Ecological Pest Control in Alpine Ecosystems: Monitoring Asteraceae Phytophages and Developing Integrated Management Protocols in the Three River Source Region
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
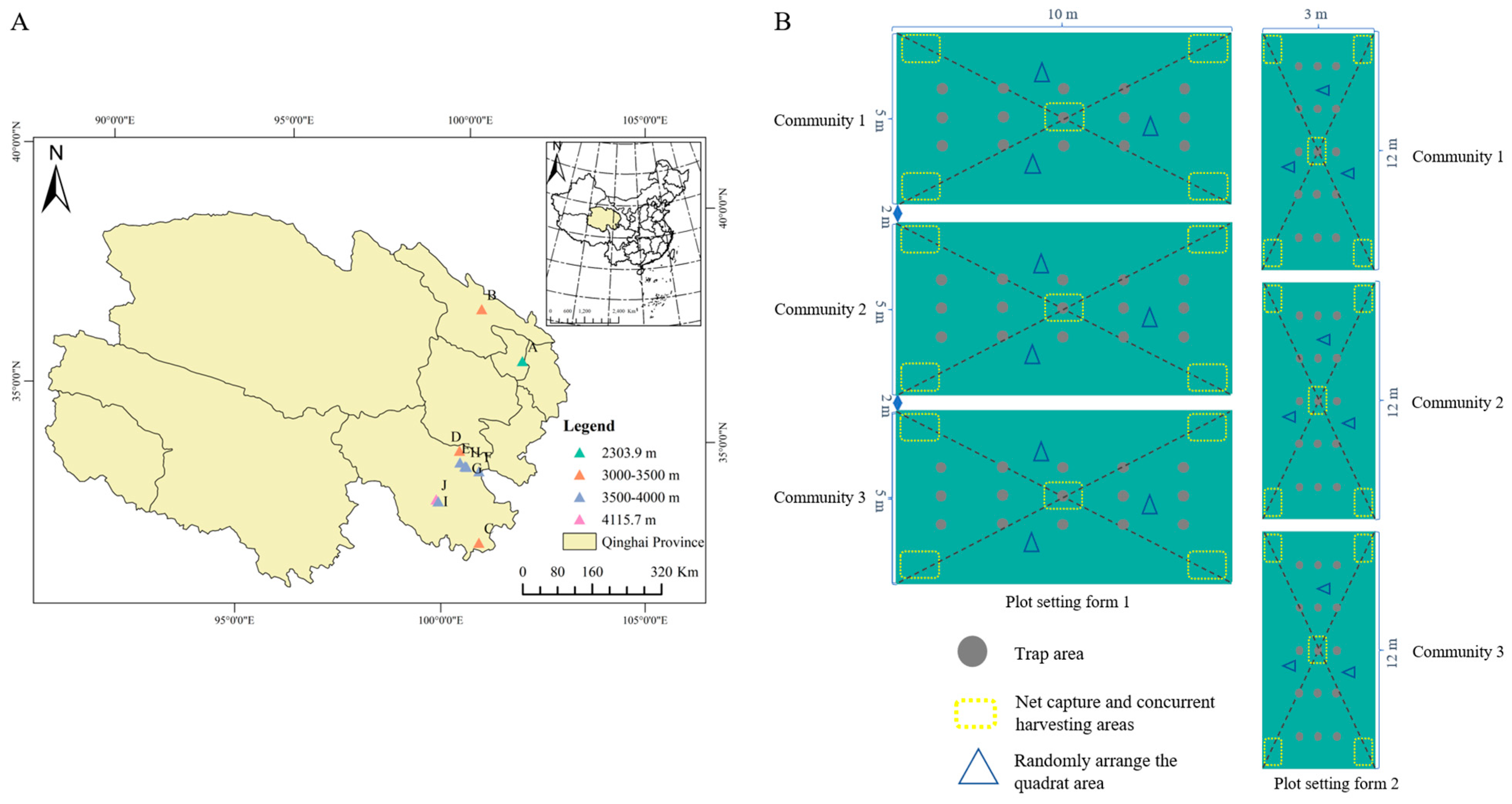
2.1. Study Area and Survey Design
2.2. Pest Identification and Molecular Analysis
2.3. Integrated Pest Management Experiments for Major Pests
2.4. Biochemical Index Detection and Data Analysis
2.5. Data Analysis
2.5.1. Calculation Methods for Experimental Indicators
2.5.2. Data Statistical Analysis
3. Results
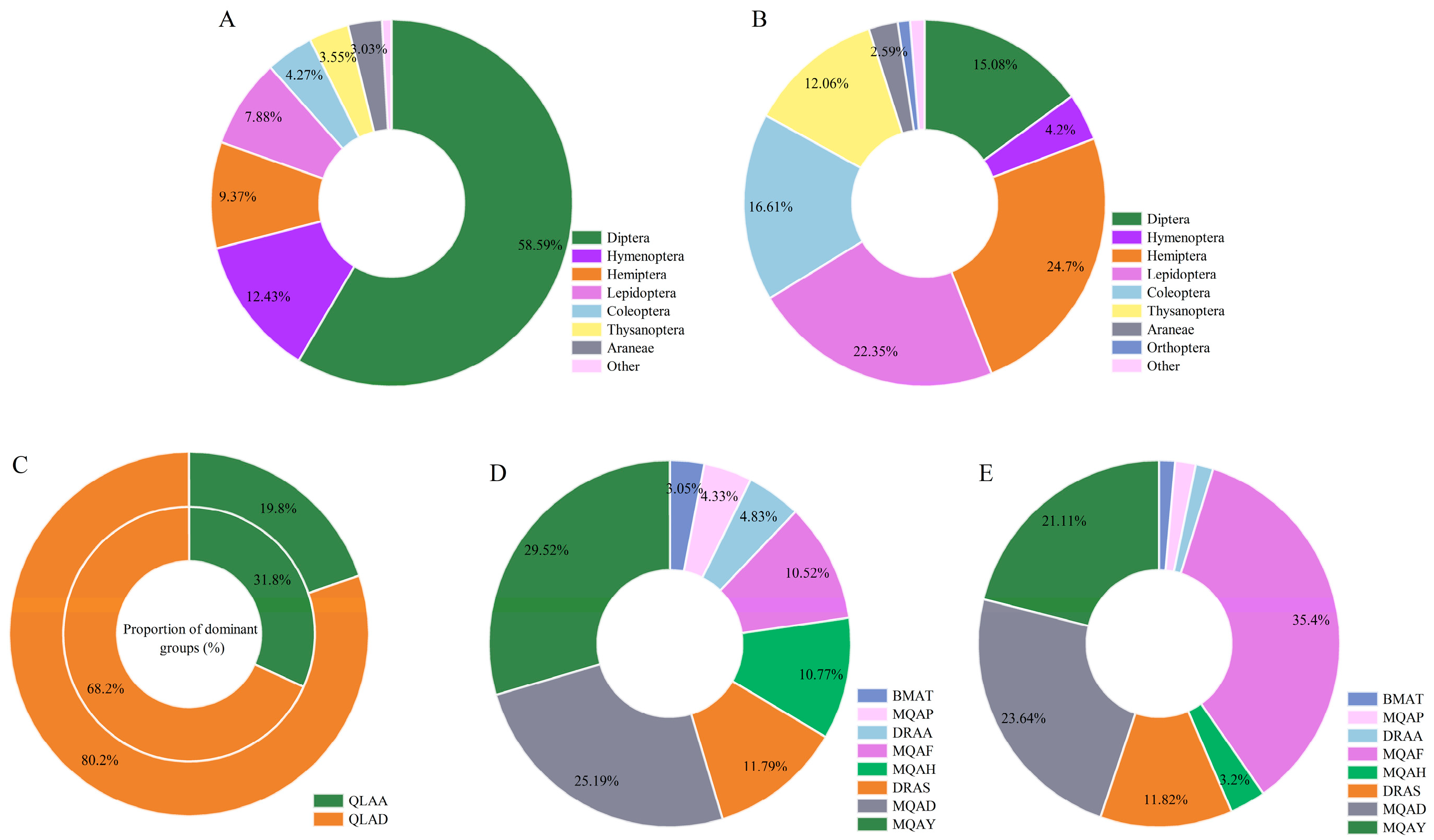
3.1. Species Diversity and Geographical Distribution Characteristics
3.2. Spatiotemporal Dynamics of Dominant Species
3.2.1. Spatiotemporal Dynamics of Insect Dominants
3.2.2. Spatiotemporal Dynamics of Spider Dominants
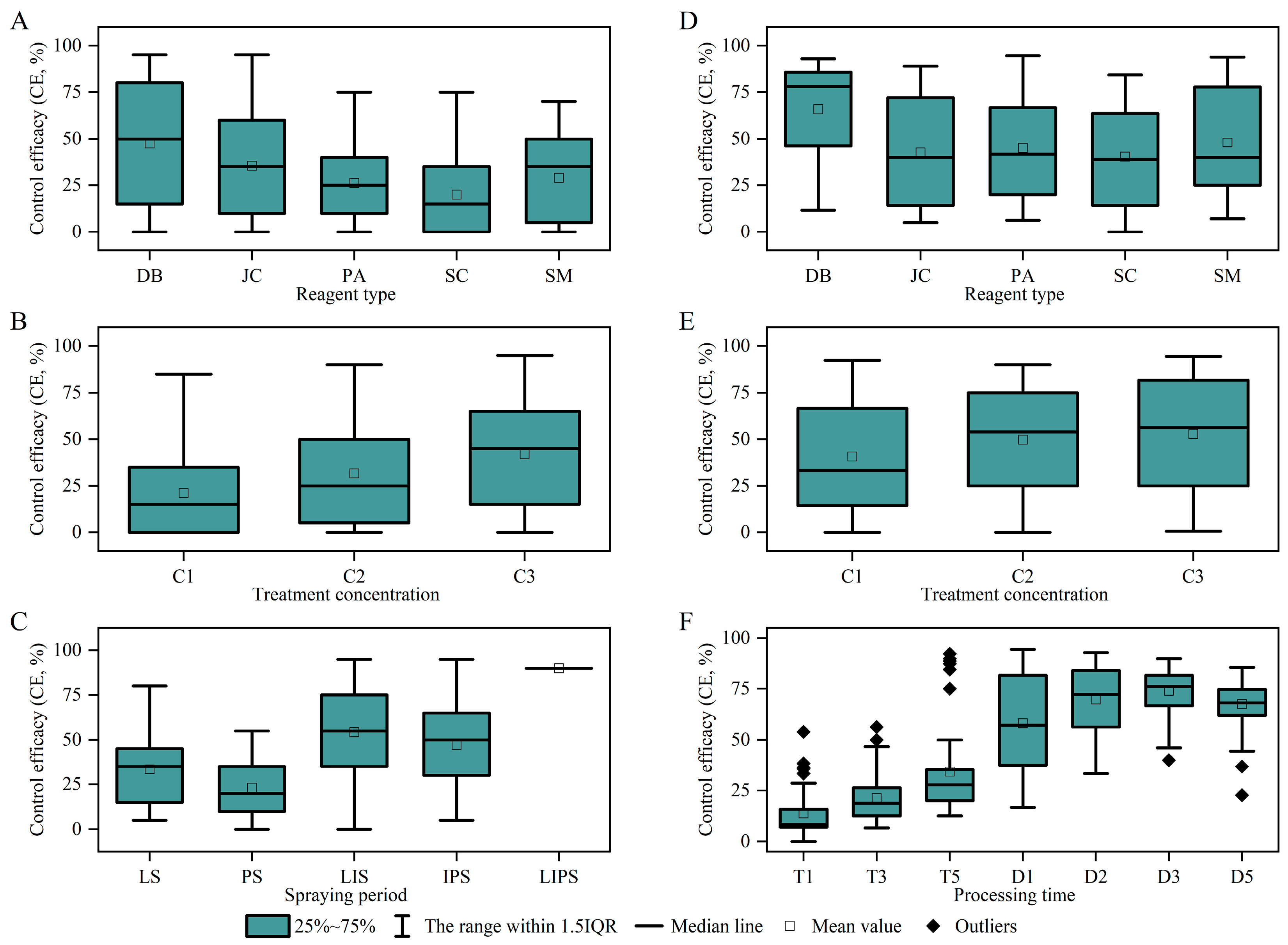
3.3. Comparison of Physical and Chemical Control Methods
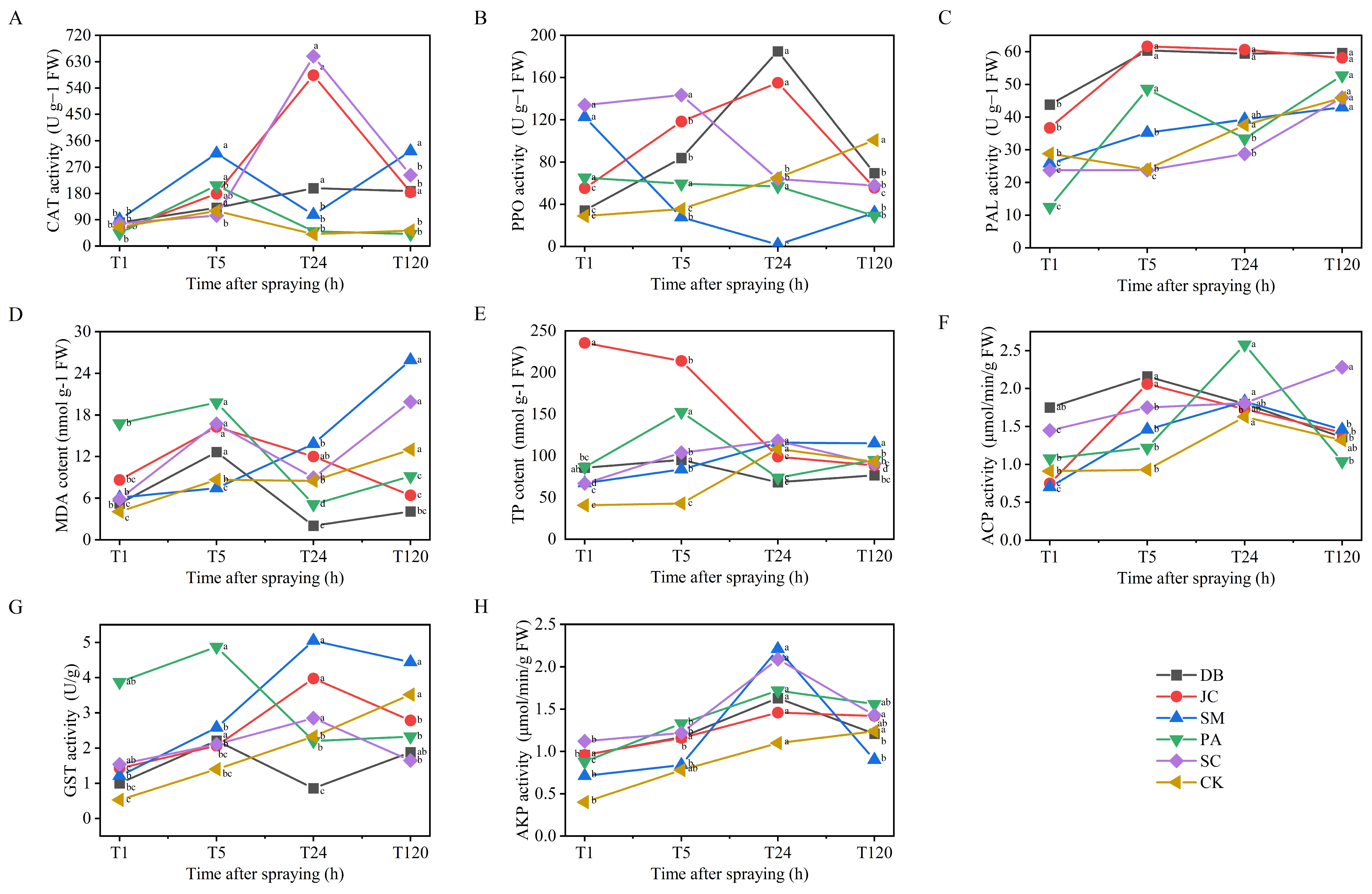
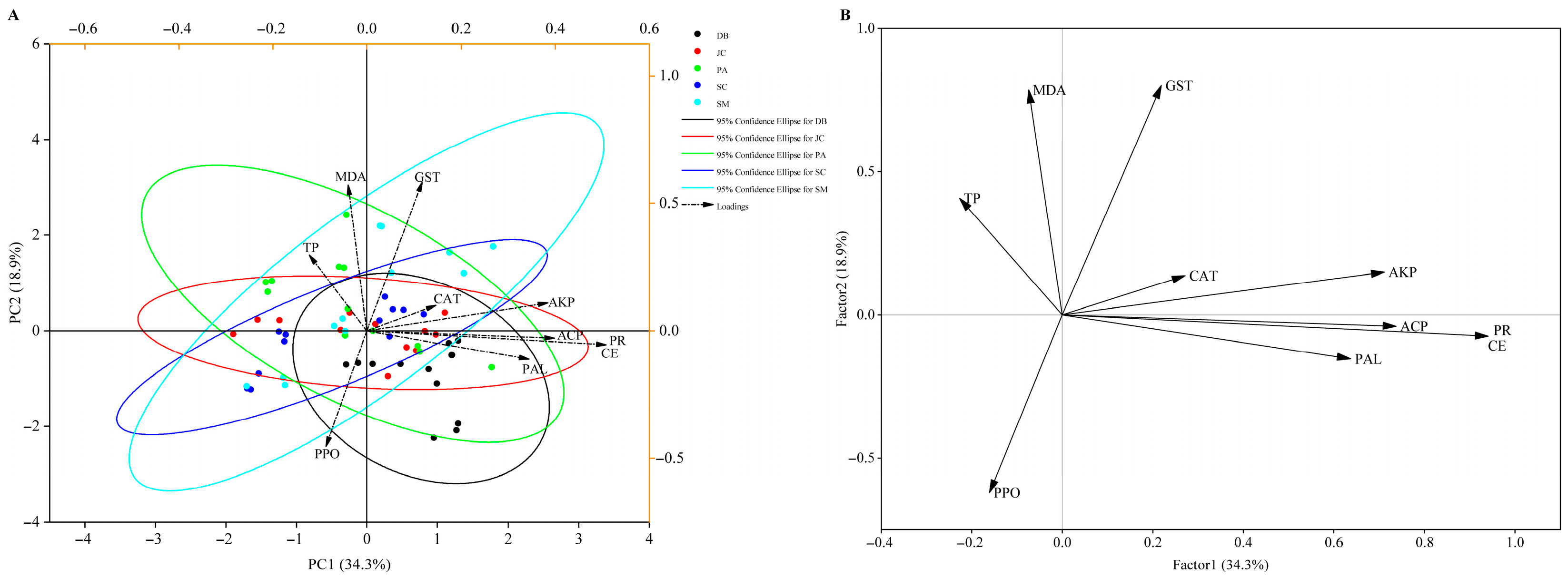
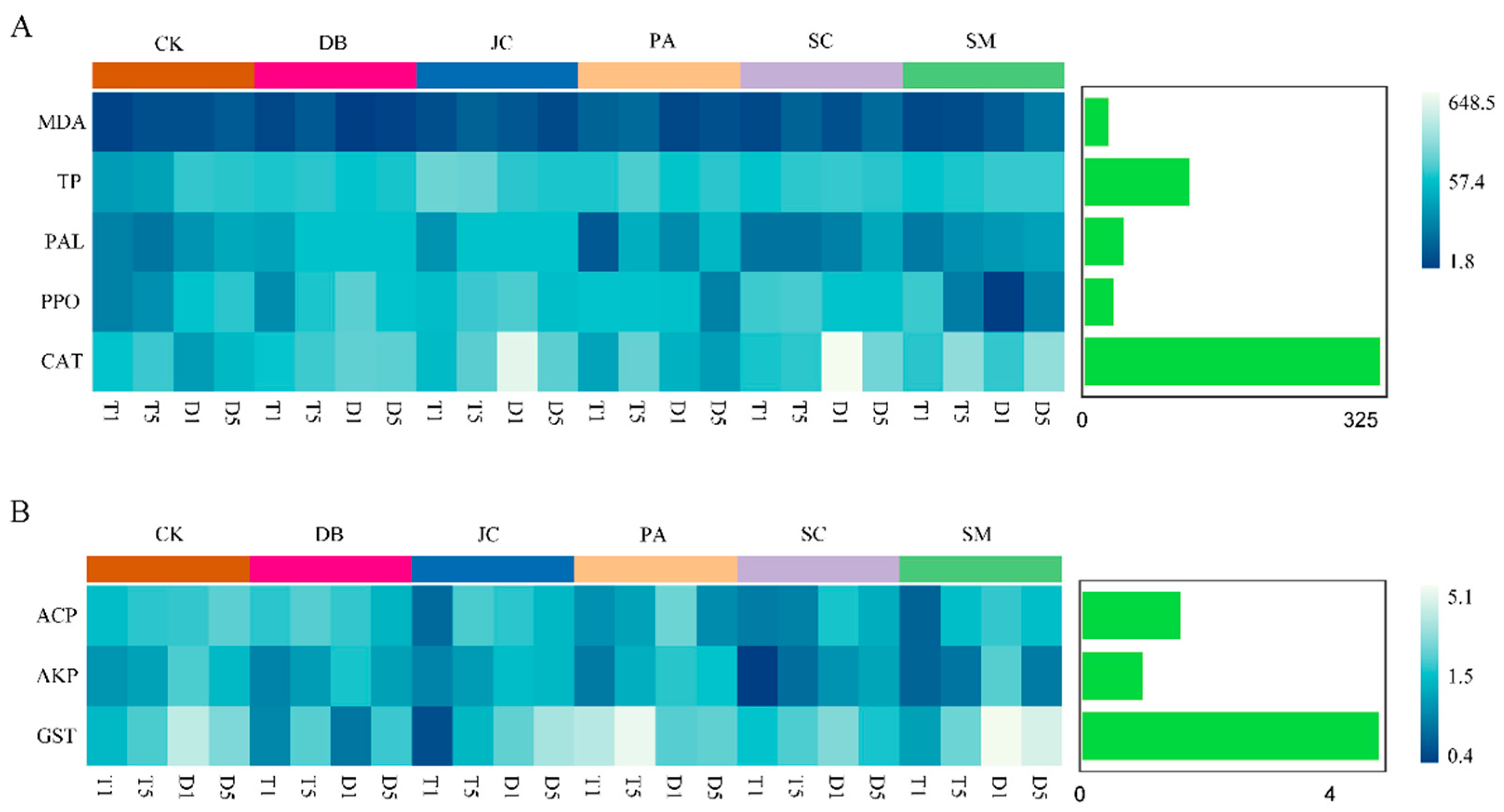
3.4. Enzyme Activity Responses and Mechanistic Analysis
3.4.1. Plant Responses
3.4.2. Pest Detoxification Responses
3.4.3. Dominant Factor Analysis
4. Discussion
4.1. Optimization and Limitations of Sampling and Identification Methods
4.2. Structural and Dynamic Drivers of Alpine Insect Communities
4.3. Efficacy and Ecological Trade-Offs of Physical–Chemical Integrated Control
4.4. Biochemical Regulatory Networks and Key Factors in Plant–Insect Interaction
4.5. Necessity for Implementing Integrated Pest Management and a Sustainable Pest Management Framework for Insects in High-Altitude Ecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Zhang, R.H. Effect of upper-level air temperature changes over the Tibetan Plateau on the genesis frequency of Tibetan Plateau vortices at interannual timescales. Clim. Dyn. 2021, 57, 341–352. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Guo, W.D.; Yao, X.P.; Du, J.; Li, W.K.; Ge, J. Tibetan Plateau vortex-associated precipitation and its link with the Tibetan Plateau heating anomaly. Int. J. Climatol. 2021, 41, 6300–6313. [Google Scholar] [CrossRef]
- Chen, H.X. Phylogenetic Relationships Between Tephritid Flies and Host Plants in Hongyuan County, Sichuan Province. Master’s Thesis, Nanjing University, Nanjing, China, 2017. [Google Scholar]
- Jiao, K.W.; Gao, J.B.; Liu, Z.H. Precipitation drives the NDVI distribution on the Tibetan Plateau while high warming rates may intensify its ecological droughts. Remote Sens. 2021, 13, 1305. [Google Scholar] [CrossRef]
- Wang, C.P.; Huang, M.T.; Zhai, P.M. Change in drought conditions and its impacts on vegetation growth over the Tibetan Plateau. Adv. Clim. Change Res. 2021, 12, 333–341. [Google Scholar] [CrossRef]
- Li, W.P.; Chen, S.M. Present status of systematic studies on Aster s. L (Astereae, Asteraceae). Life Sci. Res. 2004, 8, 93–96. [Google Scholar]
- Zhang, L.J.; Liu, Y.; Ma, Y.S.; Wang, Y.L.; Wang, X.Y.; Ma, Y.; Xie, L.L. Insect resistance responses of ten Aster varieties to damage by Tephritis angustipennis in the three rivers source region of China. Sci. Rep. 2025, 15, 6962–6980. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Y.; Qiu, Y.; Zhang, Y.; Huo, T.; Ge, L.; Yuan, T. Relationship between physiological changes and drought resistance of 5 perennial flowers under water stress. In Proceedings of the 2016 China Ornamental Horticulture Symposium, Changsha, China, 9 July 2016. [Google Scholar]
- Si, J.; Sun, H.; Zhao, X.; Yang, X.Y. Effects of salt stress on seed germinati on and immature embryo growth of Aster alpinus L. J. Seed Sci. 2019, 38, 19–23. [Google Scholar]
- Zhang, L.J.; Liu, Y.; Wang, Y.L.; Xie, L.L.; Wang, X.Y.; Ma, Y.S. Population genetic diversity and structure of Tephritis angustipennis and Campiglossa loewiana (Diptera: Tephritidae) based on COI DNA barcodes in the three-river source region, China. J. Insect Sci. 2024, 24, 7–20. [Google Scholar] [CrossRef]
- Li, W.; Qian, F.; Yang, X.; Chen, S. Systematic position of Cyathocline cass. (Asteraceae): Evidences from molecular, cytological and morphological data. In Proceedings of the Botany in the Construction of Ecological Civilization: Present and Future—The 15th Congress and 80th Anniversary Annual Meeting of the Botanical Society of China, Nanchang, China, 13 October 2013. [Google Scholar]
- Lee, S.E.; Park, S.; Jang, G.Y.; Lee, J.; Moon, M.; Ji, Y.J.; Jung, J.W.; Nam, Y.; Shin, S.J.; Lee, Y.; et al. Extract of Aster koraiensis nakai leaf ameliorates memory dysfunction via anti-inflammatory action. Int. J. Mol. Sci. 2023, 24, 5765. [Google Scholar] [CrossRef]
- Guo, T.; Qin, Y.; Li, Z. Advances in host selection and response of Tephritidae insects. Plant Prot. 2023, 49, 196–206. [Google Scholar]
- Xia, L.T.; Sheng, J.F.; Liao, J.Y.; Yi, J.L.; Zeng, Q.; Huang, C.J.; Song, T.; Chen, X.G.; Tian, B.Q. The current situation of Dacus dorsalis occurrence and comprehensive control techniques. Bull. Agric. Sci. Technol. 2025, 7, 195–197. [Google Scholar]
- Cortez, M.H. Exploiting plant–natural enemy interactions: Selection and evaluation of plants for the improvement of biological control. Insects 2025, 16, 138. [Google Scholar] [CrossRef]
- Salim, K.U.; Chan-Golston, A.M.; Naughton, C.C.; Ha, S.; Bradman, A.; Joyce, A. Sterile insect technique and incompatible insect technique, emerging alternatives to insecticides for adult mosquito control. J. Integr. Pest Manag. 2025, 16, 10. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, H.; Zhou, Y.; Zhou, X.; Guo, J. Sequence characterization and ligand binding analysis of two odorant-binding proteins in Tirathaba rufivena (Walker). J. Trop. Biol. 2025, 15, 1–12. [Google Scholar]
- Wang, J.; Sun, T.; He, J.; Chen, X.; Zhang, D.; Su, X.; Feng, Y.; Xu, X.; Zhao, Z.; Xu, L.; et al. Comparison of the attraction effects of different attractant products on Bactrocera dorsalis. South China Fruits 2023, 52, 18–20+29. [Google Scholar]
- Liu, Z.; Jiang, F.; Guo, S. Research and application progress on quarantine treatment technologies forTephritidae pests. Plant Prot. 2025, 51, 20–29+40. [Google Scholar]
- Zhang, X.Y.; Quan, Y.; Li, C.; Huang, G.Y.; Tan, A.C.; Lu, S.J.; Liu, Y.; Yang, Y.; Xu, L.X.; Gong, Z.B. Evaluation of attraction efficacy of different food lures on fruit files in pear orchards. J. Plant Prot. 2025, 51, 271–279. [Google Scholar]
- Yang, L.; Song, M.; Wang, Y.; Wang, H.S.; Zhou, R. Population characteristics of harmful organisms and their terrain influencing factors in the alpine meadow of Guoluo Prefecture, Qinghai Province. Chin. J. Ecol. 2025, 21, 2164–2173. [Google Scholar]
- Yin, Y.; Zhang, N.; Yan, C.; Cha, G.; Wang, D.M.; Guo, Y.P.; Qin, G.L.; Li, S.W.; Chang, X.; Tu, X.B.; et al. Analysis of insect diversity and its correlation with vegetation in the natural grasslands of Bayannur, Inner Mongolia. J. Plant Prot. 2024, 51, 1158–1168. [Google Scholar]
- Lane, D.E.; Walker, T.J.; Grantham, D.G. IPM adoption and impacts in the United States. J. Integr. Pest Manag. 2023, 14, 1. [Google Scholar] [CrossRef]
- Zhang, L. Feeding Range and Temporal Dynamics of Larvae of Gynaephora Menyuanensis. Master’s Thesis, Lanzhou University, Lanzhou, China, 2021. [Google Scholar]
- Luo, F. Survey on Spider Species Diversity and Population Dynamics of Dominant Dpiders in Alfalfa Fields. Master’s Thesis, Lanzhou University, Lanzhou, China, 2020. [Google Scholar]
- Liu, H. Study on the Occurrence Patterns and Control Techniques of Grassland Pests in Yulin City. Master’s Thesis, Northwest A&F University, Yulin, China, 2019. [Google Scholar]
- Mo, L. Study on the Occurrence Patterns and Control Strategies of Rice ‘Two Migratory’ Pests in Hechi City. Master’s Thesis, Guangxi University, Nanning, China, 2021. [Google Scholar]
- Qin, M.; Lai, Y. Research progress on the application of phoxim in China. Sci. Technol. Qinghai Agric. For. 2021, 35, 54–57. [Google Scholar]
- Wang, X.; Zheng, C.; Dong, Z.; Liu, H.J.; Fu, L.H.; Qian, X.J. A comparative study on male external genitalia among twelve species of grasshoppers (Orthoptera:Acridoidea) from Jiuquan Area, Gansu, Northwest China. J. Plant Prot. 2024, 67, 1239–1250. [Google Scholar]
- Prabhulinga, T.; Kranthi, S.; Raghavendra, K.P.; Kumar, R.; Suke, R.; Chawla, S.; Kranthi, K.R. Mitochondrial COI based genetic diversity and phylogeographic structure of whitefly Bemisia tabaci (Gennadius) on cotton in India. Int. J. Trop. Insect Sci. 2021, 41, 1543–1554. [Google Scholar]
- Ji, J.; Lai, Y.; Zhou, H. Structure and diversity analysis of insect community in different grassland types in Qinghai Lake. Grassl. Turf 2024, 2, 186–195. [Google Scholar]
- Guo, Y.; Shi, K.; Xing, A.; Huang, B.X.; Ling, Y.; Li, Y.Y. Analysis of interspecific interaction network between herbivorous plant bugs and host plants species in the Hulunbuir grassland. J. Ecol. Rural. Environ. 2024, 10, 770–780. [Google Scholar]
- Yue, H.; Liu, G.; Zhang, S.; Ta, F.; Han, G.; Tang, K.; Wang, N. Grassland insect diversity and its influencing factors in central Inner Mongolia. Chin. J. Grassl. 2024, 46, 124–132. [Google Scholar]
- Li, T. Phenology Synchronization Between Carduus Crispus and Tephritis hendeliana in Hongyuan and Songpan Sites: A Comparative Study. Master’s Thesis, Nanjing University, Nanjing, China, 2021. [Google Scholar]
- Wang, Y.; Yang, X.; Hao, L. Phenological of vegetation and its response to climate change in the western Sichuan Plateau. J. Chang. River Sci. Res. Inst. 2023, 40, 77–84+93. [Google Scholar]
- Zhang, Y.H.; Li, J.J.; Li, Y.; Zhang, B.H.; Wang, Y.H.; Hao, B.; Li, X.Y.; Zhang, C.T. Tachinidae (Insecta: Diptera) from the Qilian Mountains of Qinghai, China. Chin. J. Appl. Entomol. 2024, 61, 217–228. [Google Scholar]
- Liu, D. Mechanism Underlying Plant-Mediated Speciation of Parasitoid Wasps: A Preliminary Study. Master’s Thesis, Nanjing University, Nanjing, China, 2021. [Google Scholar]
- He, W. Effects of anthropogenic disturbance on Orthoptera diversity in Xishuangbanna. South China Agric. 2021, 15, 21–26. [Google Scholar]
- Hu, Z. Effects of Inflorescence Bagging on Seed Yield and Growth of Seed Predators in Eight Asteraceae Species. Master’s Thesis, Nanjing University, Nanjing, China, 2017. [Google Scholar]
- Li, Y. Study on Species and Richness of Ichneumonidae in Occurrence Area of Pine Wilt Disease in Fushun City, Liaoning Province. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Nan, Z.; Ai, N.; Yan, H.; Lai, S.; Wang, S.; Yuan, C.; Liu, C. Characteristics and ecological niche analysis of ground-dwelling spider communities in the loess hilly area of returning farmland to forest. Acta Ecol. Sin. 2024, 45, 239. [Google Scholar]
- Xu, W.H. Sublethal Effect of Broflanilide on Spodoptera litura and Eocanthecona furcellata. Master’s Thesis, Southwest University, Chongqi, China, 2023. [Google Scholar]
- Liu, Y.; Liang, X.; Wu, C.; An, X.; Li, D.; Chen, Q. Study on the control efficacy of the combination of food attractant and insecticide on megalurothrips usitatus Bagnall (Thysanoptera: Thripidae). Chin. J. Trop. Agric. 2025, 13, 1–8. [Google Scholar]
- Li, X.; Lu, Y. Effects of biological and environmental factors on the emergence and development of pest resistance. Plant protection 2023, 5, 1–12. [Google Scholar]
- Yang, J.; Zhao, K.; Tang, Z.; Li, X. Practice and reflection on greencontrol of tobacco pests and diseases. J. Agric. Catastrophology 2024, 14, 4–6. [Google Scholar]
- Wang, Y. Research on comprehensive control strategies for common diseases and pests in corn cultivation. Hebei Farm Mach. 2024, 3, 94–96. [Google Scholar]
- Dang, Y.; Wang, X.; Hou, Y. Invasive alien insects: Research progress and prospects. Acta Entomol. Sin. 2024, 67, 1585–1596. [Google Scholar]
- Ji, D.; Ou, L.; Ren, X.; Yang, X.; Tan, Y.; Zhou, X.; Jin, L. Transcriptomic and metabolomic analysis reveal possible molecular mechanisms regulating tea plant growth elicited by chitosan oligosaccharide. Int. J. Mol. Sci. 2022, 23, 5469. [Google Scholar] [CrossRef]
- Jiménez, A.; Correa, S.; Sevilla, F. Identification of Superoxide Dismutase (SOD) Isozymes in Plant Tissues; Springer: New York, NY, USA, 2024; pp. 205–212. [Google Scholar]
- Wang, J.; Zhang, Z.; Wu, J.; Han, X.; Wang-Pruski, G.; Zhang, Z. Genome-wide identification, characterization, and expression analysis related to autotoxicity of the GST gene family in Cucumis melo L. Plant Physiol. Biochem. 2020, 155, 59–69. [Google Scholar]
- Büyükgüzel, E. Effects of eicosanoid biosynthesis inhibitors on selected oxidative stress biomarkers in the midgut of Galleria mellonella (Lepidoptera: Pyralidae) Larvae. J. Entomol. Sci. 2014, 49, 144–155. [Google Scholar] [CrossRef]
- Nakka, S.; Godar, A.S.; Thompson, C.R.; Peterson, D.E.; Jugulam, M. Rapid detoxification via glutathione S-transferase (GST) conjugation confers a high level of atrazine resistance in Palmer amaranth (Amaranthus palmeri). Pest Manag. Sci. 2017, 73, 2236–2243. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Z.; Fang, C.; Liu, K. Transcriptome and metabolome analyses reveal the responses of brown planthoppers to RH resistant rice cultivar. Front. Physiol. 2022, 13, 1018470. [Google Scholar] [CrossRef]
- Benavent-Albarracin, L.; Perez-Hedo, M.; Alonso-Valiente, M.; Catalán, J.; Urbaneja, A.; González-Cabrera, J. Response of Amblyseius swirskii to deltamethrin. Pest Manag. Sci. 2025, 10, 2800–2811. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, X.; Yin, Z.; Zhao, Q.; Shen, J.; Huang, J. Population dynamics of important noctuidae pests in Nanyang city from 2021–2023. J. Environ. Entomol. 2024, 44, 1–11. [Google Scholar]
- Ritchie, F.; Sapelli, L.; Smith, J.A.; Paveley, N.D.; Lees, A.K.; Bain, R.A.; Ritchie, J.M. Testing the taguchi method to design and analyze integrated disease management strategies, for the control of late blight (Phytophthora infestans) on potato. Pest Manag. Sci. 2025, 81, 2337–2346. [Google Scholar] [CrossRef]
- Castle, S.; Naranjo, S.E. Sampling plans, selective insecticides and sustainability: The case for IPM as ‘informed pest management’. Pest Manag. Sci. 2009, 65, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Caton, B.P.; Rogers, J.S.; Marasas, C.N. Taxonomic, geographic, and diversity trends for exotic plant pests in recent biosurveillance articles. J. Pest Sci. 2022, 95, 577–591. [Google Scholar] [CrossRef]
- Noar, R.D.; Jahant-Miller, C.J.; Emerine, S.; Hallberg, R. Early warning systems as a component of integrated pest management to prevent the introduction of exotic pests. J. Integr. Pest Manag. 2021, 12, 16. [Google Scholar] [CrossRef]
- Wang, R.; Li, R.; Chen, T.; Zhang, J.; Xie, C.; Qiu, K.; Chen, P.; Du, J.; Chen, H.; Shao, F.; et al. An automatic system for pest recognition and forecasting. Pest Manag. Sci. 2022, 78, 711–721. [Google Scholar] [CrossRef] [PubMed]

| Sampling Sites | Growth Period of Golog Tibetan Autonomous Prefecture | Total Number of Samples | Sample Proportion | ||||
|---|---|---|---|---|---|---|---|
| LS | BS | IS | PS | FS | |||
| MQAF | 286 | 837 | 1069 | 1399 | 292 | 3883 | 30.87% |
| MQAD | 254 | 449 | 654 | 987 | 165 | 2509 | 19.95% |
| MQAY | 211 | 571 | 621 | 862 | 204 | 2469 | 19.63% |
| MQAA | 84 | 147 | 159 | 145 | 56 | 591 | 4.70% |
| MQAP | 15 | 35 | 54 | 111 | 21 | 236 | 1.88% |
| BMAT | 21 | 59 | 69 | 23 | 15 | 187 | 1.49% |
| DRAS | 281 | 501 | 667 | 757 | 151 | 2357 | 18.74% |
| DRAA | 57 | 67 | 85 | 89 | 49 | 347 | 2.76% |
| Total | 1209 | 2666 | 3378 | 4373 | 953 | 12,579 | 100.00% |
| Sampling sites | Growth period of Haibei Tibetan Autonomous Prefecture | Total Number of Samples | Sample Proportion | ||||
| LS | BS | IS | PS | FS | |||
| QLAD | 125 | 322 | 511 | 658 | 225 | 1841 | 72.31% |
| QLAF | 65 | 91 | 124 | 356 | 69 | 705 | 27.69% |
| Total | 190 | 413 | 635 | 1014 | 294 | 2546 | 100.00% |
| Sampling Sites | Dominant Species Name (Abbreviation) | Growth Period | ||||
|---|---|---|---|---|---|---|
| LS | BS | IS | PS | FS | ||
| MQAF | Tephritis angustipennis (Ta) | 0 | 56 ± 0.33 B | 122 ± 0.33 A | 195 ± 1.2 A | 109 ± 4.48 A |
| Campiglossa loewiana (Cl) | 0 | 58 ± 0.36 A | 33 ± 0.67 C | 56 ± 0.33 B | 46 ± 0.67 B | |
| Hylemyza partita (Hp) | 8 ± 0.33 A | 13 ± 0.19 D | 36 ± 0.58 B | 38 ± 0.33 C | 31 ± 0.67 C | |
| Rhopalomyia giraldii (Rg) | 0 | 20 ± 0.33 C | 32 ± 1.67 C | 22 ± 0.88 D | 8 ± 0.35 F | |
| Parasitoid wasp (Pw) | 3 ± 0.67 B | 9 ± 0.33 E | 16 ± 1.12 D | 23 ± 1.15 D | 19 ± 0.33 E | |
| Aproaerema anthyllidella (Aa) | 0 | 4 ± 0.23 F | 12 ± 0.88 E | 23 ± 1.53 D | 27 ± 1.67 CD | |
| Melitaea cinxia (Mc) | 0 | 1 ± 0.58 G | 13 ± 1.2 E | 16 ± 0.67 E | 23 ± 0.67 DE | |
| MQAD | Tephritis angustipennis (Ta) | 0 | 47 ± 1.12 A | 62 ± 0.33 A | 99 ± 1.2 A | 81 ± 0.58 A |
| Campiglossa loewiana (Cl) | 0 | 8 ± 0.33 C | 15 ± 0.23 E | 46 ± 0.32 B | 29 ± 0.58 B | |
| Hylemyza partita (Hp) | 0 | 6 ± 0.33 E | 25 ± 1.53 C | 33 ± 0.67 D | 19 ± 0.58 D | |
| Rhopalomyia giraldii (Rg) | 0 | 7 ± 0.19 D | 27 ± 0.33 B | 35 ± 0.33 C | 23 ± 1.23 C | |
| Parasitoid wasp (Pw) | 4 ± 0.67 A | 12 ± 0.33 B | 18 ± 0.88 D | 6 ± 0.28 F | 0 | |
| Aproaerema anthyllidella (Aa) | 0 | 0 | 7 ± 0.33 F | 9 ± 0.33 E | 13 ± 1 E | |
| Melitaea cinxia (Mc) | 0 | 0 | 1 ± 0.33 G | 1 ± 0.05 G | 2 ± 0.26 F | |
| MQAY | Tephritis angustipennis (Ta) | 0 | 35 ± 0.67 A | 46 ± 0.67 A | 109 ± 0.58 A | 53 ± 0.58 A |
| Campiglossa loewiana (Cl) | 7 ± 0.58 B | 10 ± 0.33 C | 29 ± 0.58 B | 21 ± 0.33 B | 2 ± 0.25 B | |
| Hylemyza partita (Hp) | 4 ± 0.33 C | 11 ± 0.33 C | 16 ± 1.21 D | 9 ± 0.27 D | 3 ± 0.36 B | |
| Rhopalomyia giraldii (Rg) | 7 ± 0.05 B | 30 ± 0.88 B | 23 ± 0.59 C | 19 ± 0.88 C | 4 ± 0.28 B | |
| Parasitoid wasp (Pw) | 4 ± 0.67 C | 5 ± 0.58 D | 6 ± 0.33 E | 1 ± 0.58 F | 5 ± 1.2 B | |
| Aproaerema anthyllidella (Aa) | 1 ± 0.33 D | 2 ± 0.33 E | 2 ± 0.33 F | 3 ± 0.33 E | 6 ± 0.39 B | |
| Melitaea cinxia (Mc) | 0 | 1 ± 0.33 E | 1 ± 0.33 F | 3 ± 0.01 E | 7 ± 0.47 B | |
| MQAA | Tephritis angustipennis (Ta) | 12 ± 7.25 A | 21 ± 8.01 A | 42 ± 2.58 A | 30 ± 7.67 A | 9 ± 2.59 A |
| Thysanoptera sp. (Ts) | 0 | 9 ± 0.33 AB | 18 ± 0.58 AB | 30 ± 0.33 A | 12 ± 0.33 A | |
| Rhopalomyia giraldii (Rg) | 4 ± 0.67 A | 7 ± 0.58 B | 12 ± 0.67 AB | 2 ± 0.33 B | 0 | |
| Acyrthosiphon pisum (Ap) | 0 | 2 ± 0.33 B | 4 ± 0.29 B | 1 ± 0.67 B | 1 ± 0.33 B | |
| Aphis craccivora (Ac) | 0 | 1 ± 0.33 B | 2 ± 0.33 B | 4 ± 0.33 B | 1 ± 0.25 B | |
| Apolygus lucorum (Al) | 1 ± 0.67 A | 2 ± 0.33 B | 2 ± 0.67 B | 1 ± 0.33 B | 0 | |
| Hylemyza partita (Hp) | 0 | 1 ± 0.33 B | 1 ± 0.58 B | 2 ± 0.58 B | 1 ± 0.33 B | |
| Sampling sites | Dominant species name (abbreviation) | Growth period | ||||
| LS | BS | IS | PS | FS | ||
| MQAP | Tephritis angustipennis (Ta) | 0 | 2 ± 0.33 A | 4 ± 0.33 AB | 6 ± 0.33 A | 4 ± 0.33 A |
| Apolygus lucorum (Al) | 0 | 2 ± 0.88 A | 5 ± 0.33 A | 2 ± 0.33 B | 1 ± 0.58 DE | |
| Campiglossa loewiana (Cl) | 0 | 0 | 2 ± 0.33 DE | 5 ± 0.58 A | 2 ± 0.33 BC | |
| Tephritis femoralis (Tf) | 0 | 1 ± 0.33 AB | 3 ± 0.33 BC | 3 ± 0.58 B | 1 ± 0.67 CD | |
| Melitaea cinxia (Mc) | 0 | 0 | 1 ± 0.58 E | 2 ± 0.33 B | 3 ± 0.58 AB | |
| Acyrthosiphon pisum (Ap) | 1 ± 0.67 A | 2 ± 0.33 A | 2 ± 0.33 CD | 0 | 0 | |
| Hylemyza partita (Hp) | 0 | 1 ± 0.29 AB | 2 ± 0.33 CD | 2 ± 0.63 B | 0 | |
| DRAS | Tephritis angustipennis (Ta) | 0 | 5 ± 0.33 C | 30 ± 0.33 C | 38 ± 0.33 B | 16 ± 0.33 B |
| Euodynerus dantici (Ed) | 7 ± 0.58 A | 18 ± 0.58 A | 56 ± 1.2 A | 71 ± 0.88 A | 31 ± 0.58 A | |
| Rhopalomyia giraldii (Rg) | 0 | 8 ± 0.58 B | 44 ± 0.33 B | 29 ± 0.47 C | 14 ± 0.33 B | |
| Symphoromyia crassicornis (Sc) | 2 ± 0.33 B | 7 ± 0.58 B | 12 ± 0.33 D | 29 ± 0.33 C | 0 | |
| Philonthus nitidus (Pn) | 0 | 2 ± 0.33 D | 8 ± 0.33 DE | 8 ± 0.33 E | 15 ± 0.35 B | |
| Hylemyza partita (Hp) | 0 | 2 ± 0.33 D | 6 ± 0.58 E | 12 ± 0.33 D | 3 ± 0.33 C | |
| Lygus pratensis (Lp) | 1 ± 0.33 C | 3 ± 0.33 D | 12 ± 0.33 D | 5 ± 0.01 F | 3 ± 0.33 C | |
| DRAA | Tephritis angustipennis (Ta) | 0 | 5 ± 0 A | 12 ± 0.58 A | 7 ± 0.87 A | 4 ± 0.58 A |
| Tephritis femoralis (Tf) | 1 ± 0.33 BC | 2 ± 0.67 BC | 4 ± 0.33 B | 2 ± 0.33 C | 1 ± 0.05 C | |
| Geotrupidae sp. (Gs) | 2 ± 0.33 A | 3 ± 0.33 B | 4 ± 0.58 B | 0 | 0 | |
| Aphis craccivora (Ac) | 1 ± 0.33 BC | 1 ± 0.33 C | 2 ± 0.67 C | 3 ± 0.33 B | 2 ± 0.33 B | |
| Curculionidae sp. (Cs) | 0 | 1 ± 0.33 C | 5 ± 0.67 B | 1 ± 0.33 D | 0 | |
| Rhopalomyia giraldii (Rg) | 1 ± 0.33 B | 2 ± 0.33 BC | 2 ± 0.33 C | 1 ± 0.33 D | 0 | |
| Acyrthosiphon pisum (Ap) | 0 | 1 ± 0.41 C | 1 ± 0.48 C | 3 ± 0.29 CD | 1 ± 0.29 CD | |
| BMAT | Tephritis angustipennis (Ta) | 0 | 2 ± 0.33 AB | 3 ± 0.58 A | 5 ± 0.67 A | 1 ± 0.33 B |
| Hylemyza partita (Hp) | 2 ± 0.33 A | 3 ± 0.67 A | 3 ± 0.33 A | 6 ± 0.88 A | 5 ± 0.67 A | |
| Acyrthosiphon pisum (Ap) | 1 ± 0.29 B | 2 ± 0.33 A | 3 ± 0.88 A | 1 ± 0.33 B | 1 ± 0.33 B | |
| Mantodea sp. (Ms) | 1 ± 0.05 B | 2 ± 0.33 AB | 3 ± 0.58 A | 1 ± 0.33 B | 1 ± 0.33 B | |
| Rhopalomyia giraldii (Rg) | 0 | 2 ± 0.33 AB | 2 ± 0.33 AB | 0 | 0 | |
| Campiglossa loewiana (Cl) | 0 | 1 ± 0.33 B | 1 ± 0.33 B | 2 ± 0.33 B | 1 ± 0.33 B | |
| Aphis craccivora (Ac) | 1 ± 0.29 BC | 1 ± 0.48 B | 1 ± 0.48 B | 1 ± 0.75 B | 0 | |
| Sampling Sites | Dominant Species Name (Abbreviation) | Growth Period | ||||
|---|---|---|---|---|---|---|
| LS | BS | IS | PS | FS | ||
| QLAD | Melitaea cinxia (Mc) | 0 | 0 | 9 ± 0.33 D | 15 ± 0.33 C | 19 ± 0.33 A |
| Frankliniella intonsa (Fi) | 2 ± 0.33 C | 6 ± 0.33 DE | 25 ± 0.33 B | 19 ± 0.67 B | 12 ± 0.33 C | |
| Adonia variegata (Av) | 2 ± 0.33 C | 4 ± 0.33 F | 2 ± 0.58 G | 1 ± 0.33 F | 0 | |
| Acyrthosiphon pisum (Ap) | 5 ± 0.58 AB | 7 ± 0.58 D | 13 ± 0.67 C | 3 ± 0.58 DE | 2 ± 0.33 D | |
| Adelphocoris lineolatus (Ai) | 6 ± 0.58 A | 11 ± 0.33 B | 7 ± 0.58 E | 3 ± 0.67 E | 2 ± 0.33 D | |
| Curculionidae sp. (Cs) | 2 ± 0.33 C | 6 ± 0.33 E | 9 ± 0.33 D | 4 ± 0.33 D | 0 | |
| Apolygus lucorum (Al) | 4 ± 0.58 B | 9 ± 0.33 C | 4 ± 0.33 F | 3 ± 0.33 DE | 0 | |
| Tephritis angustipennis (Ta) | 0 | 19 ± 0.33 A | 33 ± 0.67 A | 41 ± 0.33 A | 16 ± 0.33 B | |
| QLAF | Gynaephora menyuanensis (Gm) | 5 ± 0.58 A | 9 ± 0.33 A | 13 ± 0.58 A | 3 ± 0.33 B | 0 |
| Frankliniella intonsa (Fi) | 4 ± 0.33 B | 7 ± 0.33 A | 10 ± 0.58 B | 5 ± 0.33 A | 0 | |
| Adelphocoris lineolatus (Ai) | 3 ± 0.33 C | 9 ± 0.33 A | 13 ± 0.67 A | 1 ± 0.33 C | 0 | |
| Rhopalomyia giraldii (Rg) | 0 | 5 ± 0.33 B | 7 ± 0.33 C | 1 ± 0.33 C | 0 | |
| Curculionidae sp. (Cs) | 1 ± 0.33 D | 2 ± 1.2 DE | 3 ± 0.33 DE | 3 ± 0.67 B | 1 ± 0.33 A | |
| Apolygus lucorum (Al) | 3 ± 0.58 C | 4 ± 0.67 BC | 2 ± 0.33 E | 0 | 0 | |
| Hylemyza partita (Hp) | 1 ± 0.33 DE | 3 ± 0.58 CD | 4 ± 0.33 D | 0 | 0 | |
| Tephritis angustipennis (Ta) | 0 | 1 ± 0.67 E | 2 ± 0.33 E | 3 ± 0.33 B | 0 | |
| Period (Abbreviation) | Type/Combination of Chemicals | Pot Experiment | Field Experiment | ||
|---|---|---|---|---|---|
| Pest Species | Pest Quantity | Number of T. angustipennis | Male Adult Number of Gynaephora menyuanensis | ||
| Leaf unfolding stage (LS) | Ae | 1 ± 0 a | 5 ± 1.2 a | / | / |
| Ae + Su | 1 ± 0 a | 6 ± 1.15 a | / | / | |
| Ae + Ch | 1 ± 0 a | 4.67 ± 0.33 a | / | / | |
| Ae + Su + Ch | 1 ± 0 a | 3.67 ± 0.33 a | / | / | |
| Budding stage (BS) | Ae | 2 ± 0.33 a | 36 ± 4.33 a | / | / |
| Ae + Su | 2 ± 0.67 a | 39 ± 4.26 a | / | / | |
| Ae + Ch | 1 ± 0.33 a | 46 ± 6.24 a | / | / | |
| Ae + Su + Ch | 1 ± 0 a | 51 ± 3.48 a | / | / | |
| Initial flowering stage (IS) | Ae | 1 ± 0 a | 42 ± 5.29 b | 45 ± 0.67 b | 90 ± 1.53 b |
| Ae + Su | 2 ± 0.67 a | 42 ± 6.08 b | 48 ± 1.76 b | 95 ± 3.84 b | |
| Ae + Ch | 1 ± 0.33 a | 65 ± 5.03 a | 59 ± 1.33 a | 101 ± 2.96 a | |
| Ae + Su + Ch | 1 ± 0.33 a | 68 ± 3.76 a | 58 ± 2.91 a | 101 ± 3.48 a | |
| Peak bloom stage (PS) | Ae | 1 ± 0 a | 49 ± 9.06 b | 47 ± 6.67 a | 86 ± 14.93 a |
| Ae + Su | 2 ± 0.67 a | 77 ± 12.6 ab | 64 ± 6.12 a | 82 ± 6.36 a | |
| Ae + Ch | 2 ± 0.33 a | 81 ± 7.22 a | 52 ± 6.64 a | 90 ± 7.84 a | |
| Ae + Su + Ch | 2 ± 0.88 a | 107 ± 4.18 a | 57 ± 9.94 a | 102 ± 15.13 a | |
| Fruiting stage (FS) | Ae | 1 ± 0 a | 16 ± 0.88 b | 35 ± 3.33 b | 83 ± 9.02 a |
| Ae + Su | 1 ± 0 a | 16 ± 0.33 b | 46 ± 7.75 ab | 89 ± 4.58 a | |
| Ae + Ch | 1 ± 0 a | 17 ± 1.15 b | 65 ± 8.95 a | 93 ± 11.32 a | |
| Ae + Su + Ch | 1 ± 0 a | 20 ± 0.58 a | 58 ± 5.51 a | 94 ± 13.3 a | |
| Overall comparison of all periods (ALL) | Ae | 2 ± 0.58 a | 148 ± 17.01 c | 127 ± 5.21 b | 259 ± 24.58 a |
| Ae + Su | 3 ± 0.88 a | 181 ± 15.04 bc | 157 ± 5 a | 266 ± 14.42 a | |
| Ae + Ch | 2 ± 0.58 a | 213 ± 12.44 ab | 176 ± 11.62 a | 285 ± 17.44 a | |
| Ae + Su + Ch | 1 ± 0 a | 249 ± 3.84 a | 174 ± 8.19 a | 296 ± 26.44 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-J.; Ma, Y.-S.; Liu, Y.; Wang, J.-L. Ecological Pest Control in Alpine Ecosystems: Monitoring Asteraceae Phytophages and Developing Integrated Management Protocols in the Three River Source Region. Insects 2025, 16, 861. https://doi.org/10.3390/insects16080861
Zhang L-J, Ma Y-S, Liu Y, Wang J-L. Ecological Pest Control in Alpine Ecosystems: Monitoring Asteraceae Phytophages and Developing Integrated Management Protocols in the Three River Source Region. Insects. 2025; 16(8):861. https://doi.org/10.3390/insects16080861
Chicago/Turabian StyleZhang, Li-Jun, Yu-Shou Ma, Ying Liu, and Jun-Ling Wang. 2025. "Ecological Pest Control in Alpine Ecosystems: Monitoring Asteraceae Phytophages and Developing Integrated Management Protocols in the Three River Source Region" Insects 16, no. 8: 861. https://doi.org/10.3390/insects16080861
APA StyleZhang, L.-J., Ma, Y.-S., Liu, Y., & Wang, J.-L. (2025). Ecological Pest Control in Alpine Ecosystems: Monitoring Asteraceae Phytophages and Developing Integrated Management Protocols in the Three River Source Region. Insects, 16(8), 861. https://doi.org/10.3390/insects16080861








