Metabolic Remodeling of the Tricarboxylic Acid Cycle and Glycolysis Reveals Cold-Induced Respiratory Adaptations in Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae) Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Low-Temperature Treatment
2.3. Enzyme Activity Assay in Larvae at Low Temperature
2.4. Changes in Energy Metabolites Under Low-Temperature Conditions
2.4.1. Determination of Glucose Content
2.4.2. Determination of the Metabolites
2.5. Transcriptome Sequencing
2.6. Screening of Differentially Expressed Genes and Identification of Key Genes
2.7. Statistical Analysis
3. Results
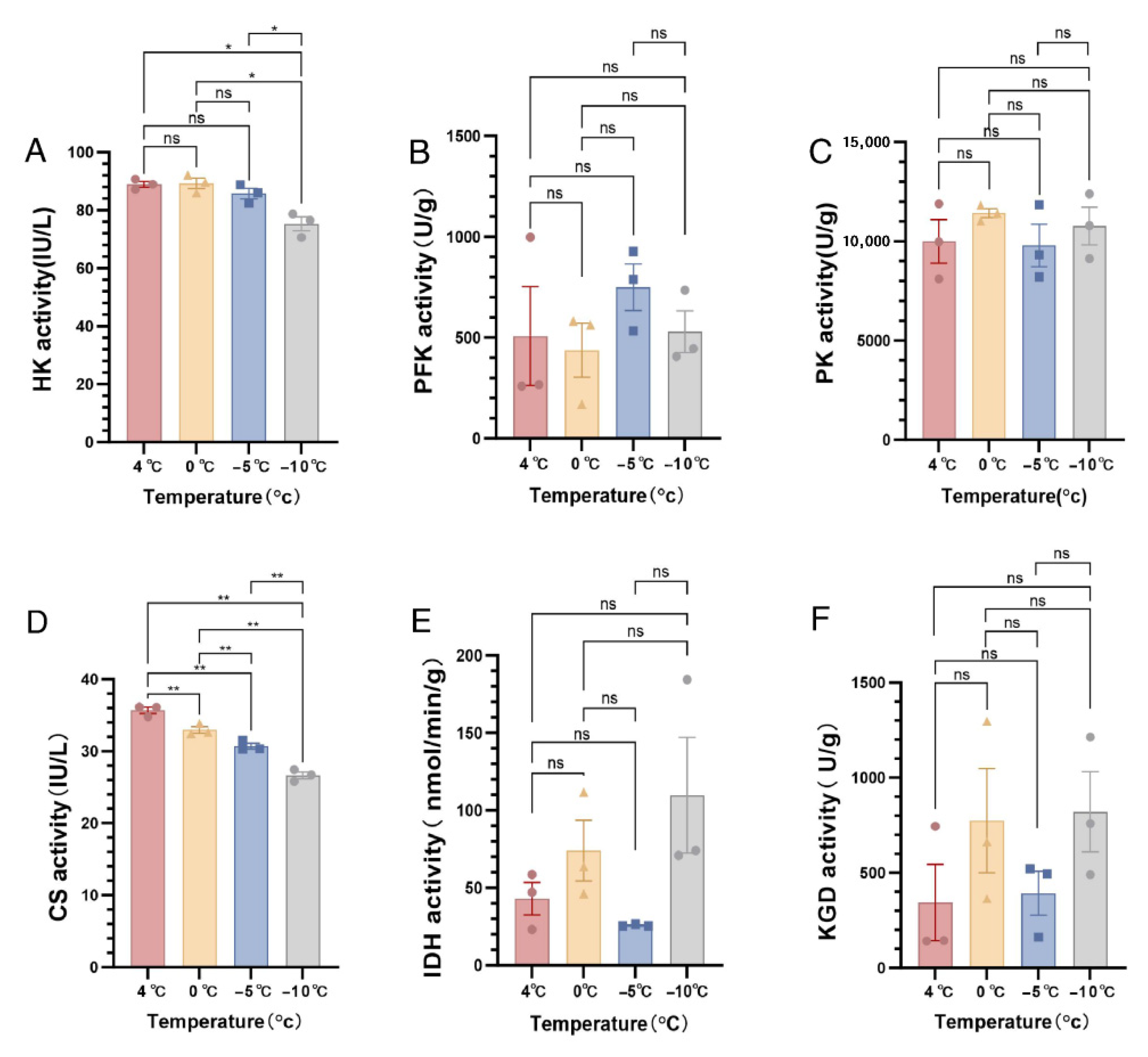
3.1. Changes in Enzyme Activity in the TCA Cycle and Glycolysis
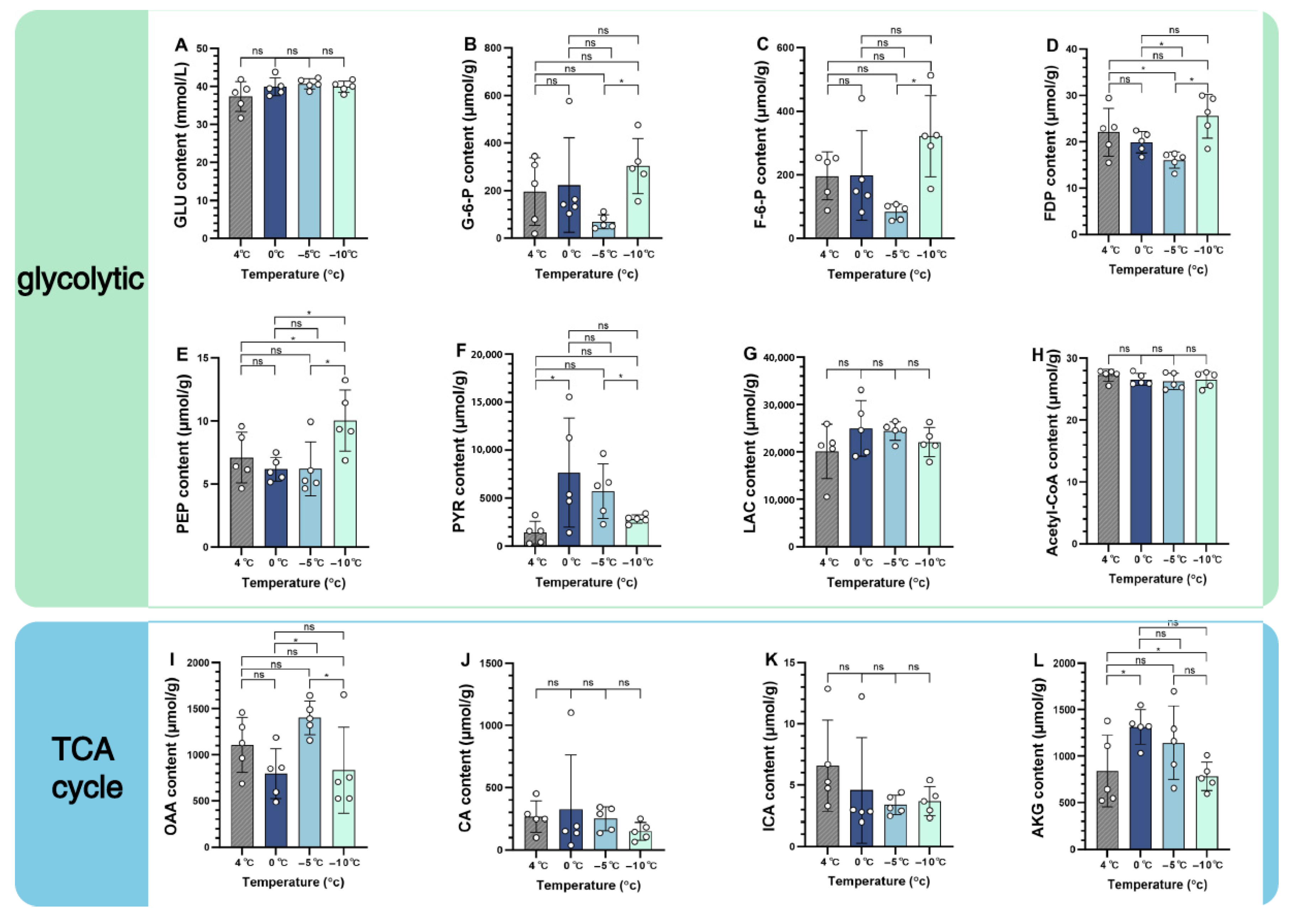
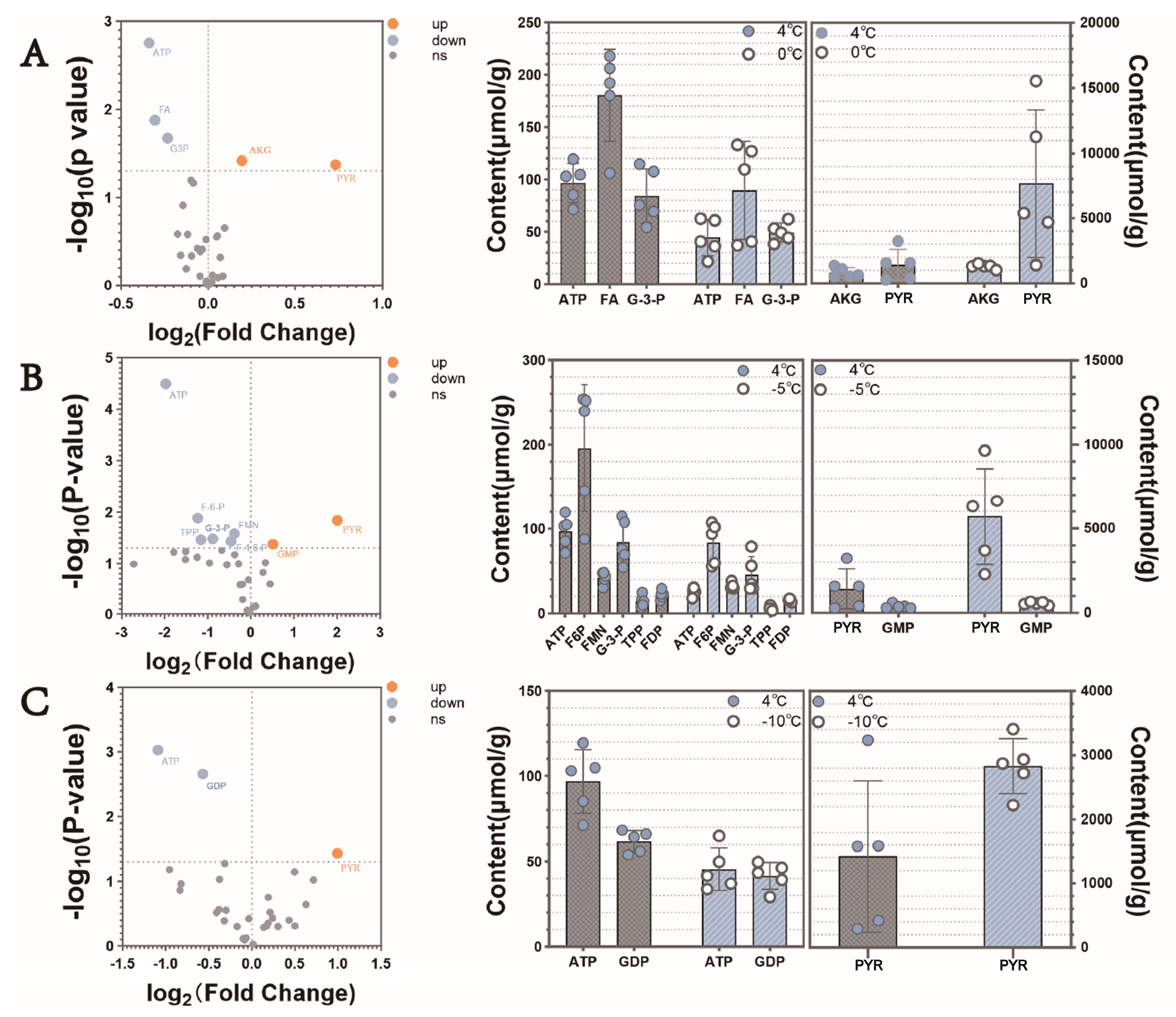
3.2. Changes in Metabolite Concentration
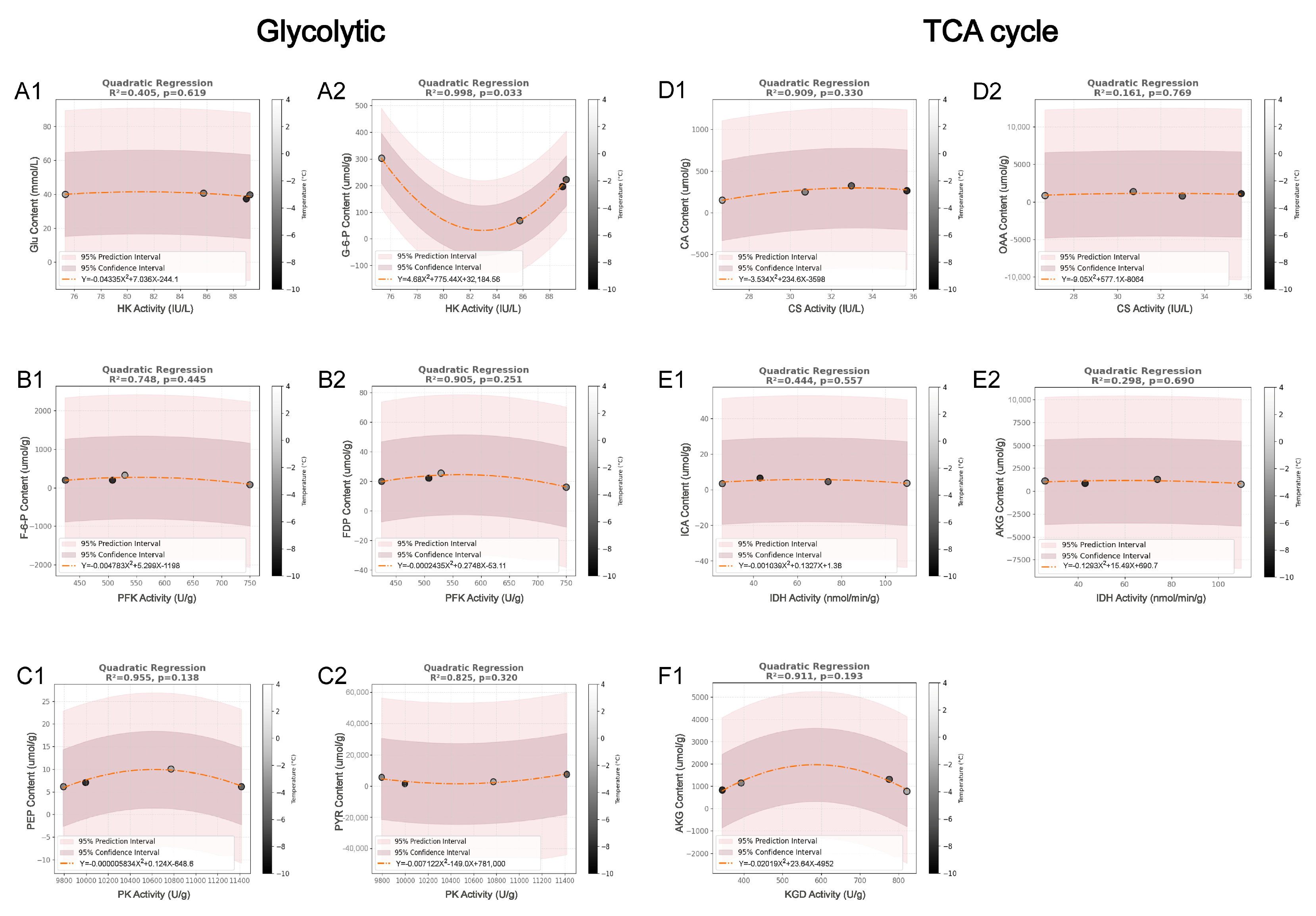
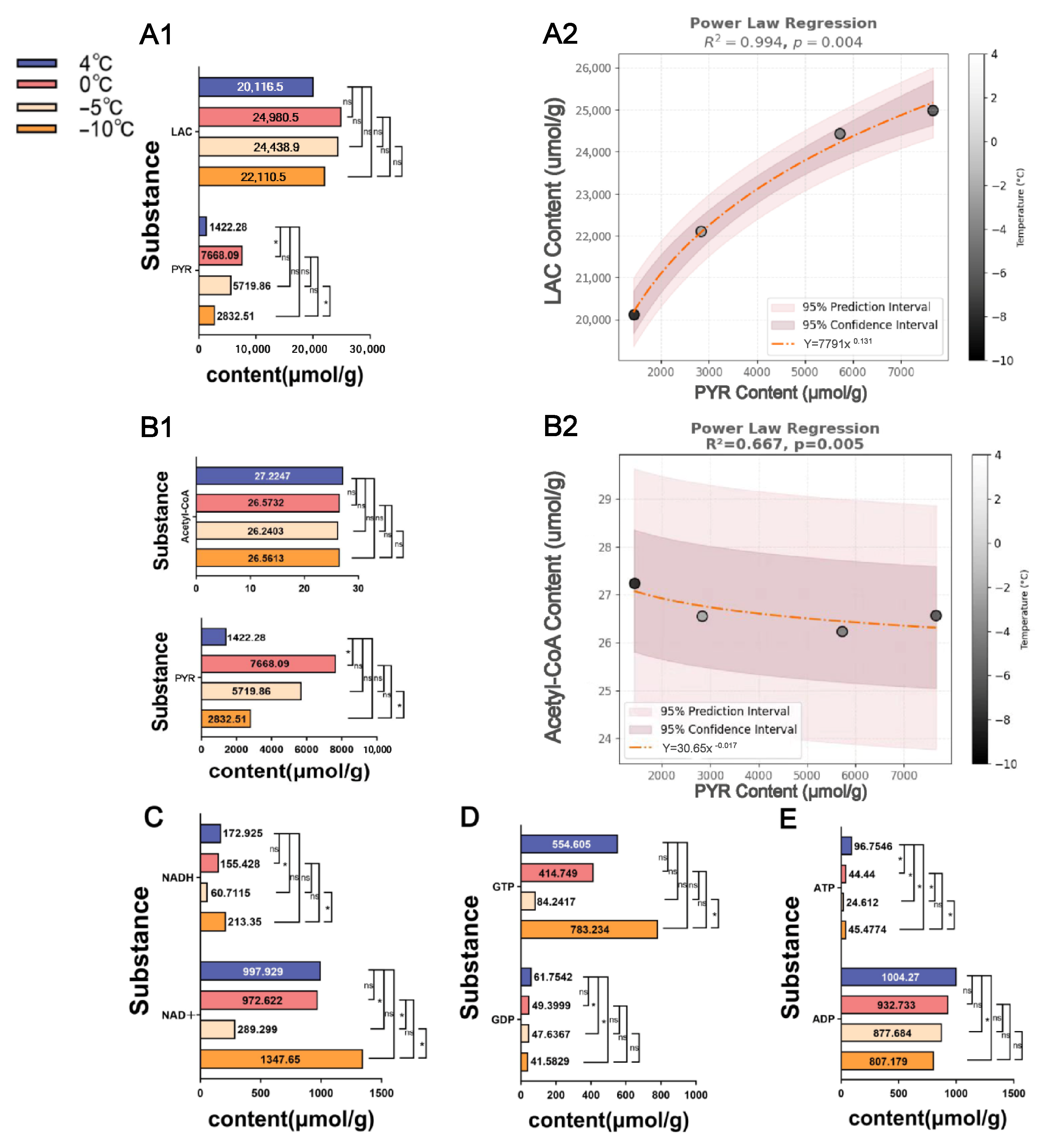
3.3. The Relationship Between Rate-Limiting Enzymes and Their Related Metabolites Under Different Temperature Conditions
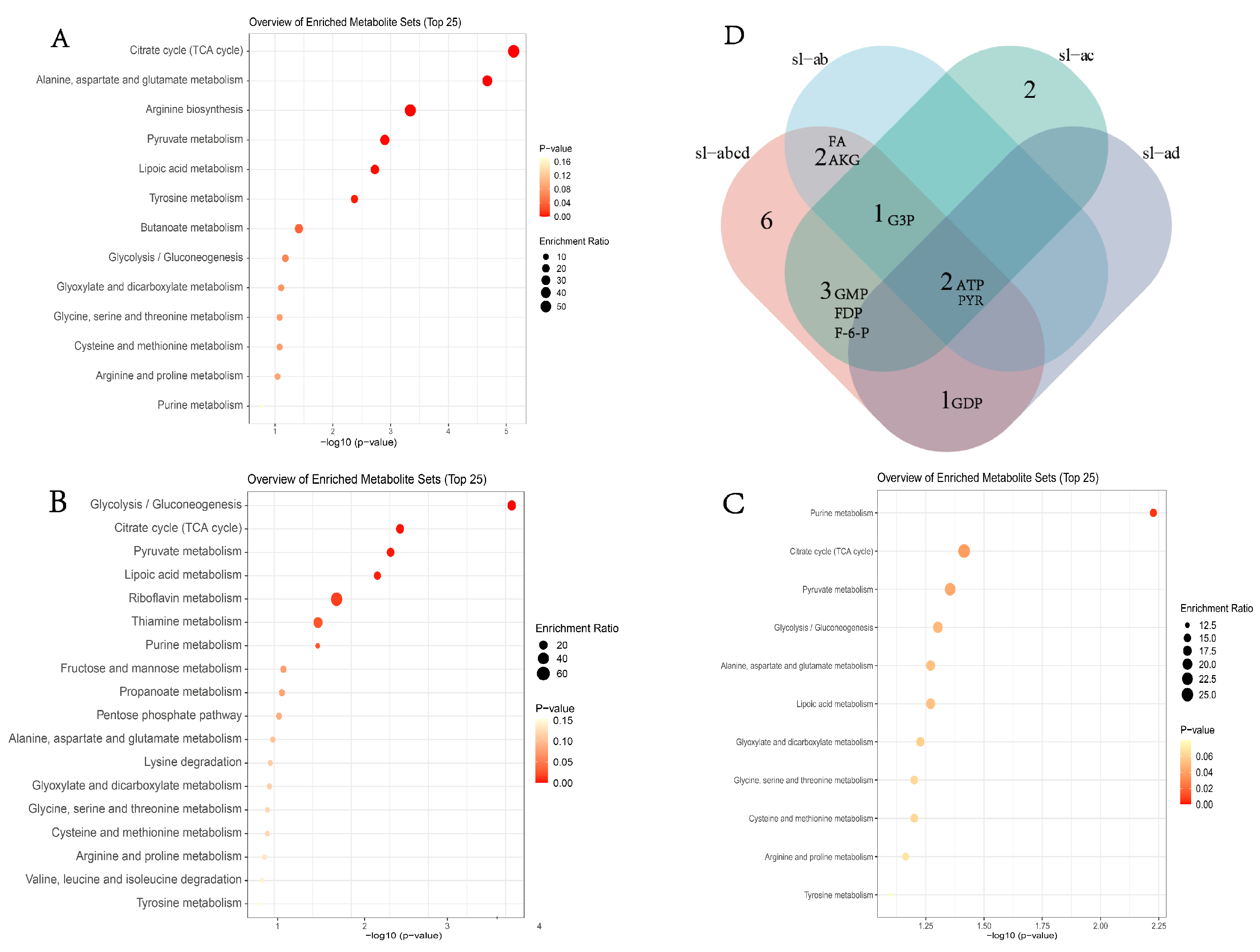
3.4. Critical Metabolites Associated with Cold Tolerance in Respiratory Metabolism
3.5. Transformation Dynamics of Key Metabolites in Response to Low-Temperature Stress
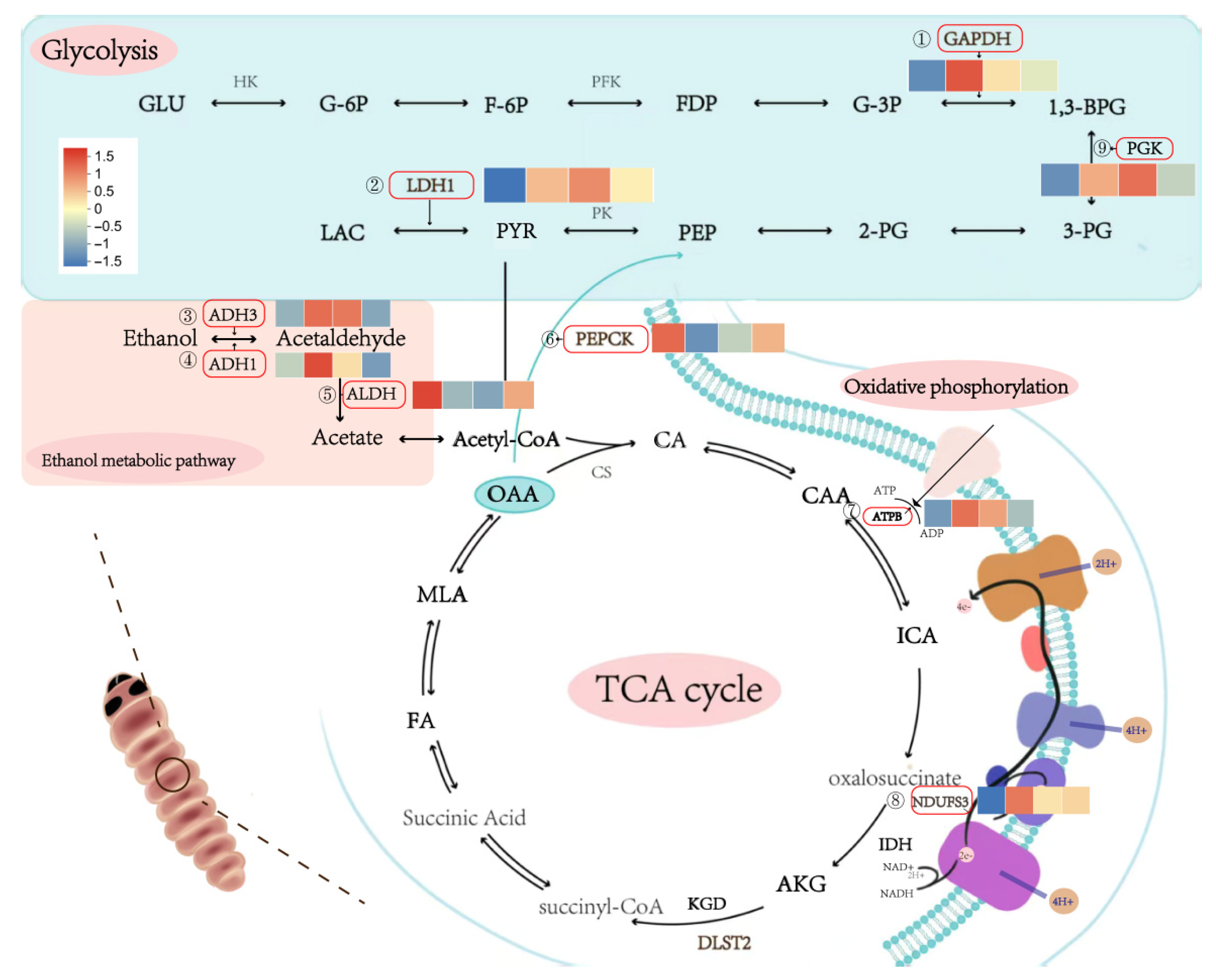
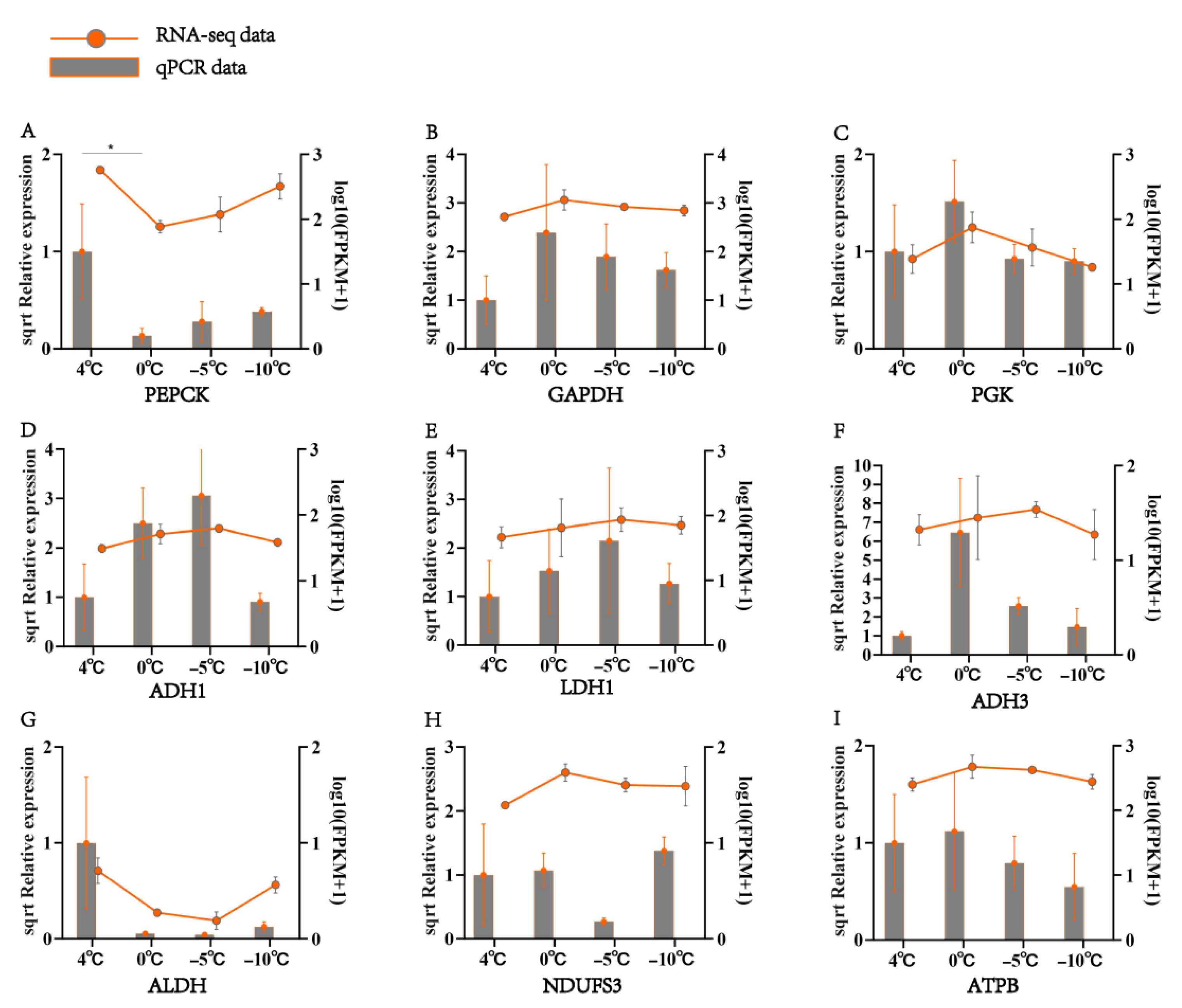
3.6. Dynamic Changes in Differentially Expressed Genes Under Low-Temperature Stress
4. Discussion
4.1. Effects of Low-Temperature Stress on Key Enzyme Activities in S. insularis Larval Glycolysis and TCA Cycle
4.2. Effects of Low-Temperature Stress on Key Metabolites in S. insularis Larval Glycolysis and TCA Cycle
4.3. Complexity of Enzyme–Substrate Relationships in Glycolysis and the TCA Cycle
4.4. Role of Differentially Expressed Metabolic Genes in S. insularis Larval Cold Tolerance
4.5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.M.; Haynes, K.J. Spatiotemporal Dynamics of Forest Insect Populations Under Climate Change. Curr. Opin. Insect Sci. 2023, 56, 101020. [Google Scholar] [CrossRef]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of Forest Insect Pests to Climate Change: Not So Simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar] [CrossRef]
- León-Quinto, T.; Antón-Ruiz, N.; Madrigal, R.; Serna, A. Experimental Evidence of a Neotropical Pest Insect Moderately Tolerant to Complete Freezing. J. Therm. Biol. 2024, 123, 103939. [Google Scholar] [CrossRef]
- Lee, R.E.J. Insect Cold-Hardiness: To Freeze or Not to Freeze: How Insects Survive Low Temperatures. Bioscience 1989, 39, 308–311. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Addo-Bediako, A.; Chown, S.L. Climatic Variability and the Evolution of Insect Freeze Tolerance. Biol. Rev. 2003, 78, 181–195. [Google Scholar] [CrossRef]
- Ullah, F.; Abbas, A.; Gul, H.; Güncan, A.; Hafeez, M.; Gadratagi, B.-G.; Cicero, L.; Ramirez-Romero, R.; Desneux, N.; Li, Z. Insect Resilience: Unraveling Responses and Adaptations to Cold Temperatures. J. Pest Sci. 2024, 97, 1153–1169. [Google Scholar] [CrossRef]
- Johnson, N.C.; Xie, S.P.; Kosaka, Y.; Li, X.C. Increasing Occurrence of Cold and Warm Extremes During the Recent Global Warming Slowdown. Nat. Commun. 2018, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Bathiany, S.; Dakos, V.; Scheffer, M.; Lenton, T.M. Climate Models Predict Increasing Temperature Variability in Poor Countries. Sci. Adv. 2018, 4, eaar5809. [Google Scholar] [CrossRef]
- Al Kindi, K.M.; Kwan, P.; Andrew, N.R.; Welch, M. Modelling the Potential Effects of Climate Factors on Dubas Bug (Ommatissus lybicus) Presence/Absence and Its Infestation Rate: A Case Study from Oman. Pest Manag. Sci. 2019, 75, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, R.; Yu, C.M.; Dang, X.P.; Sun, W.G.; Li, Q.F.; Wang, X.T.; Wan, J.Z. Risk Assessment of Insect Pest Expansion in Alpine Ecosystems Under Climate Change. Pest Manag. Sci. 2021, 77, 3165–3178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fang, D.; Chen, S. Biological Characteristics and Control Tests of Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae). Shandong Sci. Technol. 1987, 3, 32–35. [Google Scholar]
- Wang, C.; Wang, P.; Xuan, Y.; Yang, L.; Zhang, J.; He, M.; Wang, S. Investigation on the occurrence and damage of trunk-boring insects on ash trees in Kokdala City. Pest Manag. Sci. 2023, 49, 304–310. [Google Scholar] [CrossRef]
- Pei, J.H.; Li, C.C.; Ren, L.L.; Zong, S.X. Factors Influencing Cold Hardiness During Overwintering of Streltzoviella insularis (Lepidoptera: Cossidae). J. Econ. Entomol. 2020, 113, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.H.; Xu, Y.B.; Zong, S.X.; Ren, L.L. Transcriptomic and Metabolomic Data Reveal the Key Metabolic Pathways Affecting Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae) Larvae During Overwintering. Front. Physiol. 2021, 12, 655059. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Fu, N.N.; Qu, C.; Wang, R.; Xu, Y.H.; Luo, C. Understanding the Mechanisms of Dormancy in an Invasive Alien Sycamore Lace Bug, Corythucha ciliata Through Transcript and Metabolite Profiling. Sci. Rep. 2017, 7, 2631. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L.; Sørensen, J.G.; Kostál, V.; Šimek, P.; Terblanche, J.S. Cold Tolerance is Unaffected by Oxygen Availability Despite Changes in Anaerobic Metabolism. Sci. Rep. 2016, 6, 32856. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Qin, X. Preliminary Studies on the Streltzoviella insularis (Staudinger). For. Pests Dis. Newsl. 1983, 1, 3–6. [Google Scholar]
- Sinclair, B.J.; Alvarado, L.E.C.; Ferguson, L.V. An Invitation to Measure Insect Cold Tolerance: Methods, Approaches, and Workflow. J. Therm. Biol. 2015, 53, 180–197. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2013; pp. 196–243, 526–564, 606–635. [Google Scholar]
- Ge, Y.D.; Pan, W.; Wang, J.; Zhu, G.P. The Molecular Biology Research Progress of Citrate Synthase. J. Biol. 2010, 27, 59–62. [Google Scholar]
- Uzelac, I.; Avramov, M.; Celic, T.; Vukasinovic, E.; Gosic-Dondo, S.; Purac, J.; Kojic, D.; Blagojevic, D.; Popovic, Z.D. Effect of Cold Acclimation on Selected Metabolic Enzymes During Diapause in the European Corn Borer Ostrinia nubilalis (Hbn.). Sci. Rep. 2020, 10, 9085. [Google Scholar] [CrossRef]
- Bond, D.R.; Mester, T.; Nesbo, C.L.; Izquierdo-Lopez, A.V.; Collart, F.L.; Lovley, D.R. Characterization of Citrate Synthase from Geobacter sulfurreducens and Evidence for a Family of Citrate Synthases Similar to Those of Eukaryotes Throughout the Geobacteraceae. Appl. Environ. Microbiol. 2005, 71, 3858–3865. [Google Scholar] [CrossRef]
- Scaraffia, P.Y.; Gerez de Burgos, N.M. Effects of Temperature and pH on Hexokinase from the Flight Muscles of Dipetalogaster maximus (Hemiptera: Reduviidae). J. Med. Entomol. 2000, 37, 689–694. [Google Scholar] [CrossRef]
- Muise, A.M. Enzymatic Regulation of Glycerol Metabolism in the Overwintering Gall Moth Epiblema Scudderiana. Master’s Thesis, Carleton University, Ottawa, ON, Canada, 1993. [Google Scholar] [CrossRef]
- Muise, A.M.; Storey, K.B. Regulation of Hexokinase in a Freeze Avoiding Insect: Role in the Winter Production of Glycerol. Arch. Insect Biochem. Physiol. 2001, 47, 29–34. [Google Scholar] [CrossRef]
- Teets, N.M.; Denlinger, D.L. Physiological Mechanisms of Seasonal and Rapid Cold-Hardening in Insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Overgaard, J.; MacMillan, H.A. The Integrative Physiology of Insect Chill Tolerance. In Annual Review of Physiology; Julius, D., Ed.; Annual Reviews: San Mateo, CA, USA, 2017; pp. 187–208. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Cold Hardiness of the South American Tomato Pinworm Tuta absoluta (Lepidoptera: Gelechiidae): Both Larvae and Adults Are Chill-Susceptible. Pest Manag. Sci. 2021, 77, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.X.; Head, M.L.; Liu, C.; Haseeb, M.; Zhang, R.Z. The High Invasion Success of Fall Armyworm Is Related to Life-History Strategies Across a Range of Stressful Temperatures. Pest Manag. Sci. 2022, 78, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Abboud, J.; Green, S.R.; Smolinski, M.B.; Storey, K.B. Regulation of an Important Glycolytic Enzyme, Pyruvate Kinase, Through Phosphorylation in the Larvae of a Species of Freeze-Tolerant Insect, Eurosta solidaginis. Insect Mol. Biol. 2021, 30, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.Y.; Chen, Y.Q.; Wang, Z.J.; Zhou, H.S.; Sun, M.Y.; Yao, T.; Mu, W. Transcriptome, Histological, and Physiological Responses of Amur Sleeper (Perccottus glenii) During Cold Stress, Freezing, and Recovery. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101192. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.M.; Hu, J.W.; Lu, Y.L.; Wu, Z.H.; Wang, L.J.; Xu, D.D.; Zhang, P.J.; You, F. Cold Effect Analysis and Screening of SNPs Associated with Cold-Tolerance in the Olive Flounder Paralichthys olivaceus. J. Appl. Ichthyol. 2019, 35, 924–932. [Google Scholar] [CrossRef]
- Lannig, G.; Storch, D.; Pörtner, H.O. Aerobic Mitochondrial Capacities in Antarctic and Temperate Eelpout (Zoarcidae) Subjected to Warm Versus Cold Acclimation. Polar Biol. 2005, 28, 575–584. [Google Scholar] [CrossRef]
- Kühnhold, H.; Novais, S.C.; Alves, L.M.F.; Kamyab, E.; Lemos, M.F.L.; Slater, M.J.; Kunzmann, A. Acclimation Capability Inferred by Metabolic Performance in Two Sea Cucumber Species from Different Latitudes. J. Therm. Biol. 2019, 84, 407–413. [Google Scholar] [CrossRef]
- Mattice, A.M.S.; Varma, A.; Storey, K.B. Role of NADP thorn -dependent isocitrate Dehydrogenase from Muscle Tissue of Rana sylvatica in ROS Defense During Freeze-Tolerance. Biochimie 2023, 210, 14–21. [Google Scholar] [CrossRef]
- Tretter, L.; Adam-Vizi, V. Alpha-Ketoglutarate Dehydrogenase: A Target and Generator of Oxidative Stress. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Tretter, L.; Adam-Vizi, V. Generation of Reactive Oxygen Species in the Reaction Catalyzed by α-Ketoglutarate Dehydrogenase. J. Neurosci. 2004, 24, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Bertero, E.; Nickel, A.; Kohlhaas, M.; Gibson, G.E.; Heggermont, W.; Heymans, S.; Maack, C. Selective NADH Communication from α-Ketoglutarate Dehydrogenase to Mitochondrial Transhydrogenase Prevents Reactive Oxygen Species Formation Under Reducing Conditions in the Heart. Basic Res. Cardiol. 2020, 115, 53. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, C. Biochemistry, 4th ed.; Higher Education Press: Beijing, China, 2016; pp. 49–93. [Google Scholar]
- Boardman, L.; Sørensen, J.G.; Kostál, V.; Šimek, P.; Terblanche, J.S. Chilling Slows Anaerobic Metabolism to Improve Anoxia Tolerance of Insects. Metabolomics 2016, 12, 176. [Google Scholar] [CrossRef]
- Ren, L.L.; Zhang, H.X.; Zhou, J.; Wu, Y.J.; Liu, B.; Wang, S.P.; Liu, X.; Hao, X.; Zhao, L.L. Unique and Generic Crossed Metabolism in Response to Four Sub-Lethal Environmental Stresses in the Oriental Fruit Fly, Bactrocera dorsalis Hendel. Ecotoxicol. Environ. Saf. 2023, 264, 115434. [Google Scholar] [CrossRef]
- Xu, R.; Yang, F.; Sun, X.; Lu, J.; Yang, W.; Zhen, K.; Hua, J. Self-Assembled Immobilized Polyphosphate Kinase for the Synthesis of ATP and GTP. Food Ferment. Ind. 2023, 51, 77–82. [Google Scholar] [CrossRef]
- Colinet, H.; Renault, D.; Roussel, D. Cold Acclimation Allows Drosophila Flies to Maintain Mitochondrial Functioning Under Cold Stress. Insect Biochem. Mol. Biol. 2017, 80, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H. Disruption of ATP Homeostasis During Chronic Cold Stress and Recovery in the Chill Susceptible Beetle (Alphitobius diaperinus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 63–67. [Google Scholar] [CrossRef]
- MacMillan, H.A.; Williams, C.M.; Staples, J.F.; Sinclair, B.J. Metabolism and Energy Supply Below the Critical Thermal Minimum of a Chill-Susceptible Insect. J. Exp. Biol. 2012, 215, 1366–1372. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, F.L.; Yang, Q.B.; Huang, J.H.; Yang, L.S.; Jiang, S.G. Impact of Temperature Stress on Oxygen and Energy Metabolism in the Hepatopancreas of the Black Tiger Shrimp, Penaeus monodon (Crustacea: Decapoda: Penaeidae). Pak. J. Zool. 2019, 51, 141–148. [Google Scholar] [CrossRef]
- Vasquez, M.C.; Tomanek, L. Sirtuins as Regulators of the Cellular Stress Response and Metabolism in Marine Ectotherms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 236, 110528. [Google Scholar] [CrossRef]
- Kuz’mina, V.V.; Ushakova, N.V. Influence of Temperature and pH on the Effects of Zinc and Copper on Proteolytic Activities of the Intestinal Mucosa of Planktivorous and Benthophagous Fishes and Their Potential Preys. Toxicol. Environ. Chem. 2013, 95, 150–162. [Google Scholar] [CrossRef]
- Durak, R.; Dampc, J.; Kula-Maximenko, M.; Mołoń, M.; Durak, T.J. Changes in Antioxidative, Oxidoreductive and Detoxification Enzymes During Development of Aphids and Temperature Increase. Antioxidants 2021, 10, 1181. [Google Scholar] [CrossRef]
- He, F.; Murabito, E.; Westerhoff, H.V. Synthetic Biology and Regulatory Networks: Where Metabolic Systems Biology Meets Control Engineering. J. R. Soc. Interface 2016, 13, 20151046. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.E. Biological Feedback Control at the Molecular Level: Interaction Between Metabolite-Modulated Enzymes Seems to Be a Major Factor in Metabolic Regulation. Science 1965, 150, 851–857. [Google Scholar] [CrossRef]
- Lebenzon, J.E.; Overgaard, J.; Jørgensen, L.B. Chilled, Starved or Frozen: Insect Mitochondrial Adaptations to Overcome the Cold. Curr. Opin. Insect Sci. 2023, 58, 101076. [Google Scholar] [CrossRef]
- Jørgensen, L.B.; Hansen, A.M.; Willot, Q.; Overgaard, J. Balanced Mitochondrial Function at Low Temperature Is Linked to Cold Adaptation in Drosophila Species. J. Exp. Biol. 2023, 226, jeb245439. [Google Scholar] [CrossRef] [PubMed]
- Roussel, D.; Janillon, S.; Teulier, L.; Pichaud, N. Succinate Oxidation Rescues Mitochondrial ATP Synthesis at High Temperature in Drosophila melanogaster. FEBS Lett. 2023, 597, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Teets, N.M.; Kawarasaki, Y.; Potts, L.J.; Philip, B.N.; Gantz, J.; Denlinger, D.L.; Lee, R.E.J., Jr. Rapid Cold Hardening Protects Against Sublethal Freezing Injury in an Antarctic Insect. J. Exp. Biol. 2019, 222, jeb206011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, W.; Li, Y.; Chen, J.; Teets, N.M.; Zhang, L.J. Metabolic and Transcriptional Regulation of Reproductive Diapause in Arma chinensis. iScience 2025, 28, 111761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.-R.; Gao, G.-Q.; Ma, M.-Y.; Chen, H. Cold Tolerance and Silencing of Three Cold-Tolerance Genes of Overwintering Chinese White Pine Larvae. Sci. Rep. 2016, 6, 34698. [Google Scholar] [CrossRef]
- Barth, M.B.; Buchwalder, K.; Kawahara, A.Y.; Zhou, X.; Liu, S.L.; Krezdorn, N.; Rotter, B.; Horres, R.; Hundsdoerfer, A.K. Functional Characterization of the Hyles euphorbiae Hawkmoth Transcriptome Reveals Strong Expression of Phorbol Ester Detoxification and Seasonal Cold Hardiness Genes. Front. Zool. 2018, 15, 20. [Google Scholar] [CrossRef]
- Lubawy, J.; Chowański, S.; Adamski, Z.; Słocińska, M. Mitochondria as a Target and Central Hub of Energy Division During Cold Stress in Insects. Front. Zool. 2022, 19, 1. [Google Scholar] [CrossRef] [PubMed]

| Rate-Limiting Enzyme Names | Acronyms |
|---|---|
| hexokinase | HK |
| phosphofructokinase | PFK |
| pyruvate kinase | PK |
| citrate synthase | CS |
| isocitrate dehydrogenase | IDH |
| α-ketoglutarate dehydrogenase | KGD |
| Metabolic Pathway | Metabolites | Acronyms |
|---|---|---|
| Glycolytic | glucose | GLU |
| Glycolytic | glucose-6-phosphate | G-6-P |
| Glycolytic | fructose-6-phosphate | F-6-P |
| Glycolytic | 1,6-fructose-diphosphate | FDP |
| Glycolytic | phosphoenolpyruvic acid | PEP |
| Glycolytic | pyruvic acid | PYR |
| Glycolytic | lactic acid | LAC |
| Glycolytic | adenosine triphosphate | ATP |
| Glycolytic | adenosine diphosphate | ADP |
| TCA cycle | acetoacetyl-CoA | Acetyl-CoA |
| TCA cycle | oxaloacetic acid | OAA |
| TCA cycle | citric acid | CA |
| TCA cycle | isocitric acid | ICA |
| TCA cycle | α-ketoglutaric acid | AKG |
| TCA cycle | guanosine triphosphate | GTP |
| TCA cycle | guanosine diphosphate | GDP |
| TCA cycle | nicotinamide adenine dinucleotide (reduced) | NADH |
| TCA cycle | nicotinamide adenine dinucleotide (oxidized) | NAD+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, L.; Li, R.; Zhang, B.; Zhang, Y.; Pei, J.; Zong, S. Metabolic Remodeling of the Tricarboxylic Acid Cycle and Glycolysis Reveals Cold-Induced Respiratory Adaptations in Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae) Larvae. Insects 2025, 16, 864. https://doi.org/10.3390/insects16080864
Zhi L, Li R, Zhang B, Zhang Y, Pei J, Zong S. Metabolic Remodeling of the Tricarboxylic Acid Cycle and Glycolysis Reveals Cold-Induced Respiratory Adaptations in Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae) Larvae. Insects. 2025; 16(8):864. https://doi.org/10.3390/insects16080864
Chicago/Turabian StyleZhi, Lingxu, Ruixin Li, Baosheng Zhang, Yan Zhang, Jiahe Pei, and Shixiang Zong. 2025. "Metabolic Remodeling of the Tricarboxylic Acid Cycle and Glycolysis Reveals Cold-Induced Respiratory Adaptations in Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae) Larvae" Insects 16, no. 8: 864. https://doi.org/10.3390/insects16080864
APA StyleZhi, L., Li, R., Zhang, B., Zhang, Y., Pei, J., & Zong, S. (2025). Metabolic Remodeling of the Tricarboxylic Acid Cycle and Glycolysis Reveals Cold-Induced Respiratory Adaptations in Streltzoviella insularis (Staudinger) (Lepidoptera: Cossidae) Larvae. Insects, 16(8), 864. https://doi.org/10.3390/insects16080864







