Efficient Conversion of Mushroom and Sawdust Residues in Protaetia brevitarsis Biosystem: Characterization of Humic Acid and Bacterial Communities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Frass Collection
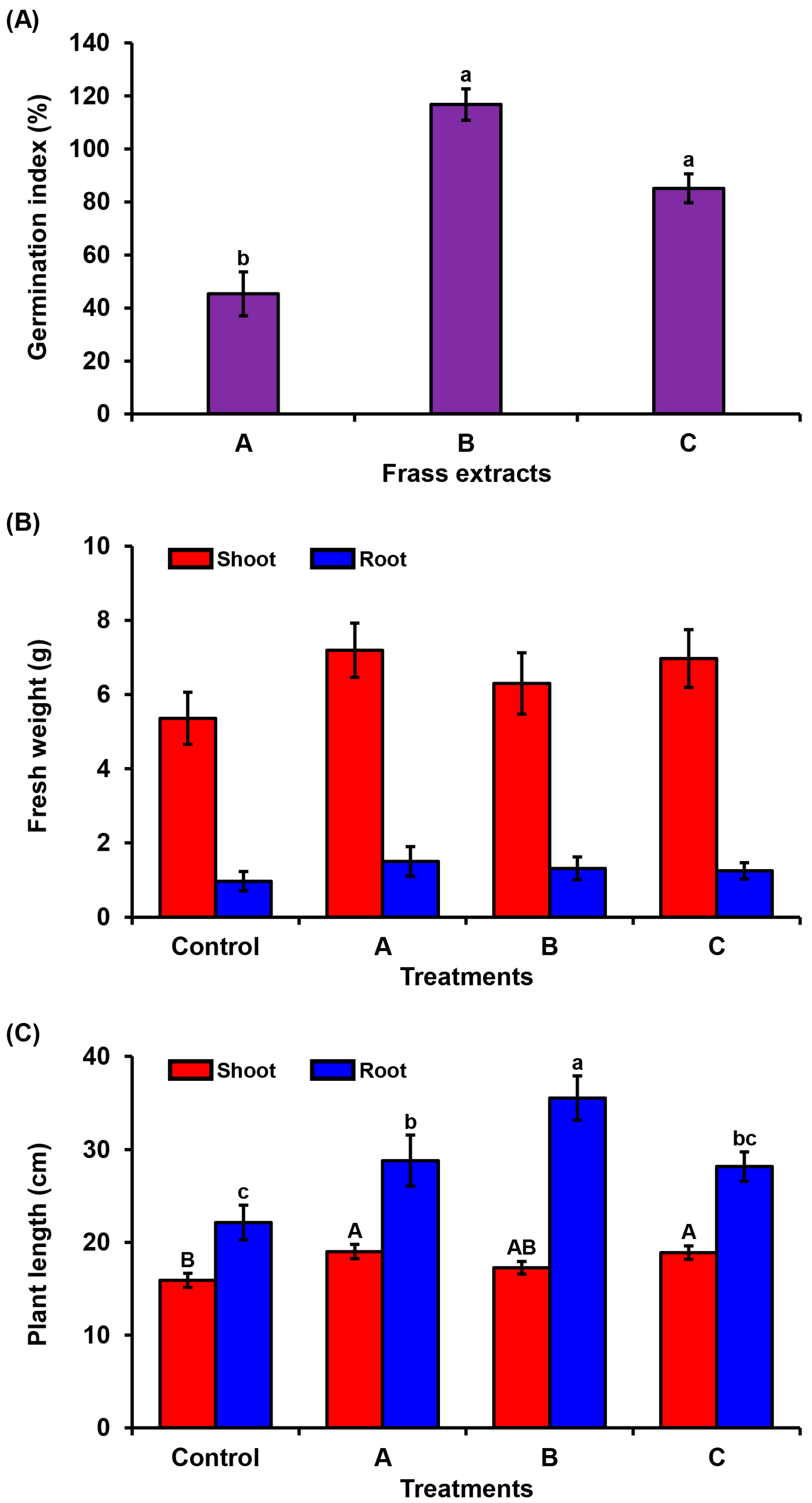
2.2. Phytotoxicity Assessment
2.3. Plant Growth Assay
2.4. Humic Acid Extraction
2.5. UV-Spectrophotometer
2.6. DNA Extraction and Sequencing
2.7. Bioinformatic Analysis
2.8. Statistical Analysis
3. Results
3.1. Larval Performance Across Feeding Substrates
3.2. Phytotoxicity Assay of PBL Frass on Tomato
3.3. PBL-Based Bioconversion Led to Increasing HA Quantity and Quality
3.4. Changes in Microbial Diversity and Composition Between Diet and Frass Due to PBL-Based Bioconversion
3.4.1. Changes in Microbial Diversity and Richness
3.4.2. Changes in Microbial Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBL | Protaetia brevitarsis larva |
| HA | Humic acid |
| LCB | Lignocellulosic biomass |
| SMSs | Spent mushroom substrates |
| PGP | Plant growth-promoting |
References
- Shaboon, A.M.; Qi, X.; Omar, M.A. Insect-mediated waste conversion. In Waste-to-Energy: Recent Developments and Future Perspectives Towards Circular Economy; Springer International Publishing: Cham, Switzerland, 2022; pp. 479–509. [Google Scholar]
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent substrate from mushroom cultivation: Exploitation potential toward various applications and value-added products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef]
- Alam, N.; Amin, R.; Khan, A.; Ara, I.; Shim, M.J.; Lee, M.W.; Lee, T.S. Nutritional analysis of cultivated mushrooms in Bangladesh–Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology 2008, 36, 228–232. [Google Scholar] [CrossRef]
- Deepalakshmi, K.; Sankaran, M. Pleurotus ostreatus: An oyster mushroom with nutritional and medicinal properties. J. Biochem Technol. 2014, 5, 718–726. [Google Scholar]
- Choi, U.K.; Bajpai, V.K.; Lee, N.H. Influence of calcinated starfish powder on growth, yield, spawn run and primordial germination of king oyster mushroom (Pleurotus eryngii). Food Chem. Toxicol. 2009, 47, 2830–2833. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Inter. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef]
- Jeong, D.; Min, N.; Kim, Y.; Kim, S.R.; Kwon, O. The effects of feed materials on the nutrient composition of Protaetia brevitarsis larvae. Entomol. Res. 2020, 50, 23–27. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Zhou, J.; Ge, W.; Zhang, X.; Wu, J.; Chen, Q.; Ma, D.; Chai, C. Effects of spent mushroom substrate on the dissipation of polycyclic aromatic hydrocarbons in agricultural soil. Chemosphere 2020, 259, 127462. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Sun, Y.; Bian, S.; Baig, S.A.; Hu, B.; Xu, X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 2017, 169, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.B.; Malviya, D.; Khan, W.; Singh, S.; Karthikeyan, N.; Imran, M.; Oh, J.W. Earthworm grazed-Trichoderma harzianum biofortified spent mushroom substrates modulate accumulation of natural antioxidants and bio-fortification of mineral nutrients in tomato. Front. Plant Sci. 2018, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Li, Y.; Lai, D.; Geng, L.; Liu, C.; Zhang, J.; Liu, R. Protaetia brevitarsis larvae can feed on and convert spent mushroom substrate from Auricularia auricula and Lentinula edodes cultivation. Waste Manag. 2020, 114, 234–239. [Google Scholar] [CrossRef]
- Li, Y.; Fu, T.; Geng, L.; Shi, Y.; Chu, H.; Liu, F.; Liu, C.; Song, F.; Zhang, J.; Shu, C. Protaetia brevitarsis larvae can efficiently convert herbaceous and ligneous plant residues to humic acids. Waste Manag. 2019, 83, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Khil’Ko, S.L.; Efimova, I.V.; Smirnova, O.V. Antioxidant properties of humic acids from brown coal. Solid Fuel Chem. 2011, 45, 367–371. [Google Scholar] [CrossRef]
- Ghani, M.J.; Akhtar, K.; Khaliq, S.; Akhtar, N.; Ghauri, M.A. Characterization of humic acids produced from fungal liquefaction of low-grade Thar coal. Process Biochem. 2021, 107, 1–12. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Engin. C. 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z.; Romic, D. Humic acids decrease uptake and distribution of trace metals, but not the growth of radish exposed to cadmium toxicity. Ecotoxicol. Environ. Saf. 2018, 151, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Zuo, X.; Ouyang, Z.; Liao, J.; Ding, R.; Zhang, W.; Zhang, C.; Guo, X.; Zhu, L. Novel insights into the relationship between the functional groups and photoactivity of biochar-derived dissolved organic matter. Water Res. 2024, 260, 121892. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Della Mura, B.; Moliterni, S.; Spagnoletta, A.; Dimatteo, S.; Bassi, D.; Cortimiglia, C.; Rebuzzi, R.; Palazzo, S.; et al. Tenebrio molitor frass: A cutting-edge biofertilizer for sustainable agriculture and advanced adsorbent precursor for environmental remediation. Agronomy 2025, 15, 758. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Zhang, S.; Xu, A.; Meng, Z.; Ge, H.; Ma, D. Transformation capability optimization and product application potential of Proteatia brevitarsis (Coleoptera: Cetoniidae) larvae on cotton stalks. Insects 2022, 13, 1083. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Zhou, T.; Zhao, W.; Li, D.; Liu, Y.; Wang, L. An integrated system of Pleurotus pulmonarius and Protaetia brevitarsis larvae promotes the efficient and high-value utilization of lignocellulosic biomass. Waste Biomass Valor. 2023, 14, 277–286. [Google Scholar] [CrossRef]
- Xuan, H.; Gao, P.; Du, B.; Geng, L.; Wang, K.; Huang, K.; Shu, C. Characterization of microorganisms from Protaetia brevitarsis larva frass. Microorganisms 2022, 10, 311. [Google Scholar] [CrossRef]
- Wang, K.; Gao, P.; Geng, L.; Liu, C.; Zhang, J.; Shu, C. Lignocellulose degradation in Protaetia brevitarsis larvae digestive tract: Refining on a tightly designed microbial fermentation production line. Microbiome 2022, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, T.; Geng, L.; Zhang, C.; Xiang, W.; Zhang, J.; Shu, C. Characterization and evaluation of actinomycete from the Protaetia brevitarsis Larva Frass. Front. Microbiol. 2024, 15, 1385734. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Luan, Y.; Sun, F. Variance adjusted weighted UniFrac: A powerful beta diversity measure for comparing communities based on phylogeny. BMC Bioinform. 2011, 12, 118. [Google Scholar] [CrossRef]
- Du, B.; Xuan, H.; Geng, L.; Li, W.; Zhang, J.; Xiang, W.; Shu, C. Microflora for improving the Auricularia auricula spent mushroom substrate for Protaetia brevitarsis production. iScience 2022, 25, 105307. [Google Scholar] [CrossRef]
- Wrap, T.C.A. Public Available Specification 100—Specification for Composted Material. Annex D: Method Assess Contam. Weed Propagules Phytotoxins Compost. Mater. The Waste and Resources Action Program (WRAP). 2002. Available online: https://scholar.google.com/scholar?q=Public%20available%20specification%20100%20%20specification%20for%20composted%20material (accessed on 26 February 2025).
- Meng, L.; Li, W.; Zhang, S.; Wu, C.; Lv, L. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Biores. Technol. 2017, 226, 39–45. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Jie, L.I.; Liu, J.J.; Lü, Y.C.; Wang, X.F.; Cui, Z.J. Microbial community dynamics during biogas slurry and cow manure compost. J. Integra. Agric. 2013, 12, 1087–1097. [Google Scholar] [CrossRef]
- Li, Y.; Shen, F.; Guo, H.; Wang, Z.; Yang, G.; Wang, L.; Deng, S. Phytotoxicity assessment on corn stover biochar, derived from fast pyrolysis, based on seed germination, early growth, and potential plant cell damage. Environ. Sci. Pollut. Res. 2015, 22, 9534–9543. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F.; Pera, A.; Forte, M.; de Bertoldi, M. Evaluating toxicity of immature compost. BioCycle 1981, 2, 54–57. [Google Scholar]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2006, 25, 2811. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Shirshova, L.T.; Ghabbour, E.A.; Davies, G. Spectroscopic characterization of humic acid fractions isolated from soil using different extraction procedures. Geoderma 2006, 133, 204–216. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soci. Amer. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Hanc, A.; Enev, V.; Hrebeckova, T.; Klucakova, M.; Pekar, M. Characterization of humic acids in a continuous-feeding vermicomposting system with horse manure. Waste Manag. 2019, 99, 1–11. [Google Scholar] [CrossRef]
- Mannaa, M.; Mansour, A.; Park, I.; Lee, D.W.; Seo, Y.S. Insect-based agri-food waste valorization: Agricultural applications and roles of insect gut microbiota. Environ. Sci. Ecotechnol. 2024, 17, 100287. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Ann. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Akami, M.; Ren, X.; Wang, Y.; Mansour, A.; Cao, S.; Qi, X.; Niu, C.Y. Host fruits shape the changes in the gut microbiota and development of Bactrocera dorsalis (Diptera: Tephritidae) larvae. Inter. J. Trop. Insect Sci. 2022, 42, 2127–2141. [Google Scholar] [CrossRef]
- Tippelt, A.; Nett, M.; Vela Gurovic, M.S. Complete genome sequence of the lignocellulose-degrading actinomycete Streptomyces albus CAS922. Microbiol. Resour. Announc. 2020, 9, e00227-20. [Google Scholar] [CrossRef] [PubMed]
- Zakalyukina, Y.V.; Zaytsev, A.R.; Biryukov, M.V. Study of cellulose-destroying activity of actinobacteria associated with ants. Mosc. Univ. Biol. Sci. Bull. 2021, 76, 20–27. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, B.; Chu, W.; Mao, J.; Zhang, J. Chemical nature of humic substances in two typical Chinese soils (upland vs. paddy soil): A comparative advanced solid state NMR study. Sci. Total Environ. 2017, 576, 444–452. [Google Scholar] [CrossRef]
- Huda, N.U.; Tanvir, R.; Badar, J.; Ali, I.; Rehman, Y. Arsenic-resistant plant growth promoting Pseudoxanthomonas mexicana S254 and Stenotrophomonas maltophilia S255 isolated from agriculture soil contaminated by industrial effluent. Sustainability 2022, 14, 10697. [Google Scholar] [CrossRef]
- Wu, L.; Che, S.; Qin, X.; Xu, Y.; Tian, S.; Zhu, Y.; Yang, M. Identification, characteristics and rice growth promotion of a highly efficient cellulolytic bacterial strain, Cellulomonas iranensis ZJW-6, isolated from paddy soil in central China. Front. Microbiol. 2023, 14, 1152966. [Google Scholar] [CrossRef] [PubMed]
- Kolton, M.; Frenkel, O.; Elad, Y.; Cytryn, E. Potential role of Flavobacterial gliding-motility and type IX secretion system complex in root colonization and plant defense. Mol. Plant-Microbe Interact. 2014, 27, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Kraut-Cohen, J.; Shapiro, O.H.; Dror, B.; Cytryn, E. Pectin induced colony expansion of soil-derived Flavobacterium strains. Front. Microbiol. 2021, 12, 651891. [Google Scholar] [CrossRef]
- Seo, H.; Kim, J.H.; Lee, S.M.; Lee, S.W. The Plant-Associated Flavobacterium: A Hidden Helper for Improving Plant Health. Plant Pathol. J. 2024, 40, 251. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Subramanian, S.; Smith, D.L. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci. Rep. 2020, 10, 12740. [Google Scholar] [CrossRef]
- Fan, D.; Smith, D.L. Mucilaginibacter sp. K improves growth and induces salt tolerance in nonhost plants via multilevel mechanisms. Front. Plant Sci. 2022, 13, 938697. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, A.; Lee, J.; Jeong, T.; Mannaa, M.; Kim, S.Y.; Song, J.-H.; Seo, Y.-S.; Lee, D.-W. Efficient Conversion of Mushroom and Sawdust Residues in Protaetia brevitarsis Biosystem: Characterization of Humic Acid and Bacterial Communities. Insects 2025, 16, 893. https://doi.org/10.3390/insects16090893
Mansour A, Lee J, Jeong T, Mannaa M, Kim SY, Song J-H, Seo Y-S, Lee D-W. Efficient Conversion of Mushroom and Sawdust Residues in Protaetia brevitarsis Biosystem: Characterization of Humic Acid and Bacterial Communities. Insects. 2025; 16(9):893. https://doi.org/10.3390/insects16090893
Chicago/Turabian StyleMansour, Abdelaziz, Junbeom Lee, Taeho Jeong, Mohamed Mannaa, Sun Young Kim, Jeong-Hun Song, Young-Su Seo, and Dae-Weon Lee. 2025. "Efficient Conversion of Mushroom and Sawdust Residues in Protaetia brevitarsis Biosystem: Characterization of Humic Acid and Bacterial Communities" Insects 16, no. 9: 893. https://doi.org/10.3390/insects16090893
APA StyleMansour, A., Lee, J., Jeong, T., Mannaa, M., Kim, S. Y., Song, J.-H., Seo, Y.-S., & Lee, D.-W. (2025). Efficient Conversion of Mushroom and Sawdust Residues in Protaetia brevitarsis Biosystem: Characterization of Humic Acid and Bacterial Communities. Insects, 16(9), 893. https://doi.org/10.3390/insects16090893








