus using SEM. Zool. Anz. 2020, 289, 18–25. [Google Scholar] [CrossRef]
- Zacharuk, R.Y.; Albert, P.J.; Bellamy, F.W. Ultrastructure and function of digitiform sensilla on the labial palp of a larval elaterid (Coleoptera). Can. J. Zool. 1977, 55, 569–578. [Google Scholar]
- Li, X.M. Phylogenetic Relationship Analysis of Coleoptera Based on Antenna Character. Master Dissertation, Hebei University, Tianjin, China, 2010; pp. 1–75. (In Chinese). [Google Scholar]
- Zhao, H.Y.; Gao, Y.; Ma, X.Q.; Yu, W.J.; Sun, Y. Ultrastructure of Tarsal Sensilla of the Asian Longhorned Beetle Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae). Chin. Agric. Sci. Bull. 2016, 32, 40–43. [Google Scholar]
- Zhao, W.W.; Xie, G.L.; Wang, W.K. Ultrastructure of sensilla on the antennae, mouthparts and tarsi of Aromia bungii (Cerambycidae: Cerambycinae). J. Environ. Entomol. 2024, 46, 281–295. [Google Scholar]
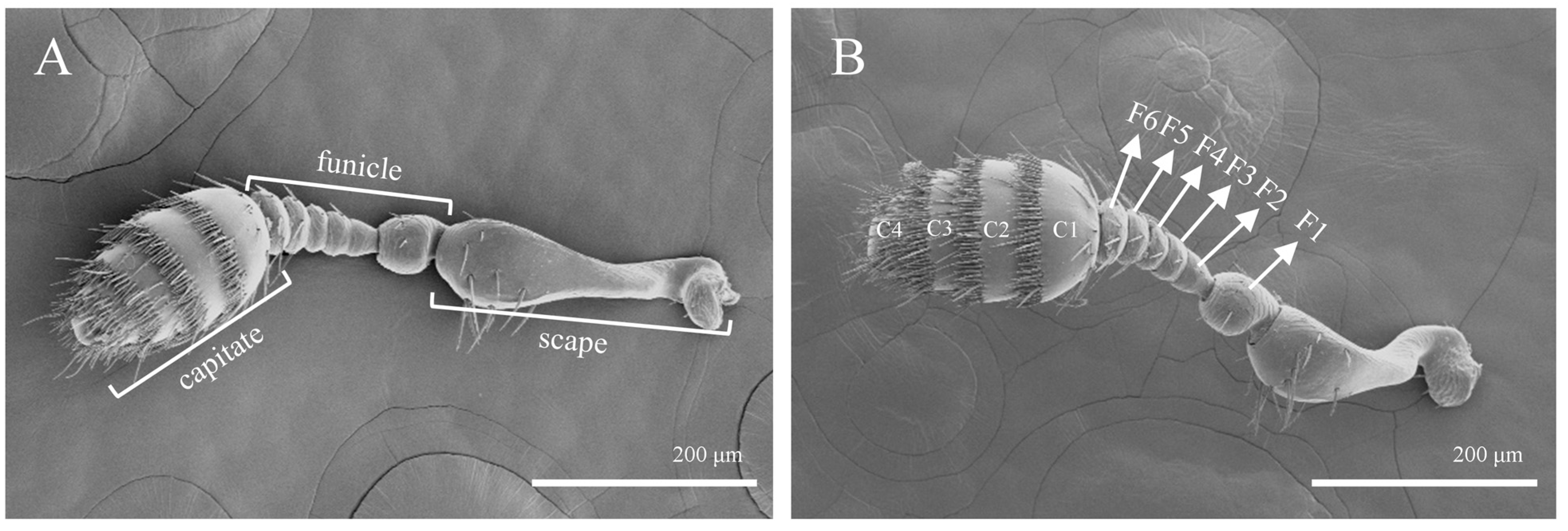


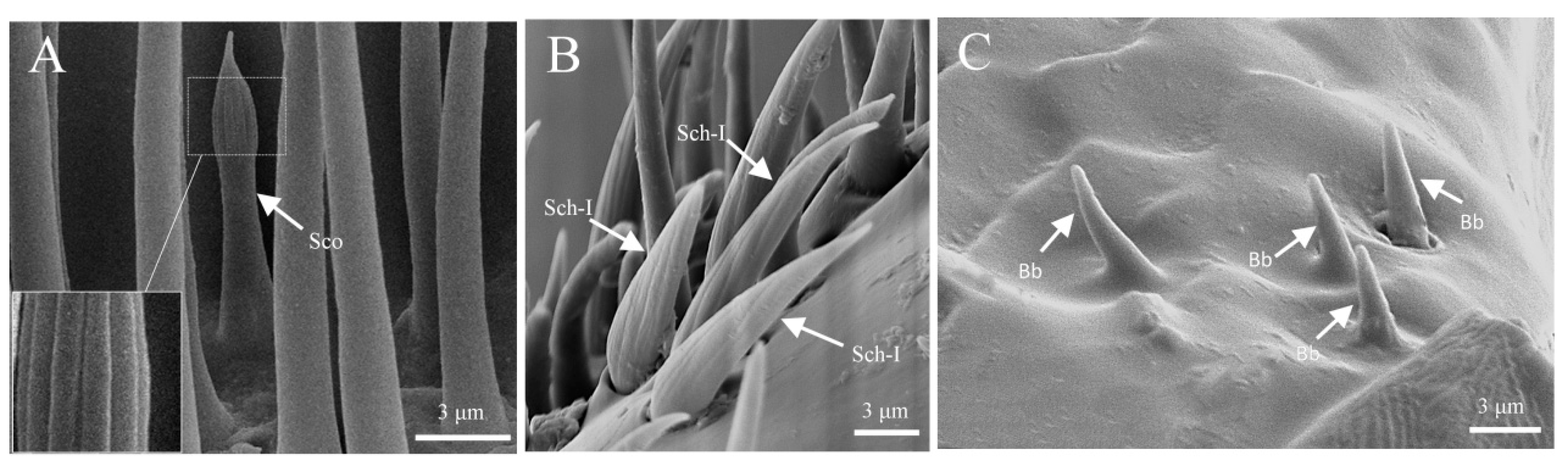


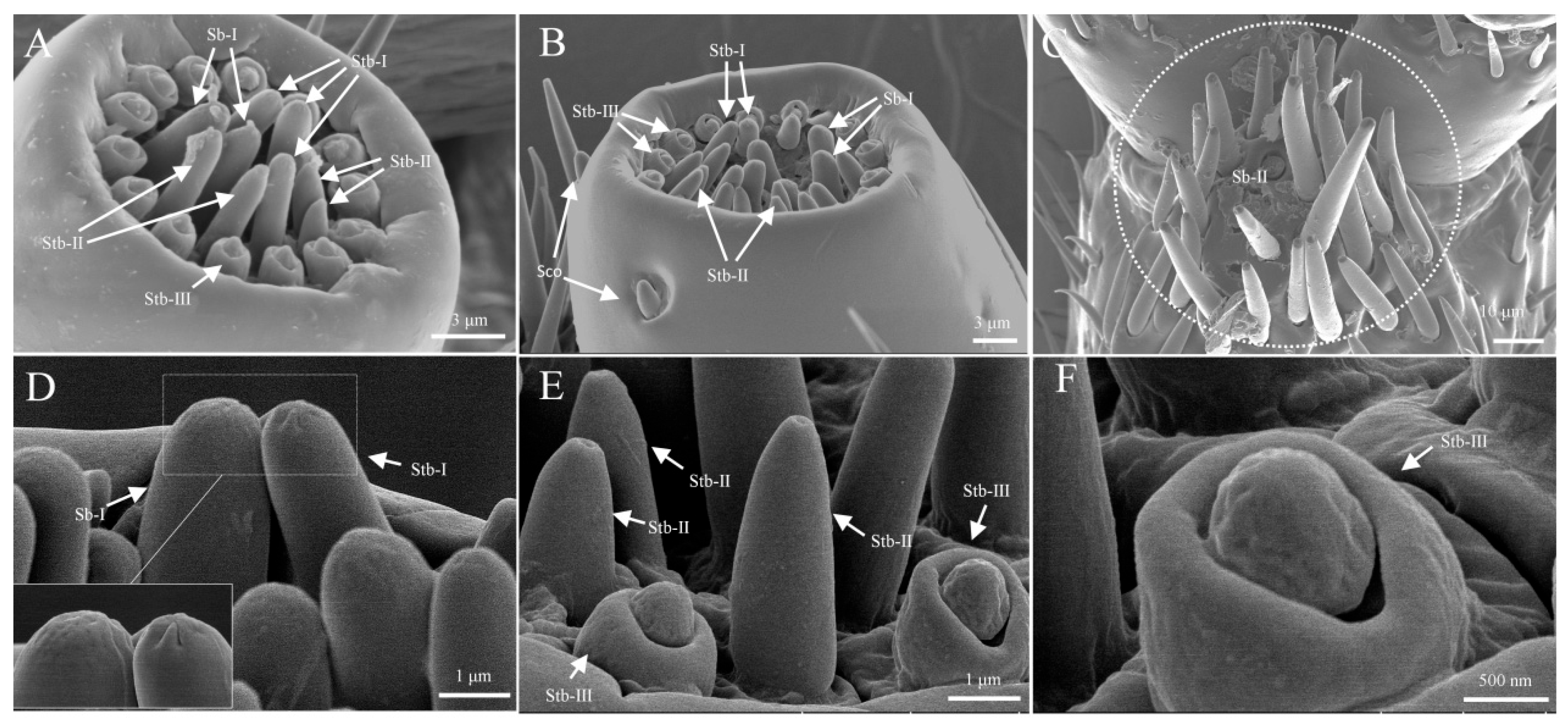

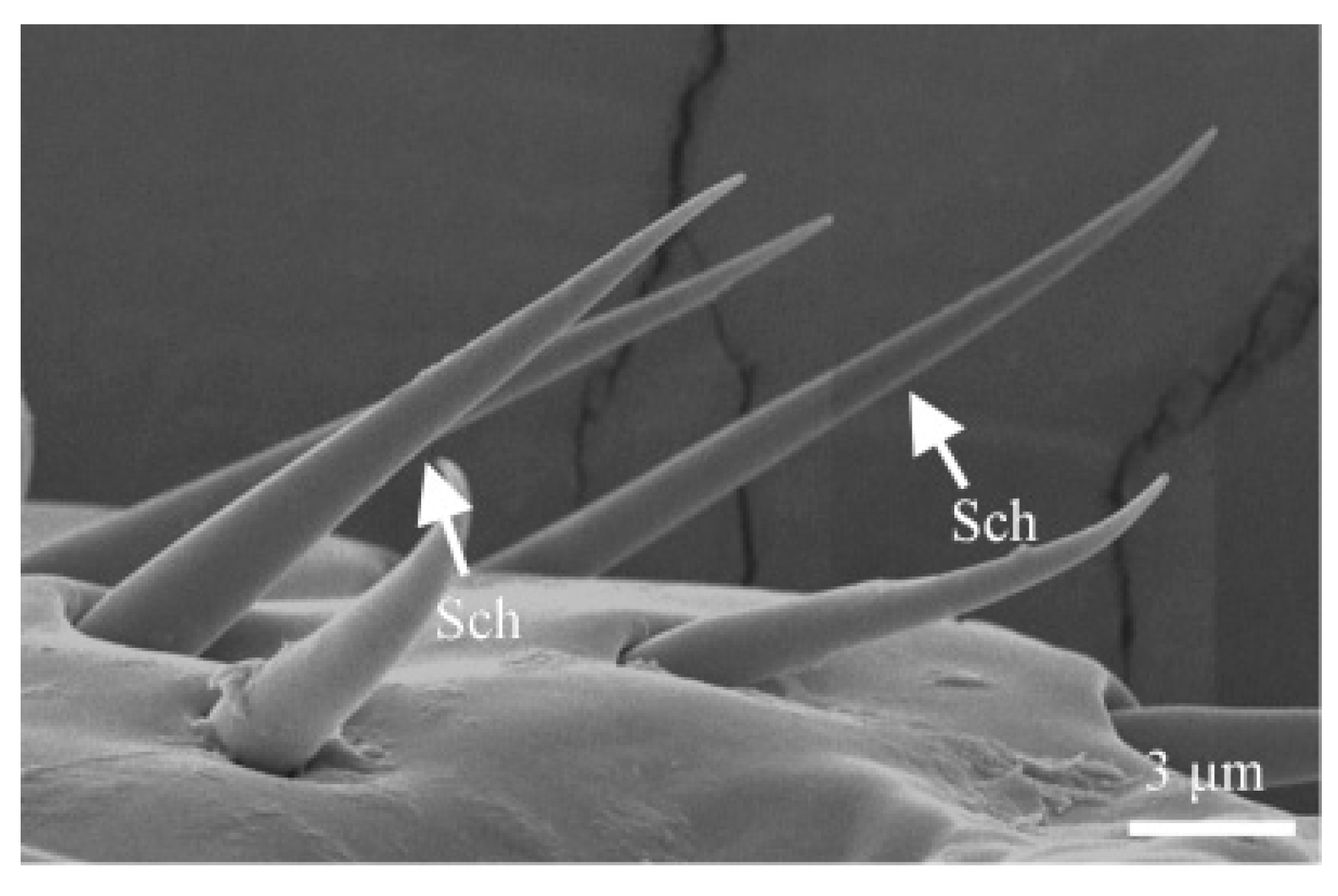




| ST I | ST Ⅱ | SZ I | SZ Ⅱ | Sco | Sch | Bb | Sb I | Sb Ⅱ | Sb Ⅲ | Sb Ⅳ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ||
| Scape | - | - | - | - | 17.3 ± 2.8 | 18.1 ± 2.3 | - | - | - | - | - | - | 14.7 ± 0.8 | 14.3 ± 1.1 | - | - | - | - | - | - | - | - | |
| Funicle | F1 | - | - | - | - | 4.7 ± 0.6 | 4.3 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.1 | - | - | 2.3 ± 0.2 | 2.7 ± 0.5 | - | - | - | - | - | - | - | - | - | - |
| F2 | - | - | - | - | - | - | - | - | - | - | - | 2.3 ± 0.2 | - | - | - | - | - | - | - | - | - | - | |
| F3 | - | - | - | - | 2.1 ± 0.2 | 2.3 ± 0.2 | 1.3 ± 0.1 | 1.7 ± 0.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| F4 | - | - | - | - | 3.7 ± 0.6 | 3.9 ± 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| F5 | - | - | - | - | 1.9 ± 0.3 | 1.7 ± 0.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| F6 | - | - | - | 7.8 ± 1.6 | 8.1 ± 1.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Capitate | C1 | 62.9 ± 11.3 | 59.7 ± 9.7 | - | - | 38.7 ± 6.2 | 41.2 ± 5.7 | - | - | 8.7 ± 2.1 | 9.6 ± 2.4 | - | - | - | - | 127.3 ± 13.9 | 121.2 ± 11.6 | 33.7 ± 5.9 | 36.8 ± 7.4 | - | - | - | - |
| C2 | 51.5 ± 9.2 | 47.8 ± 8.7 | - | - | 18.5 ± 3.1 | 16.4 ± 2.7 | - | - | 13.2 ± 2.5 | 13.7 ± 2.7 | - | - | - | - | 117.6 ± 17.3 | 114.8 ± 15.3 | 27.1 ± 3.1 | 31.8 ± 3.5 | - | - | - | - | |
| C3 | 47.1 ± 6.8 | 46.7 ± 7.2 | 3.6 ± 0.7 | 3.7 ± 0.4 | - | - | - | - | 9.1 ± 1.5 | 8.6 ± 1.7 | 18.2 ± 2.3 | 17.5 ± 2.7 | - | - | 41.2 ± 3.7 | 42.7 ± 3.5 | 21.2 ± 3.7 | 22.7 ± 3.5 | - | - | - | - | |
| C4 | 12.9 ± 2.7 | 13.7 ± 3.2 | 6.9 ± 1.5 | 6.7 ± 1.2 | - | - | - | - | - | - | 13.5 ± 1.2 | 13.8 ± 1.7 | 9.5 ± 0.5 | 9.2 ± 0.7 | 14.7 ± 2.9 | 13.1 ± 2.6 | 14.7 ± 2.9 | 13.1 ± 2.6 | - | 28.6 ± 6.1 | 8.7 ± 1.2 | 9.1 ± 1.4 | |
| Sensillum Type | Sex | Length (μm) | Basal Width (μm) | Exine | Tip | Shape | Total |
|---|---|---|---|---|---|---|---|
| ST Ⅰ | ♂ | 15.6 ± 1.7 | 1.4 ± 0.3 | stripe | sharp | erect | 174.4 ± 29.7 |
| ♀ | 15.7 ± 1.5 | 1.4 ± 0.2 | stripe | sharp | erect | 167.9 ± 28.8 | |
| ST Ⅱ | ♂ | 34.3 ± 3.2 | 2.1 ± 0.4 | stripe | sharp | bend | 10.5 ± 2.2 |
| ♀ | 34.1 ± 3.1 | 2.1 ± 0.4 | stripe | sharp | bend | 10.4 ± 1.6 | |
| SZ Ⅰ | ♂ | 23.1 ± 12.7 | 2.7 ± 0.4 | sawtooth | sharp | erect | 94.7 ± 15.4 |
| ♀ | 23.2 ± 12.7 | 2.6 ± 0.4 | sawtooth | sharp | erect | 95.9 ± 13.5 | |
| SZ Ⅱ | ♂ | 15.3 ± 1.9 | 1.3 ± 0.4 | sawtooth | blunt | erect | 3.1 ± 0.4 |
| ♀ | 15.3 ± 1.8 | 1.5 ± 0.4 | sawtooth | blunt | erect | 3.3 ± 0.4 | |
| Sco | ♂ | 8.6 ± 0.7 | 3.7 ± 0.2 | stripe | sharp | erect | 30.8 ± 6.1 |
| ♀ | 8.8 ± 0.7 | 3.5 ± 0.3 | stripe | sharp | erect | 31.9 ± 6.8 | |
| Sch | ♂ | 7.6 ± 0.7 | 2.9 ± 0.2 | stripe | sharp | erect | 36.9 ± 8.7 |
| ♀ | 7.7 ± 0.7 | 2.8 ± 0.3 | stripe | sharp | erect | 36.4 ± 8.2 | |
| Bb | ♂ | 2.4 ± 0.3 | 1.1 ± 0.1 | smooth | sharp | erect | 14.7 ± 0.8 |
| ♀ | 2.4 ± 0.2 | 1.3 ± 0.1 | smooth | sharp | erect | 14.3 ± 0.1 | |
| Sb Ⅰ | ♂ | 11.2 ± 0.9 | 2.1 ± 0.2 | pore | blunt | bend | 295.3 ± 17.1 |
| ♀ | 11.7 ± 1.1 | 2.1 ± 0.3 | pore | blunt | bend | 293.8 ± 16.1 | |
| Sb Ⅱ | ♂ | 11.6 ± 1.3 | 1.8 ± 0.2 | pore | blunt | bend | 96.4 ± 19.3 |
| ♀ | 11.3 ± 1.5 | 1.9 ± 0.2 | pore | blunt | bend | 93.8 ± 16.1 | |
| Sb Ⅲ | ♂ | 7.7 ± 0.6 | 3.1 ± 0.2 | pore | blunt | bend | 28.6 ± 6.1 |
| ♀ | - | - | - | - | - | - | |
| Sb Ⅳ | ♂ | 5.7 ± 0.6 | 1.6 ± 0.2 | pore | blunt | bend | 8.7 ± 0.6 |
| ♀ | 5.8 ± 0.8 | 1.6 ± 0.3 | pore | blunt | bend | 9.1 ± 1.4 |
| Sensilla | Total | Length (μm) | Basal Width (μm) | Location | |||
|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ||
| Sb Ⅰ | 13.2 ± 0.6 | 13.7 ± 0.6 | 5.2 ± 1.2 | 5.1 ± 0.9 | 0.8 ± 0.2 | 0.8 ± 0.3 | Mpt, Lpt |
| Sb Ⅱ | 42.7 ± 5.9 | 43.1 ± 5.7 | 28.7 ± 5.3 | 28.4 ± 5.2 | 5.1 ± 1.2 | 5.3 ± 1.3 | Lig |
| Stb Ⅰ | 14.9 ± 1.7 | 15.3 ± 1.7 | 2.4 ± 0.2 | 2.3 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 | Mpt, Lpt |
| Stb Ⅱ | 27.4 ± 3.7 | 27.1 ± 3.2 | 3.1 ± 0.2 | 3.2 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | Mpt, Lpt |
| Stb Ⅲ | 58.7 ± 6.7 | 58.3 ± 6.3 | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.2 ± 0.1 | 1.2 ± 0.1 | Mpt, Lpt |
| Sco | 5.4 ± 0.3 | 5.2 ± 0.3 | 1.7 ± 0.2 | 1.7 ± 0.3 | 0.8 ± 0.1 | 0.8 ± 0.1 | Mpt, Lpt |
| ST Ⅰ | 96.7 ± 11.5 | 101.4 ± 11.7 | 87.1 ± 21.2 | 86.6 ± 21.3 | 5.1 ± 1.2 | 5.3 ± 1.3 | Mx, Lr, Li |
| ST Ⅱ | 137.5 ± 12.9 | 135.1 ± 12.2 | 14.2 ± 2.6 | 13.9 ± 3.1 | 2.3 ± 0.7 | 2.3 ± 0.5 | Glc |
| Sch | 119.7 ± 8.4 | 119.2 ± 8.7 | 14.2 ± 2.7 | 14.5 ± 2.5 | 1.7 ± 0.4 | 1.7 ± 0.4 | Md, Mx, Li |
| SZ Ⅰ | 51.1 ± 7.2 | 48.6 ± 7.8 | 34.8 ± 2.1 | 34.2 ± 2.6 | 1.9 ± 0.3 | 1.8 ± 0.4 | Mx, Li |
| SZ Ⅱ | 18.7 ± 1.7 | 18.2 ± 1.6 | 107.3 ± 19.6 | 105.9 ± 16.2 | 5.9 ± 0.7 | 5.7 ± 0.6 | Mx |
| SZ Ⅲ | 13.2 ± 2.3 | 11.6 ± 2.7 | 7.5 ± 0.7 | 7.2 ± 0.7 | 2.8 ± 0.4 | 2.6 ± 0.5 | Li |
| Sdi | 12.7 ± 0.6 | 13.1 ± 0.7 | 13.7 ± 0.8 | 13.2 ± 0.7 | 1.3 ± 0.2 | 1.3 ± 0.2 | Mpl |
| Sensilla | Forelegs | Midlegs | Hindlegs | |||
|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | |
| SZ Ⅰ | 687.9 ± 57.4 | 677.5 ± 53.2 | 685.7 ± 49.6 | 681.2 ± 51.3 | 691.2 ± 53.2 | 682.3 ± 51.7 |
| SZ Ⅱ | 43.2 ± 7.6 | 45.3 ± 7.2 | 43.9 ± 6.8 | 43.1 ± 6.9 | 44.9 ± 7.3 | 43.7 ± 7.0 |
| ST Ⅰ | 132.2 ± 19.6 | 131.7 ± 19.2 | 129.7 ± 21.3 | 131.4 ± 20.7 | 131.9 ± 19.7 | 131.5 ± 19.4 |
| ST Ⅱ | 87.2 ± 9.7 | 88.5 ± 9.2 | 87.6 ± 9.2 | 87.5 ± 9.5 | 86.5 ± 10.1 | 87.3 ± 9.8 |
| Sch Ⅰ | 76.5 ± 11.6 | 79.7 ± 10.2 | 77.4 ± 10.8 | 77.6 ± 11.1 | 78.2 ± 10.9 | 77.9 ± 10.7 |
| Sch Ⅱ | 31.4 ± 6.7 | 30.8 ± 6.3 | 31.7 ± 6.5 | 32.4 ± 6.9 | 32.7 ± 6.2 | 31.8 ± 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, Q.; Luo, Y.; Yan, S. Ultrastructural Morphology and Descriptive Analysis of Cuticular Sensilla in Adult Tomicus pilifer (Coleoptera: Curculionidae). Insects 2025, 16, 890. https://doi.org/10.3390/insects16090890
Wang L, Wang Q, Luo Y, Yan S. Ultrastructural Morphology and Descriptive Analysis of Cuticular Sensilla in Adult Tomicus pilifer (Coleoptera: Curculionidae). Insects. 2025; 16(9):890. https://doi.org/10.3390/insects16090890
Chicago/Turabian StyleWang, Longzheng, Qi Wang, Yanan Luo, and Shanchun Yan. 2025. "Ultrastructural Morphology and Descriptive Analysis of Cuticular Sensilla in Adult Tomicus pilifer (Coleoptera: Curculionidae)" Insects 16, no. 9: 890. https://doi.org/10.3390/insects16090890
APA StyleWang, L., Wang, Q., Luo, Y., & Yan, S. (2025). Ultrastructural Morphology and Descriptive Analysis of Cuticular Sensilla in Adult Tomicus pilifer (Coleoptera: Curculionidae). Insects, 16(9), 890. https://doi.org/10.3390/insects16090890





