Does Glycerin Used in Varroa Treatments Alter Propolis Quality?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Samples
2.2. Extraction and Sample Derivatization
2.3. GC-MS
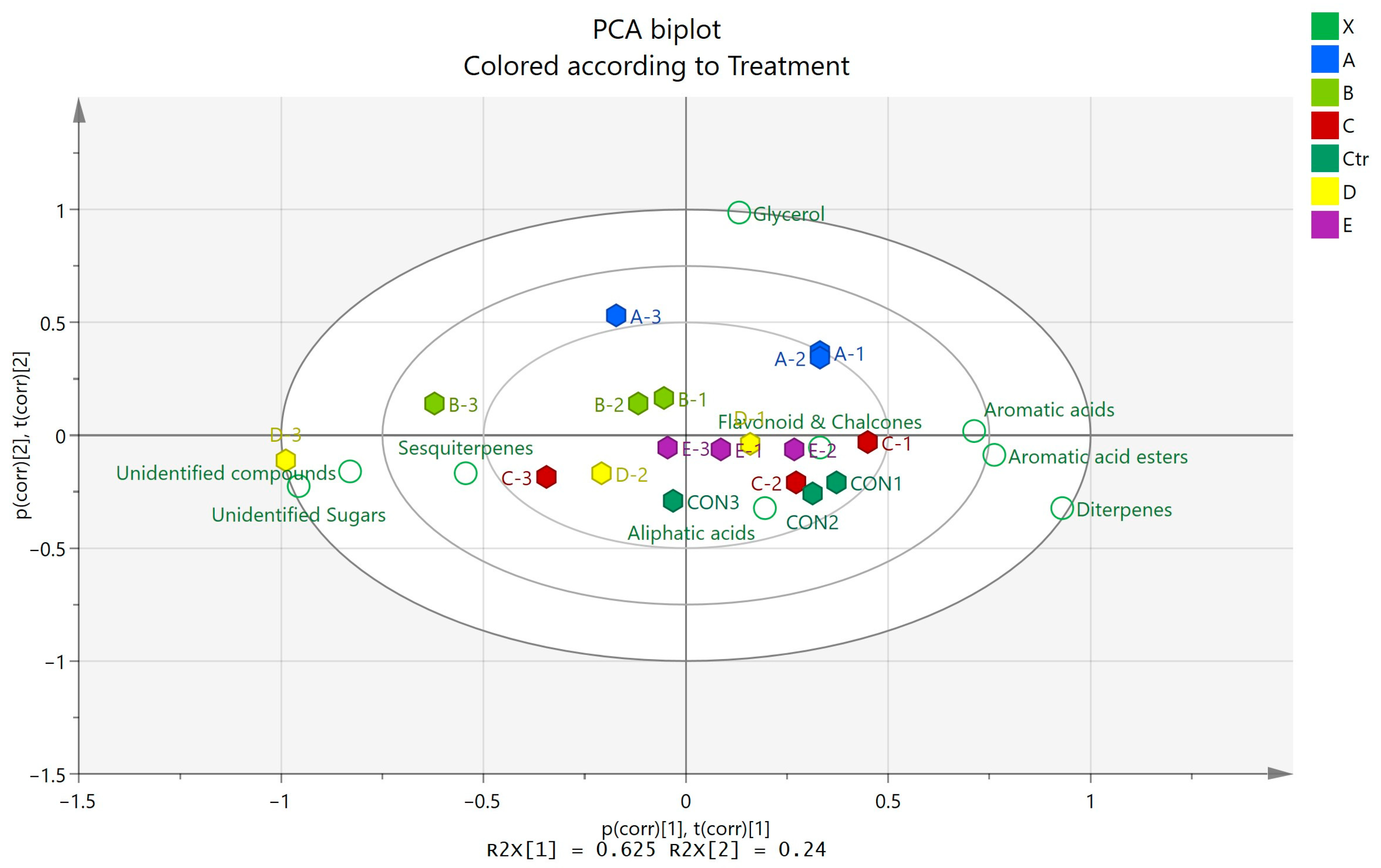
2.4. Principal Component Analysis
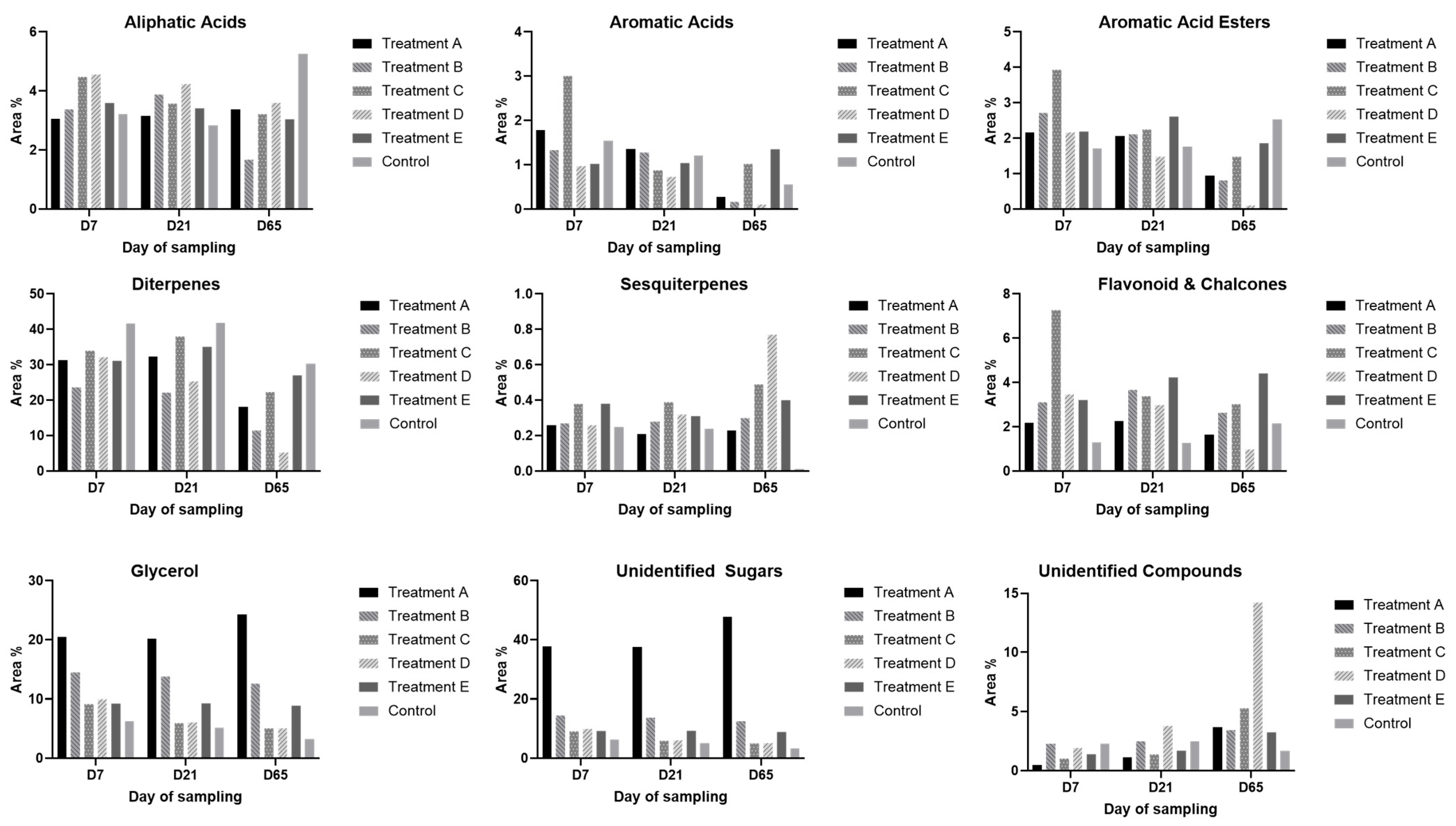
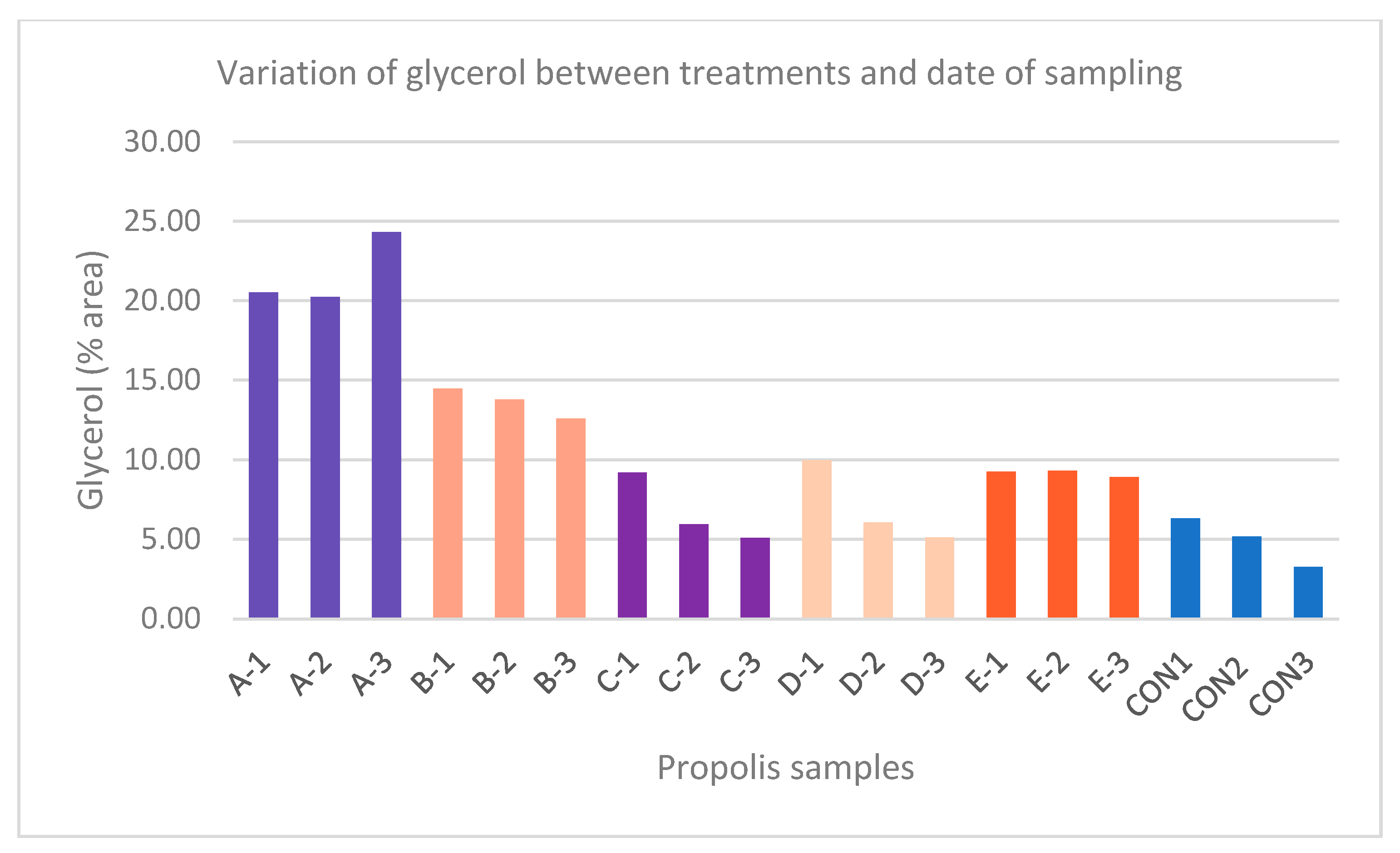
3. Results
GC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pyrgioti, E.; Graikou, K.; Cheilari, A.; Chinou, I. Assessment of Antioxidant and Antimicrobial Properties of Selected Greek Propolis Samples (North East Aegean Region Islands). Molecules 2022, 27, 8198. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, R.; Widelski, J.; Okińczyc, P.; Truszkiewicz, W.; Glous, J.; Sieniawska, E. Exposure to Nepalese Propolis Alters the Metabolic State of Mycobacterium Tuberculosis. Front. Microbiol. 2022, 13, 929476. [Google Scholar] [CrossRef] [PubMed]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and Biological Evaluation of Selected Mediterranean Propolis Samples. Is It a New Type? LWT 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef]
- Ghosh, S.; Al-Sharify, Z.T.; Maleka, M.F.; Onyeaka, H.; Maleke, M.; Maolloum, A.; Godoy, L.; Meskini, M.; Rami, M.R.; Ahmadi, S.; et al. Propolis Efficacy on SARS-CoV Viruses: A Review on Antimicrobial Activities and Molecular Simulations. Environ. Sci. Pollut. Res. 2022, 29, 58628–58647. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.M.; Connor, L.J. Honey Bee Biology and Beekeeping; Wicas press: Kalamazoo, MI, USA, 2013; ISBN 1-878075-29-2. [Google Scholar]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Milani, N.; Barbattini, R. Effectiveness of Apistan (Fluvalinate) in the Control of Varroa jacobsoni Oudemans and Its Tolerance by Apis mellifera Linnaeus. Apicoltura 1988, 4, 39–58. [Google Scholar]
- Milani, N.; Lob, M. Plastic Strips Containing Organophosphorous Acaricides to Control Varroa jacobsoni. Am. Bee J. 1998, 138, 612–615. [Google Scholar]
- Ritter, W. Medications Registered in Western Europe for Varroatosis Control. Apidologie 1988, 19, 113–116. [Google Scholar] [CrossRef]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Rodopoulou, M.-A. Evaluation of Oxalic Acid with Glycerin Efficacy against Varroa destructor (Varroidae): A Four Year Assay. J. Apic. Res. 2023, 61, 847–855. [Google Scholar] [CrossRef]
- Laboratorios Calier S.A. APITRAZ 500 mg Bee-Hive Strips for Honey Bees. 2017. Available online: https://medicines.health.europa.eu/veterinary/en/600000039327 (accessed on 7 November 2024).
- Medici, S.K.; Maggi, M.D.; Sarlo, E.G.; Ruffinengo, S.; Marioli, J.M.; Eguaras, M.J. The Presence of Synthetic Acaricides in Beeswax and Its Influence on the Development of Resistance in Varroa destructor. J. Apic. Res. 2016, 54, 267–274. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Sara Hernández-Rodríguez, C.; González-Cabrera, J. Assessing the Resistance to 472 Acaricides in Varroa destructor from Several Spanish Locations. Parasitol. Res. 2020, 119, 3595–3601. [Google Scholar] [CrossRef]
- Lodesani, M.; Costa, C.; Serra, G.; Colombo, R.; Sabatini, A.G. Acaricide Residues in Beeswax after Conversion to Organic Beekeeping Methods. Apidologie 2008, 39, 324–333. [Google Scholar] [CrossRef]
- Milani, N. The Resistance of Varroa jacobsoni Oud. to Acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; Official Journal of the European Union: Luxembourg, 2010; pp. 1–72.
- Imdorf, A.; Charriere, J.-D.; Bachofen, B. Efficiency Checking of the Varroa jacobsoni Control Methods by Means of Oxalic Acid. Apiacata 1997, 32, 89–91. [Google Scholar]
- Brødsgaard, C.; Jansen, S.; Hansen, C.; Hansen, H. Spring Treatment with Oxalic Acid in Honey Bee Colonies as Varroa Control. DIAS Rep. Hortic. 1999, 6, 16. [Google Scholar]
- Maggi, M.; Tourn, E.; Negri, P.; Szawarski, N.; Marconi, A.; Gallez, L.; Medici, S.; Ruffinengo, S.; Brasesco, C.; De Feudis, L.; et al. A New Formulation of Oxalic Acid for Varroa destructor Control Applied in Apis Mellifera Colonies in the Presence of Brood. Apidologie 2016, 47, 596–605. [Google Scholar] [CrossRef]
- Segur, J.B.; Oberstar, H.E. Viscosity of Glycerol and Its Aqueous Solutions. Ind. Eng. Chem. 1951, 43, 2117–2120. [Google Scholar] [CrossRef]
- De Groot, A. Propolis: A Review of Properties, Applications, Chemical Composition, Contact Allergy, and Other Adverse Effects. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the Skin: Holistic Approach to Its Origin and Functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Dehaibes, S.R.; Otero-Colina, G.; Sedas, V.P.; Jiménez, J.A.V. Resistance to Amitraz and Flumethrin in Varroa destructor Populations from Veracruz, Mexico. J. Apic. Res. 2005, 44, 124–125. [Google Scholar] [CrossRef]
- Papaefthimiou, C.; Papachristoforou, A.; Theophilidis, G. Biphasic Responses of the Honeybee Heart to Nanomolar Concentrations of Amitraz. Pestic. Biochem. Physiol. 2013, 107, 132–137. [Google Scholar] [CrossRef]
- O’Neal, S.T.; Brewster, C.C.; Bloomquist, J.R.; Anderson, T.D. Amitraz and Its Metabolite Modulate Honey Bee Cardiac Function and Tolerance to Viral Infection. J. Invertebr. Pathol. 2017, 149, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Bowen, I.D. Histochemical Characterization of Cell Death in Honeybee Larvae Midgut after Treatment with Paenibacillus larvae, Amitraz and Oxytetracycline. Cell Biol. Int. 2000, 24, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Rangel, J. Exposure to Pesticides during Development Negatively Affects Honey Bee (Apis mellifera) Drone Sperm Viability. PLoS ONE 2018, 13, e0208630. [Google Scholar] [CrossRef]
- Papežíková, I.; Palíková, M.; Navrátil, S.; Heumannová, R.; Fronc, M. The Effect of Oxalic Acid Applied by Sublimation on Honey Bee Colony Fitness: A Comparison with Amitraz. Acta Vet. Brno 2016, 85, 255–260. [Google Scholar] [CrossRef]
- Rademacher, E.; Fahlberg, A.; Raddatz, M.; Schneider, S.; Voigt, K. Galenics: Studies of the Toxicity and Distribution of Sugar Substitutes on Apis Mellifera. Apidologie 2013, 44, 222–233. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A Promising and Abundant Carbon Source for Industrial Microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemical Analysis and Antimicrobial Activity of Greek Propolis. Planta Medica 2004, 70, 515–519. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and Bee Health: The Natural History and Significance of Resin Use by Honey Bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
| Bee Colonies | Propolis Samples | Anti-Varroa Treatments |
|---|---|---|
| Colony A | A-1/A-2/A-3 | 4 strips with oxalic acid and glycerin (500 g/L of glycerin) |
| Colony Β | Β-1/Β-2/Β-3 | Instillation of oxalic acid (80 g of oxalic acid in a 1:1 solution of sugar–water) |
| Colony C | C-1/C-2/C-3 | 2 strips with amitraz (Apitraz® 500 mg/strip) |
| Colony D | D-1/D-2/D-3 | Sublimation of oxalic acid (2.5 g/colony) |
| Colony E | E-1/E-2/E-3 | 4 strips with formic acid, water, and glycerin (50:40:10) |
| Colony F | CON1/CON2/CON3 | No treatment |
| Area % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Category | A-1 | A-2 | A-3 | B-1 | B-2 | B-3 | C-1 | C-2 | C-3 | D-1 | D-2 | D-3 | E-1 | E-2 | E-3 | CON1 | CON2 | CON3 |
| Aliphatic acids | 3.05 | 3.15 | 3.38 | 3.38 | 3.88 | 1.67 | 4.48 | 3.57 | 3.22 | 4.56 | 4.23 | 3.60 | 3.59 | 3.41 | 3.04 | 3.22 | 2.84 | 5.25 |
| Aromatic acids | 1.78 | 1.36 | 0.27 | 1.33 | 1.28 | 0.16 | 3.01 | 0.88 | 1.02 | 0.97 | 0.73 | <0.1 | 1.02 | 1.04 | 1.35 | 1.54 | 1.21 | 0.56 |
| Aromatic acid esters | 2.17 | 2.06 | 0.95 | 2.72 | 2.11 | 0.81 | 3.94 | 2.25 | 1.48 | 2.16 | 1.48 | <0.1 | 2.19 | 2.61 | 1.86 | 1.72 | 1.77 | 2.53 |
| Diterpenes | 31.41 | 32.27 | 18.07 | 23.59 | 22.16 | 11.50 | 34.04 | 38.02 | 22.32 | 32.13 | 25.34 | 5.21 | 31.09 | 35.12 | 27.00 | 41.65 | 41.87 | 30.24 |
| Sesquiterpenes | 0.26 | 0.21 | 0.23 | 0.27 | 0.28 | 0.30 | 0.38 | 0.39 | 0.49 | 0.26 | 0.32 | 0.77 | 0.38 | 0.31 | 0.40 | 0.25 | 0.24 | - |
| Flavonoid and Chalcones | 2.19 | 2.25 | 1.66 | 3.10 | 3.66 | 2.64 | 7.27 | 3.39 | 3.02 | 3.46 | 2.99 | 0.98 | 3.21 | 4.23 | 4.41 | 1.30 | 1.26 | 2.16 |
| Glycerol | 20.51 | 20.23 | 24.30 | 14.48 | 13.78 | 12.60 | 9.19 | 5.95 | 5.09 | 9.98 | 6.07 | 5.12 | 9.25 | 9.30 | 8.91 | 6.30 | 5.18 | 3.27 |
| Unidentified Sugars | 37.72 | 37.63 | 47.70 | 49.23 | 50.59 | 63.89 | 36.19 | 44.41 | 58.36 | 45.17 | 55.51 | 66.39 | 48.21 | 42.69 | 50.06 | 41.28 | 44.10 | 52.86 |
| Unidentified compounds | 0.51 | 1.16 | 3.69 | 2.28 | 2.51 | 3.46 | 1.03 | 1.39 | 5.29 | 1.92 | 3.80 | 14.22 | 1.41 | 1.70 | 3.26 | 2.30 | 2.47 | 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakosta, F.; Graikou, K.; Charistos, L.; Cheilari, A.; Hatjina, F.; Chinou, I. Does Glycerin Used in Varroa Treatments Alter Propolis Quality? Insects 2025, 16, 871. https://doi.org/10.3390/insects16090871
Papakosta F, Graikou K, Charistos L, Cheilari A, Hatjina F, Chinou I. Does Glycerin Used in Varroa Treatments Alter Propolis Quality? Insects. 2025; 16(9):871. https://doi.org/10.3390/insects16090871
Chicago/Turabian StylePapakosta, Freideriki, Konstantia Graikou, Leonidas Charistos, Antigoni Cheilari, Fani Hatjina, and Ioanna Chinou. 2025. "Does Glycerin Used in Varroa Treatments Alter Propolis Quality?" Insects 16, no. 9: 871. https://doi.org/10.3390/insects16090871
APA StylePapakosta, F., Graikou, K., Charistos, L., Cheilari, A., Hatjina, F., & Chinou, I. (2025). Does Glycerin Used in Varroa Treatments Alter Propolis Quality? Insects, 16(9), 871. https://doi.org/10.3390/insects16090871









