Binary Mixtures of Essential Oils: Potent Housefly Adulticides That Are Safe Against Non-Target Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils and Other Materials
2.2. Fly Management
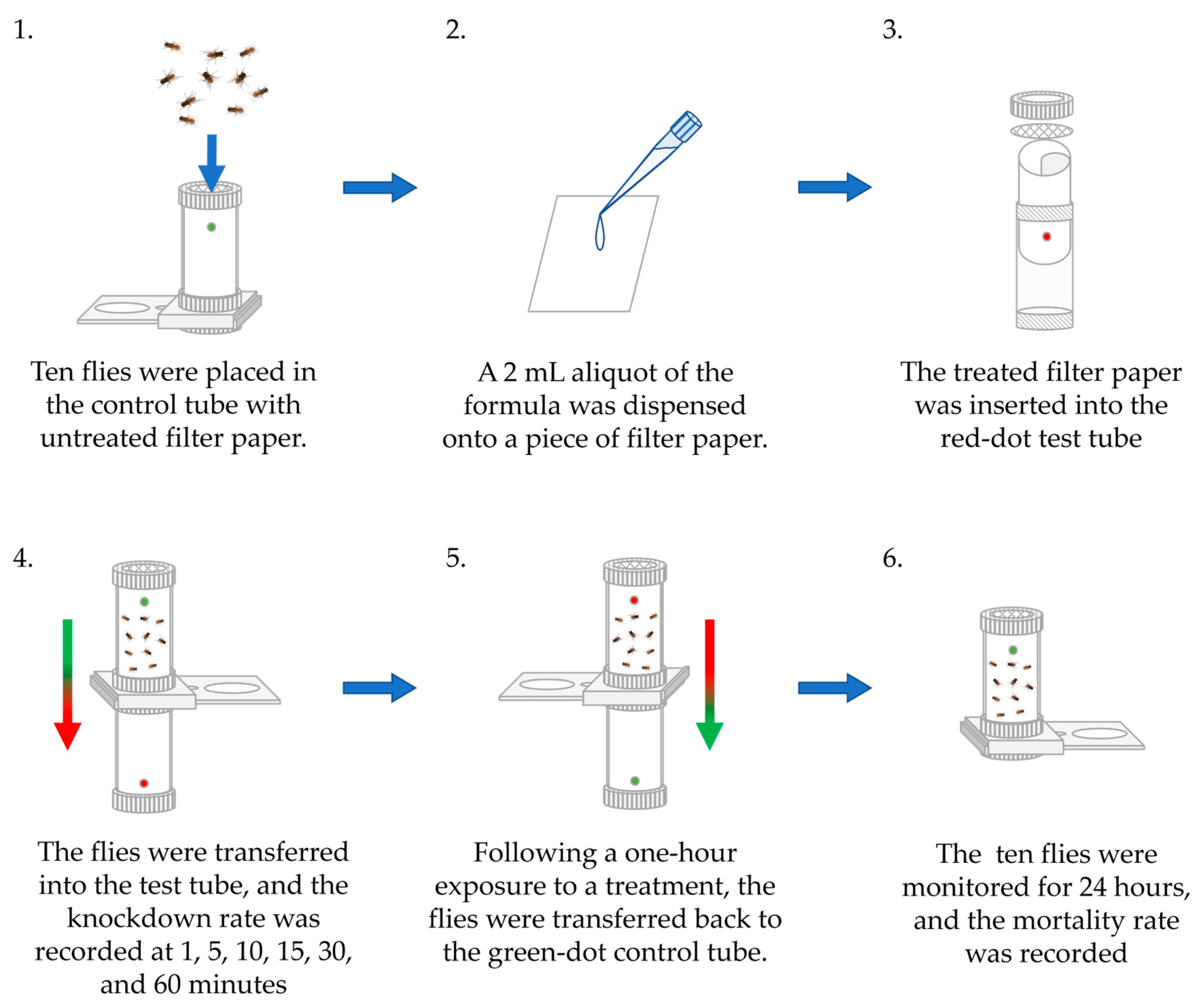
2.3. Flies’ Adulticidal Activities
2.4. Safety Test on the Dwarf Honeybee (A. florea) and Guppy (P. reticulata) Non-Target Organisms
2.5. Data Analysis
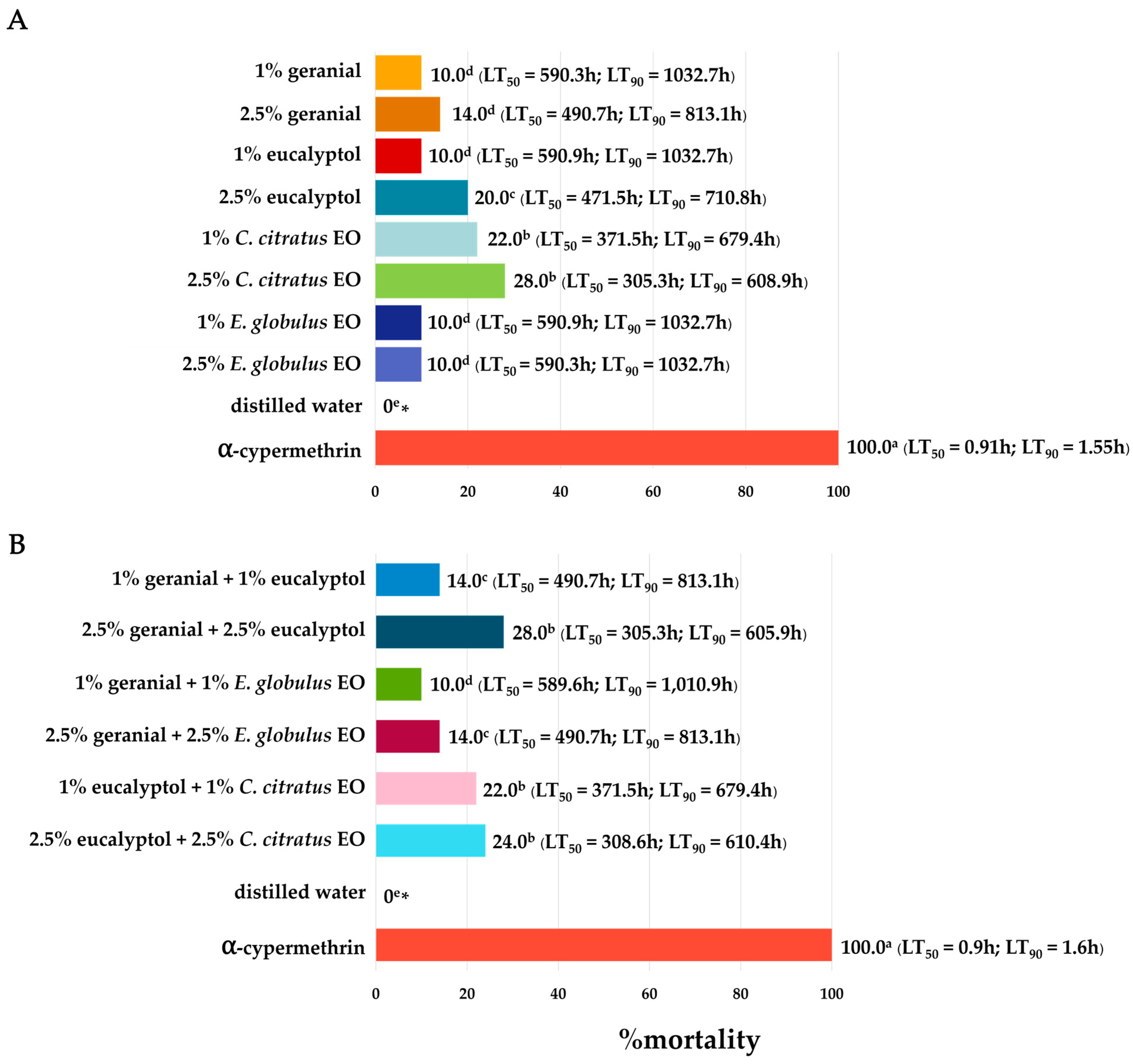
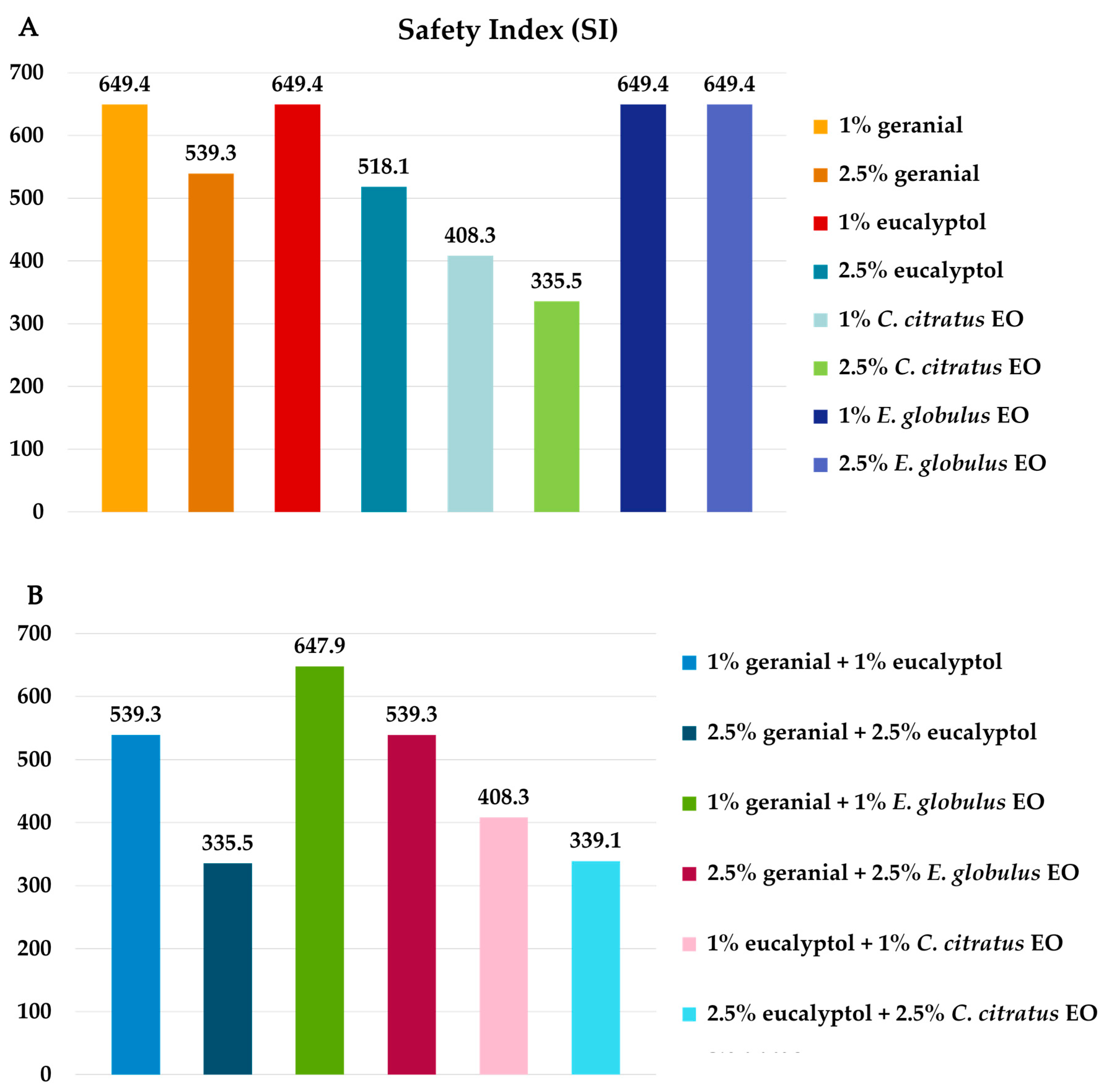
3. Results
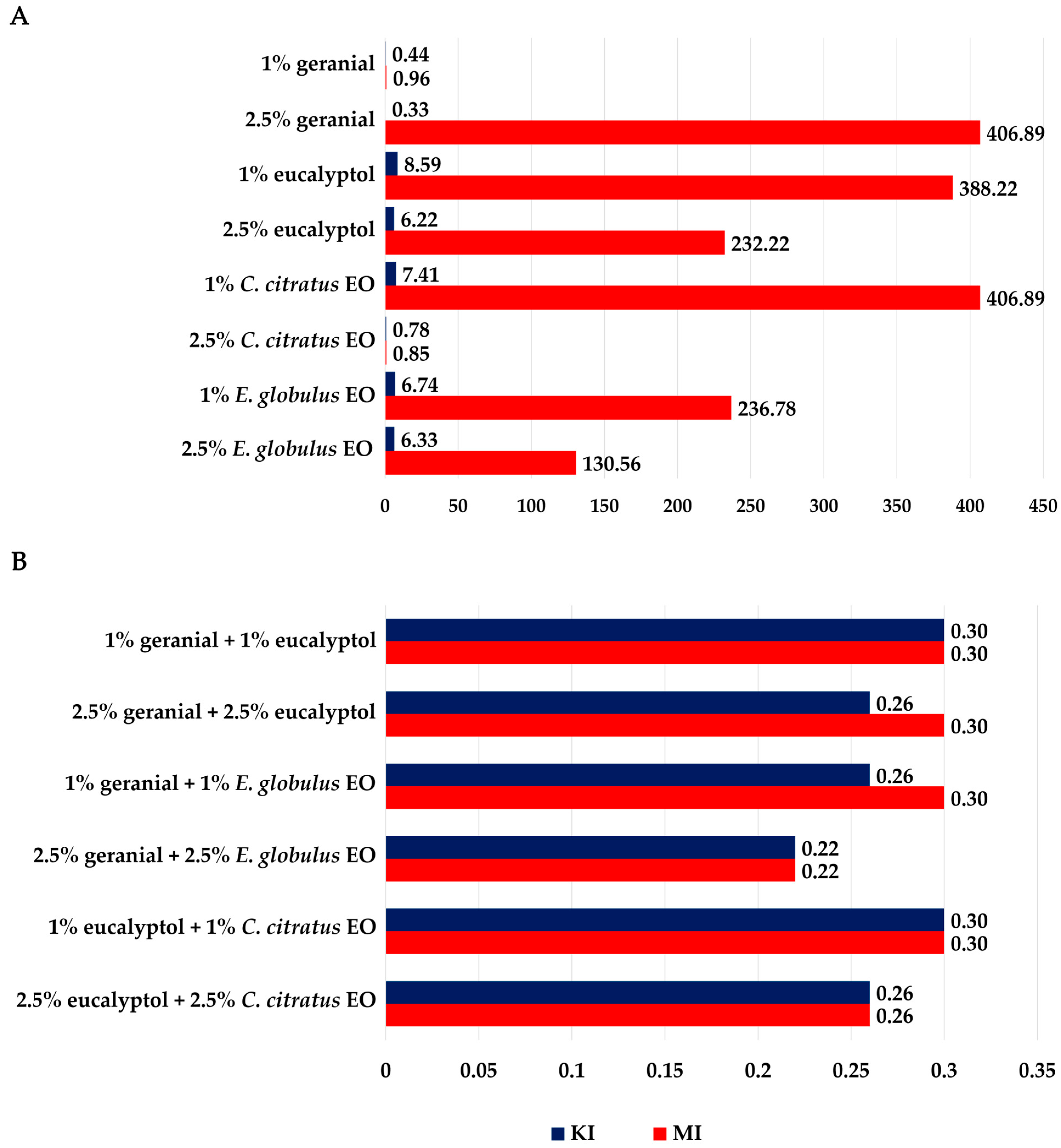
3.1. Bioassay for Adulticidal Activity
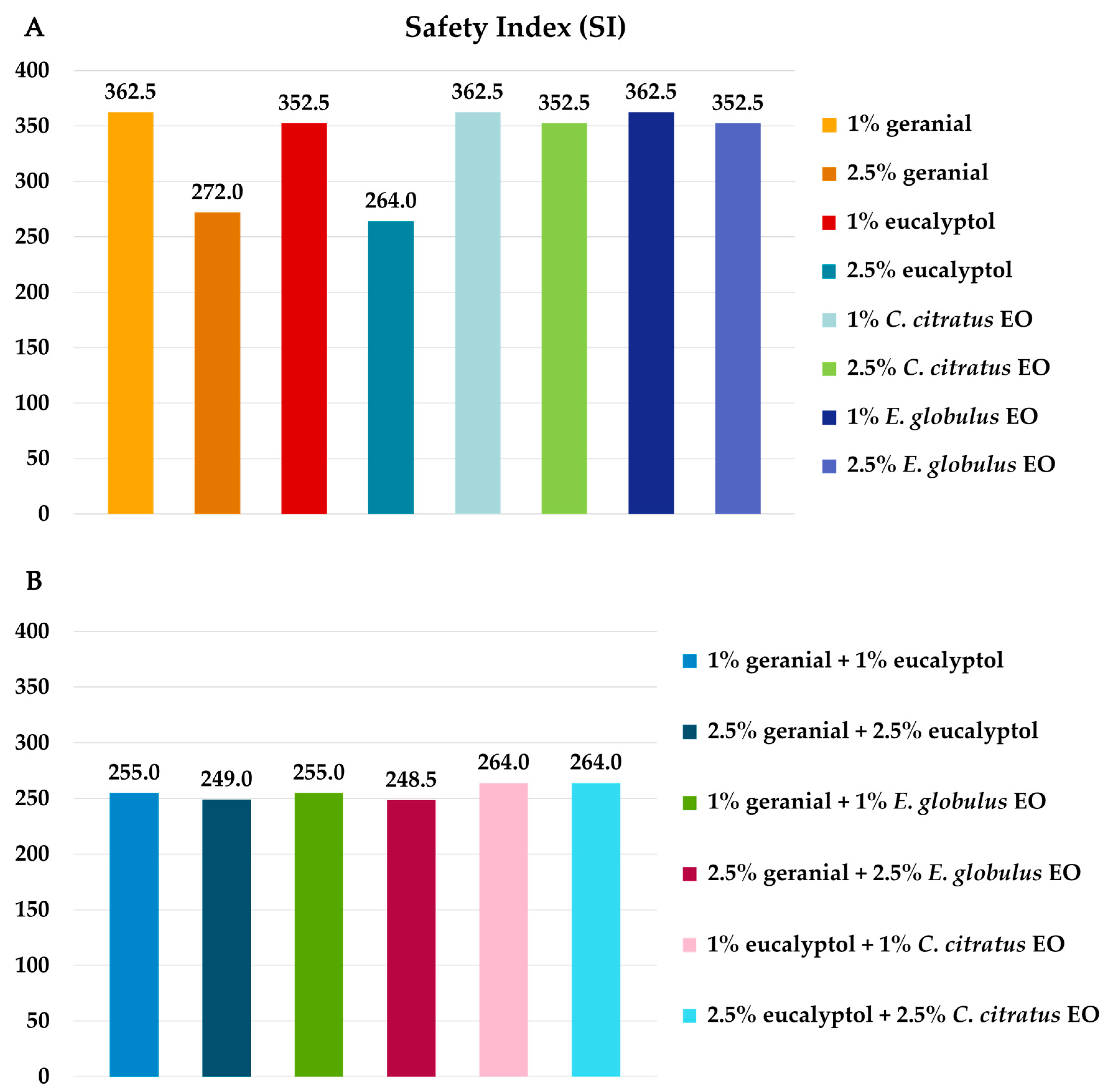
3.2. Benign Non-Target Bioassay Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortinhas, L.B.; Mendonça, P.M.; Braga, M.V.; Queiroz, M.M.C. Ultrastructure of the immature stages of Musca domestica (Diptera: Muscidae: Muscinae). J. Med. Entomol. 2020, 57, 1712–1721. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess, E.R.; Gerry, A.C.; Kaufman, P.E.; Thomson, J.; Pickens, V.; Machtinger, E.T. House fly (Diptera: Muscidae): Biology, pest status, current management prospects, and research needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Issa, R. Musca domestica acts as transport vector hosts. Bull. Natl. Res. Cent. 2019, 43, 73. [Google Scholar] [CrossRef]
- Adenusi, A.A.; Adewoga, T.S. Human intestinal parasites in non-biting synanthropic flies in Ogun state, Nigeria. Travel Med. Infect. Dis. 2013, 11, 181–189. [Google Scholar] [CrossRef]
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef]
- Park, R.; Dzialo, M.C.; Spaepen, S.; Nsabimana, D.; Gielens, K.; Devriese, H.; Crauwels, S.; Tito, R.Y.; Raes, J.; Lievens, B.; et al. Microbial communities of the house fly Musca domestica vary with geographical location and habitat. Microbiome 2019, 7, 147. [Google Scholar] [CrossRef]
- Haselton, A.T.; Acevedo, A.; Kuruvilla, J.; Werner, E.; Kiernan, J.; Dhar, P. Repellency of α-pinene against against the house fly, Musca domestica. Phytochemistry 2015, 117, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Chaiwong, T.; Srivoramas, T.; Sueabsamran, P.; Sukontason, K.; Sanford, M.R.; Sukontason, K.L. The blow fly, Chrysomya megacephala, and the house fly, Musca domestica, as mechanical vectors of pathogenic bacteria in Northeast Thailand. Trop. Biomed. 2014, 31, 336–346. [Google Scholar] [PubMed]
- Hafez, A.M. First evaluation of field evolved resistance to commonly used insecticides in house fly populations from Saudi Arabian dairy farms. Insects 2021, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Milutinović, M.; Savić, J. When Scent Becomes a Weapon—Plant essential oils as potent bioinsecticides. Sustainability 2022, 14, 6847. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Chellappandian, M.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Karthi, S.; Thanigaivel, A.; Ponsankar, A.; Kalaivani, K.; Hunter, W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018, 113, 214–230. [Google Scholar] [CrossRef]
- George, D.R.; Finn, R.D.; Graham, K.M.; Sparagano, O.A. Present and future potential of plant-derived products to control arthropods of veterinary and medical significance. Parasites Vectors 2014, 7, 28. [Google Scholar] [CrossRef]
- Naqqash, M.N.; Gökçe, A.; Bakhsh, A.; Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016, 115, 1363–1373. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control. 2022, 176, 105071. [Google Scholar] [CrossRef]
- Selvakumaran, J.; Ragavendran, K.; Ignacimuthu, S.; Sivanandhan, S.; Reegan, A.D.; Aremu, A.O.; Ganesan, P.; Alharbi, N.S.; Thiruvengadam, M. Mosquitocidal susceptibility and non-target effects of essential oil from Brassica nigra W.D.J. Koch seeds on immature stages of Aedes aegypti L., Anopheles stephensi Liston and Culex quinquefasciatus Say. S. Afr. J. Bot. 2024, 167, 578–584. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Daungchana, N.; Sisubalan, N.; Chaiyasut, C. Composition, cioactivities, microbiome, safety concerns, and impact of essential oils on the health status of domestic animals. Appl. Sci. 2024, 14, 6882. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Li, Y.; Zhong, G. Prospects of botanical compounds and pesticides as sustainable management strategies against Spodoptera frugiperda. J. Econ. Entomol. 2022, 115, 1834–1845. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Atkovska, K.; Kuvendziev, S.; Mustafa, E.; Marinkovski, M.; Ghaffari, P.; Lisichkov, K. Essential oils as green repellents against mosquito vectors. Qual. Life-APEIRON 2021, 12, 51–60. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise. Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Aungtikun, J.; Soonwera, M.; Sittichok, S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verum Hook. f. and their major active constituents. Ind. Crops Prod. 2021, 164, 113386. [Google Scholar] [CrossRef]
- Sinthusiri, J.; Soonwera, M. Efficacy of herbal essential oils as insecticides against the housefly, Musca domestica L. Southeast Asian J. Trop. Med. Public Health 2013, 44, 188–196. [Google Scholar]
- Soonwera, M.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Sinthusiri, J.; Passara, H. Adulticidal synergy of two plant essential oils and their major constituents against the housefly Musca domestica and bioassay on non-target species. Heliyon 2024, 10, e26910. [Google Scholar] [CrossRef]
- Passara, H.; Sittichok, S.; Sinthusiri, J.; Moungthipmalai, T.; Puwanard, C.; Murata, K.; Soonwera, M. Ovicidal toxicity andmorphological changes in housefly eggs induced by the essential oils of star anise and lemongrass and their main constituents. Insects 2024, 15, 481. [Google Scholar] [CrossRef]
- Passara, H.; Sittichok, S.; Puwanard, C.; Sinthusiri, J.; Moungthipmalai, T.; Murata, K.; Soonwera, M. Anise and fennel essential oils and their combination as natural and safe housefly repellents. Insects 2024, 16, 23. [Google Scholar] [CrossRef]
- Khanikor, B.; Parida, P.; Yadav, R.N.S.; Bora, D. Comparative mode of action of some terpene compounds against octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modeling and Docking studies. J. Appl. Pharm. Sci. 2013, 3, 006–012. [Google Scholar] [CrossRef]
- Yoon, J.; Tak, J.H. Synergistic modes of interaction between the plant essential oils and the respiratory bloker chlorfenapyr. Pestic. Biochem. Physiol. 2022, 188, 105274. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Soonwera, M. Adulticidal activity against housefly (Musca domestica L.; Muscidae: Diptera) of eucalyptol, limonene, and their combined formulation. Int. J. Agric. Technol. 2022, 18, 271–280. [Google Scholar]
- Puwanard, C.; Soonwera, M. Ovicidal and adulticidal activities of Cymbopogon citratus (DC.) Stapf and Illicium verum Hook. against Aedes aegypti (Linn.). Int. J. Agric. Technol. 2022, 18, 319–328. [Google Scholar]
- Rossi, Y.E.; Palacios, S.M. Fumigant toxicity of citrus sinensis essential oil on Musca domestica L. adults in the absence and presence of a P450 inhibitor. Acta Trop. 2013, 127, 33–37. [Google Scholar] [CrossRef]
- Raffiudin, R.; Shullia, N.I.; Damayanti, A.U.; Wahyudi, D.T.; Febiriani, T.V.; Atmowidi, T.; Lamerkabel, J.S.A.; Widjaja, M.C. New haplotypes of Apis cerana in Indonesia: Identification from mitochondrial and major royal jelly protein 2 genes. Int. J. Trop. Insect Sci. 2022, 42, 389–401. [Google Scholar] [CrossRef]
- Bommuraj, V.; Chen, Y.; Klein, H.; Sperling, R.; Barel, S.; Shimshoni, J.A. Pesticide and trace element residues in honey and beeswax combs from Israel in association with human risk assessment and honey adulteration. Food Chem. 2019, 299, 125123. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, A.L.; Rosa-Fontana, A.S.; dos Santos, C.F.; Blochtein, B. Larvae of stingless bee Scaptotrigona bipunctata exposed to organophosphorus pesticide develop into lighter, smaller and deformed adult workers. Environ. Pollut. 2021, 272, 116414. [Google Scholar] [CrossRef]
- Queiroz, L.G.; do Prado, C.C.A.; da Silva, D.C.V.R.; Gomes, L.E.T.; Marassi, R.J.; Almeida, É.C.; Pinto, E.; da Silva, F.T.; de Paiva, T.C.B. Ecological risk of imidacloprid on the Brazilian non-target freshwater organisms Chironomus sancticaroli and Poecilia reticulata. Environ. Monit. Assess. 2022, 194, 751. [Google Scholar] [CrossRef] [PubMed]
- WHO. Standard Operating Procedure for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Tube Tests. 2022. Available online: https://www.who.int/publications/i/item/9789240043831 (accessed on 16 July 2025).
- Matos, W.B.; Santos, A.C.C.; Lima, A.P.S.; Santana, E.D.R.; Silva, J.E.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Potential source of ecofriendly insecticides: Essential oil induces avoidance and cause lower impairment on the activity of a stingless bee than organosynthetic insecticides, in laboratory. Ecotoxicol. Environ. Saf. 2021, 209, 111764. [Google Scholar] [CrossRef]
- Soonwera, M.; Sinthusiri, J.; Passara, H.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Murata, K. Combinations of lemongrass and star anise essential oils and their main constituent: Synergistic housefly repellency and safety against non-target organism. Insects 2024, 15, 210. [Google Scholar] [CrossRef]
- Barathi, S.; Sabapathi, N.; Kandasamy, S.; Lee, J. Present status of insecticide impacts and eco-friendly approaches for remediation-a review. Environ. Res. 2024, 240, 117432. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect cuticle and insecticide development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef]
- Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Haro-Vázquez, M.D.P.; Córdova-Guerrero, I. Applications of plant essential oils in pest control and their encapsulation for controlled release: A review. Agriculture 2024, 14, 1766. [Google Scholar] [CrossRef]
- Bedini, S.; Djebbi, T.; Ascrizzi, R.; Farina, P.; Pieracci, Y.; Echeverría, M.C.; Flamini, G.; Trusendi, F.; Ortega, S.; Chiliquinga, A.; et al. Repellence and attractiveness: The hormetic effect of aromatic plant essential oils on insect behavior. Ind. Crops Prod. 2024, 210, 118122. [Google Scholar] [CrossRef]
- Chauhan, N.; Malok, A.; Sharma, S. Repellency potential of essential oils against housefly Musca domestica L. Environ. Sci. Pollut. Res. 2018, 25, 4707–4714. [Google Scholar] [CrossRef]
- Rasidi, M.; Jahanifard, E.; Soltani, A.; Sharififard, M. Evaluating the lethal toxicity of eucalyptus and yellow essential oils against the house fly (Musca domestica L.) under laboratory conditions. J. Health Res. Commun. 2023, 9, 39–47. Available online: https://dor.isc.ac/dor/20.1001.1.24236772.1402.9.3.5.9 (accessed on 14 August 2025).
- de Araújo, G.A.; Morais Oliveira Tintino, C.D.; Pereira, R.L.S.; Araújo, I.M.; Paulo, C.L.R.; de Oliveira Borges, J.A.; de Sousa Rodrigues, E.Y.; da Silva, Â.E.; Bezerra da Cunha, F.A.; de Sousa Silveira, Z.; et al. Toxicological assessment of citral and geraniol: Efflux pump inhibition in Staphylococcus aureus and invertebrate toxicity. Toxicol. Rep. 2025, 14, 101917. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.A.A.L.; Dos Santos, J.R.; Melo, T.C.S.; de Souza, M.F.; Santos, L.G.; de Gois, A.M.; Cintra, R.R.; Lins, L.C.R.F.; Ribeiro, A.M.; Marchioro, M. Depressant effect of geraniol on the central nervous system of rats: Behavior and ECoG power spectra. Biomed. J. 2018, 41, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Selles, S.M.A.; Kouidri, M.; González, M.G.; González, J.; Sánchez, M.; González-Coloma, A.; Sanchis, J.; Elhachimi, L.; Olmeda, A.S.; Tercero, J.M.; et al. Acaricidal and repellent effects of essential oils against ticks: A review. Pathogens 2021, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Pang, Y.P. Insect acetylcholinesterase as a target for effective and environmentally safe insecticides. Adv. Insect Physiol. 2014, 46, 435–494. [Google Scholar] [CrossRef]
- Soonwera, M.; Sittichok, S. Adulticidal activities of Cymbopogon citratus (Stapf.) and Eucalyptus globulus (Labill.) essential oils and of their synergistic combinations against Aedes aegypti (L.), Aedes albopictus (Skuse), and Musca domestica (L.). Environ. Sci. Pollut. Res. 2020, 27, 20201–20214. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Compositional analysis and insecticidal activity of Eucalyptus globulus (family: Myrtaceae) essential oil against housefly (Musca domestica). Acta Trop. 2012, 122, 212–218. [Google Scholar] [CrossRef]
- Khan, S.; Tabing, C.N.T.; Bonneure, E.; Mangelinckx, S.; Smagghe, G.; Shan, M.M. Insecticidal activity of plant-derived extracts against different economically important pest insects. Phytoparasitica 2017, 45, 113–124. [Google Scholar] [CrossRef]
- Tennyson, S.; Samraj, D.A.; Jeyasundar, D.; Chali, K. Larvicidal efficacy of plant oils against the dengue vector Aedes aegypti (L.) (Diptera: Culicidae). Middle-East J. Sci. Res. 2013, 13, 64–68. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Shamakhi, L.; Sahebzadeh, N.; Naseri, D.; Hoda, H. Bio-efficacy and physiological effects of Eucalyptus globulus and Allium sativum essential oils against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Toxin Rev. 2019, 39, 422–433. [Google Scholar] [CrossRef]
- Kumrungsee, N.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar] [CrossRef]
- Cruz-Castillo, A.U.; Rodríguez-Valdez, L.M.; Correa-Basurto, J.; Nogueda-Torres, B.; Andrade-Ochoa, S.; Nevárez-Moorillón, G.V. Terpenic constituents of essential oils with larvicidal activity against Aedes Aegypti: A QSAR and docking molecular study. Molecules 2023, 28, 2454. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolina, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B.; et al. Essential oils for a sustainable control of honeybee varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef]
- EI Zayyat, E.A.; Soliman, M.I.; Elleboudy, N.A.; Ofaa, S.E. Musca domestica laboratory susceptibility to three ethnobotanicalculinary plants. Environ. Sci. Pollut. Res. 2015, 22, 15844–15852. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Hou, J.; Wu, Y.Y.; Guo, S.; Liu, Q.M.; Li, T.Q.; Gong, Z.Y. Resistance of house fly, Musca domestica L. (Diptera: Muscidae), to five insecticides in Zhejiang Province, China: The situation in 2017. Can. J. Infect. Dis. Med. Microbiol. 2019, 1, 4851914. [Google Scholar] [CrossRef]
- Akıner, M.M.; Cağlar, S.S. Monitoring of five different insecticide resistance status in Turkish house fly Musca domestica L. (Diptera: Muscidae) populations and the relationship between resistance and insecticide usage profile. Turk. J. Parasitol. 2012, 36, 87–91. [Google Scholar] [CrossRef]
- Shah, R.M.; Shad, S.A.; Abbas, N. Methoxyfenozide resistance of the housefly, Musca domestica L. (Diptera: Muscidae): Cross-resistance patterns, stability and associated fitness costs. Pest Manag. Sci. 2017, 73, 254–261. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, Y.; Ma, Z.; Gao, X. Inheritance of propoxur resistance in a near-isogenic line of Musca domestica (Diptera:Muscidae). J. Econ. Entomol. 2016, 109, 873–878. [Google Scholar] [CrossRef]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N.; et al. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Lorn, S.; Klakankhai, W.; Nusen, P.; Sumarnrote, A.; Tainchum, K. Pyrethroid Susceptibility in Stomoxyscalcitrans and Stomoxys indicus (Diptera: Muscidae) Collected from Cattle Farms in Southern Thailand. Insects 2022, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Oz, E.; Cetin, H. Synergistic effect of piperonyl butoxide on the toxicity of alpha-cypermethrin and deltamethrin against pyrethroid-resistant German cockroach Blattella germanica (Blattodea: Ectobiidae) strains in Turkey. Int. J. Trop. Insect Sci. 2022, 42, 3017–3022. [Google Scholar] [CrossRef]
- Prasad, T.P.; Shetty, N.J. Autosomal inheritance of alphamethrin, a synthetic pyrethroid, resistance in Anopheles stephensi-Liston, a malaria mosquito. Bull. Entomol. Res. 2013, 103, 547–554. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef]
- Abbas, N.; Ijaz, M.; Shad, S.A.; Binyameen, M. Assessment of resistance risk to fipronil and cross resistance to other insecticides in the Musca domestica L. (Diptera: Muscidae). Vet. Parasitol. 2016, 223, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.M.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J.C. Resistance affects the field performance of insecticides used for control of Choristoneura rosaceana in michigan apples and cherries. Insects 2021, 12, 846. [Google Scholar] [CrossRef]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Yuan, J. Status and preliminary mechanism of resistance to insecticides in a field strain of housefly (Musca domestica, L). Rev. Bras. Entomol. 2018, 62, 311–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, N.; Lun, X.; Zhao, C.; Liu, Q.; Meng, F. Long-term trends in housefly (Musca domestica L.) insecticide resistance in China. Pestic. Biochem. Physiol. 2024, 201, 105880. [Google Scholar] [CrossRef]
- Abbas, N.; Hafez, A.M. Alpha-cypermethrin resistance in Musca domestica: Resistance instability, realized heritability, risk assessment, and insecticide cross-resistance. Insects 2023, 14, 233. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Resistance risk assessment: Realized heritability of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae), tobacco budworm (Lepidoptera: Noctuidae), and colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1992, 85, 1551–1559. [Google Scholar] [CrossRef]
- Abbas, N.; Abubakar, M.; Hassan, M.W.; Shad, S.A.; Hafez, A.M. Risk assessment of flonicamid resistance in Musca domestica (Diptera: Muscidae): Resistance monitoring, inheritance, and cross-resistance potential. J. Med. Entomol. 2021, 58, 1779–1787. [Google Scholar] [CrossRef]
- Ijaz, M.; Shad, S.A. Realized heritability, cross-resistance and high risk of resistance development to spirotetramat in dusky cotton bug, Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae), an emerging threat to BT cotton in Pakistan. Phytoparasitica 2022, 50, 453–463. [Google Scholar] [CrossRef]
- Mansoor, M.M.; Abbas, N.; Shad, S.A.; Pathan, A.K.; Razaq, M. Increased fitness and realized heritability in emamectin benzoate-resistant Chrysoperla carnea (Neuroptera: Chrysopidae). Ecotoxicology 2013, 22, 1232–1240. [Google Scholar] [CrossRef]
- Abbas, N.; Abbas, N.; Ejaz, M.; Shad, S.A.; Asghar, I.; Irum, A.; Binyameen, M. Resistance in field populations of Amrasca devastans (Hemiptera: Cicadellidae) to new insecticides in Southern Punjab, Pakistan. Phytoparasitica 2018, 46, 533–539. [Google Scholar] [CrossRef]
- Khan, H.A.A. Side effects of insecticidal usage in rice farming system on the non-target house fly Musca domestica in Punjab, Pakistan. Chemosphere 2020, 241, 125056. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Jin, B.; Tang, L.; Yang, J.; Li, B.; Zhuang, G.; Bai, Z. Effect of cypermethrin insecticide on the microbial community in cucumber phyllosphere. J. Environ. Sci. 2008, 20, 1356–1362. [Google Scholar] [CrossRef]
- Hafez, A.M. Risk assessment of resistance to diflubenzuron in Musca domestica: Realized heritability and cross-resistance to fourteen insecticides from different classes. PLoS ONE 2022, 17, e0268261. [Google Scholar] [CrossRef]
- AlSalhi, S.M.; Elumalai, K.; Devanesan, S.; Govindarajan, M.; Krishnappa, K.; Maggi, F. The aromatic ginger Kaempferia galanga L. (Zingiberaceae) essential oil and its main compounds are effective larvicidal agents against Aedes vittatus and Anopheles maculatus without toxicity on the non-target aquatic fauna. Ind. Crops Prod. 2020, 158, 113012. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Larvicidal activity of the essential oil from Amomum subulatum Roxb. (Zingiberaceae) against Anopheles subpictus, AedesAlbopictus and Culex tritaeniorhynchus (Diptera: Culicidae), and non-target impact on four mosquito natural enemies. Physiol. Mol. Plant Pathol. 2018, 101, 219–224. [Google Scholar] [CrossRef]
- Nararak, J.; Sanguanpong, U.; Sukkanon, C.; Manguin, S.; Chareonviriyaphap, T. Synergistic repellent and irritant effects of a mixture of β-Caryophyllene oxide and vetiver oil against mosquito vectors. Insects 2023, 14, 773. [Google Scholar] [CrossRef] [PubMed]
- Rajeswary, M.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Zingiber cernuum (Zingiberaceae) essential oil as effective larvicide and oviposition deterrent on six mosquito vectors, with little non-target toxicity on four aquatic mosquito predators. Environ. Sci. Pollut. Res. 2018, 25, 10307–10316. [Google Scholar] [CrossRef] [PubMed]
- McArt, S.; Fersch, A.; Milano, N.; Truitt, L.L.; Böröczky, K. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 2017, 7, 46554. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Puwanard, C.; Aungtikun, J.; Sittichok, S.; Soonwera, M. Ovicidal toxicity of plant essential oils and their major constituents against two mosquito vectors and their non-target aquatic predators. Sci. Rep. 2023, 13, 2119. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Mujchariyakul, W.; Laksanavilat, N.; Junhirun, P. Acute toxicity of essential oil compounds (thymol and 1,8-cineole) to insectivorous guppy, Poecilia reticulata Peters, 1859. Agric. Nat. Resour. 2018, 52, 190–194. [Google Scholar] [CrossRef]
- Sittichok, S.; Passara, H.; Sinthusiri, J.; Moungthipmalai, T.; Puwanard, C.; Murata, K.; Soonwera, M. Synergistic larvicidal and pupicidal toxicity and the morphological impact of the dengue vector (Aedes aegypti) induced by geranial and trans-cinnamaldehyde. Insects 2024, 15, 714. [Google Scholar] [CrossRef]
- Caren, J.; Zhu, Y.-C.; Read, Q.D.; Du, Y. Risk assessment of effects of essential oils on honey Bees (Apis mellifera L.). Insects 2025, 16, 303. [Google Scholar] [CrossRef]
- Chibee, G.U.; Ojelabi, O.M.; Fajana, H.O.; Akinpelu, B.A.; Kehinde, T.O.; Awodiran, O.M.; Obuotor, E.M.; Owojori, O.J. Effects of cypermethrin as a model chemical on life cycle and biochemical responses of the tropical stingless bee Meliponula bocandei Spinola, 1853. Environ. Adv. 2021, 5, 100074. [Google Scholar] [CrossRef]
- Antwi, F.B.; Reddy, G.V.P. Toxicological effects of pyrethroids on non-target aquatic insects. Environ. Toxicol. Pharmacol. 2015, 40, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Lidova, J.; Buric, M.; Kouba, A.; Velisek, J. Acute toxicity of two pyrethroid insecticides for five non-indigenous crayfish species in Europe. Vet. Med. 2019, 64, 125–133. [Google Scholar] [CrossRef]
- Ranatunga, M.; Kellar, C.; Pettigrove, V. Toxicological impacts of synthetic pyrethroids on non-target aquatic organisms: A review. Environ. Adv. 2023, 12, 100388. [Google Scholar] [CrossRef]
- Tome, H.V.V.; Barbosa, W.F.; Correa, A.S.; Gontijo, L.M.; Martins, G.F.; Guedes, R.N.C. reduced-risk insecticides in neotropical stingless bee species: Impact on survival and activity. Ann. Appl. Biol. 2015, 167, 186–196. [Google Scholar] [CrossRef]
- Macieira, O.J.D.; Hebling-Beraldo, M.J.A. Laboratory toxicity of insecticides to workers of Trigona spinipes (F., 1793) (Hymenoptera, Apidae). J. Apic. Res. 1989, 28, 3–6. [Google Scholar] [CrossRef]
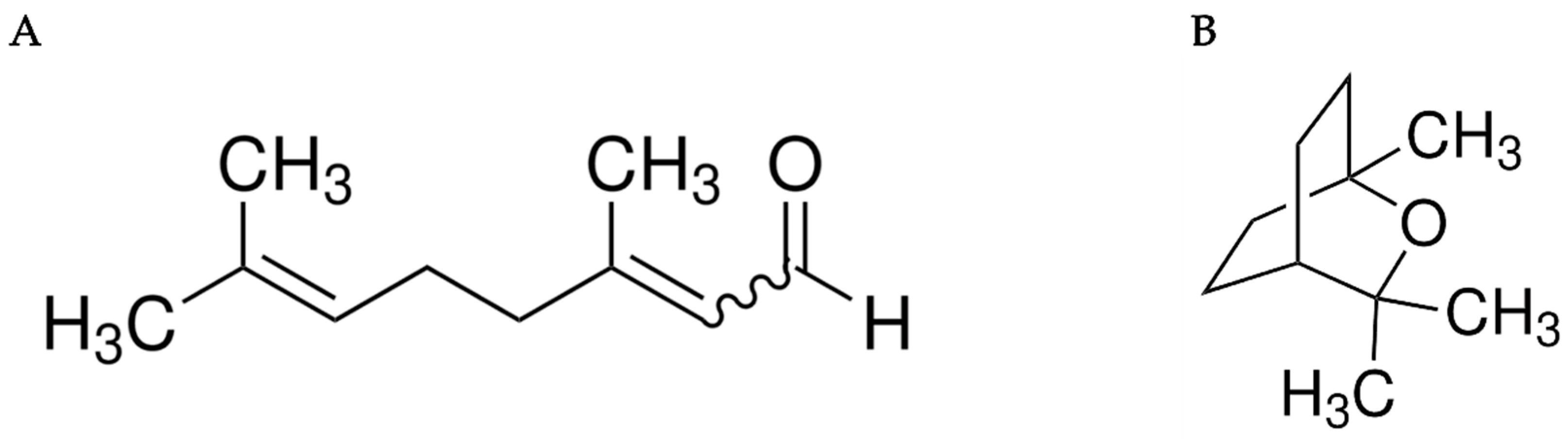




| EOs and Their Main Chemical Compound | Against Organisms | Action | Ref. |
|---|---|---|---|
| Geranial | M. domestica | Inhibited acetylcholinesterase activity | [24] |
| Trans-anethole | M. domestica | Induced cell membrane damage | [24] |
| Trans-cinnamaldehyde | M. domestica and Aede aegypti | Inhibited cytokinesis activity and excitatory to the neurons | [29,30] |
| Eucalyptol | M. domestica | Against the octopamine receptor and acetylcholine | [31] |
| α-pinene | M. domestica | Inhibited AChE activity | [7] |
| Illicium verum Hook. f. EO | M. domestica and Ae. Aegypti | Caused nervous toxicity | [24,32] |
| E. globulus EO | M. domestica | Inhibited AChE activity | [31] |
| C. citratus EO | M. domestica and Ae. Aegypti | Inhibited AChE activity and the toxicity of the olfactory | [25,32] |
| EO Constituents and Their Combinations | Formulas |
|---|---|
| Geranial | 1% geranial in 70% ethyl alcohol |
| 2.5% geranial in 70% ethyl alcohol | |
| Eucalyptol | 1% eucalyptol in 70% ethyl alcohol |
| 2.5% eucalyptol in 70% ethyl alcohol | |
| C. citratus EO | 1% C. citratus EO in 70% ethyl alcohol |
| 2.5% C. citratus EO in 70% ethyl alcohol | |
| E. globulus EO | 1% E. globulus EO in 70% ethyl alcohol |
| 2.5% E. globulus EO in 70% ethyl alcohol | |
| Geranial + eucalyptol | 1% geranial + 1% eucalyptol in 70% ethyl alcohol |
| 2.5% geranial + 2.5% eucalyptol in 70% ethyl alcohol | |
| Geranial + E. globulus EO | 1% geranial + 1% E. globulus EO in 70% ethyl alcohol |
| 2.5% geranial + 2.5% E. globulus EO in 70% ethyl alcohol | |
| Eucalyptol + C. citratus EO | 1% eucalyptol + 1% C. citratus EO in 70% ethyl alcohol |
| 2.5% eucalyptol + 2.5% C. citratus EO + in 70% ethyl alcohol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passara, H.; Sittichok, S.; Moungthipmalai, T.; Laosinwattana, C.; Murata, K.; Soonwera, M. Binary Mixtures of Essential Oils: Potent Housefly Adulticides That Are Safe Against Non-Target Species. Insects 2025, 16, 855. https://doi.org/10.3390/insects16080855
Passara H, Sittichok S, Moungthipmalai T, Laosinwattana C, Murata K, Soonwera M. Binary Mixtures of Essential Oils: Potent Housefly Adulticides That Are Safe Against Non-Target Species. Insects. 2025; 16(8):855. https://doi.org/10.3390/insects16080855
Chicago/Turabian StylePassara, Hataichanok, Sirawut Sittichok, Tanapoom Moungthipmalai, Chamroon Laosinwattana, Kouhei Murata, and Mayura Soonwera. 2025. "Binary Mixtures of Essential Oils: Potent Housefly Adulticides That Are Safe Against Non-Target Species" Insects 16, no. 8: 855. https://doi.org/10.3390/insects16080855
APA StylePassara, H., Sittichok, S., Moungthipmalai, T., Laosinwattana, C., Murata, K., & Soonwera, M. (2025). Binary Mixtures of Essential Oils: Potent Housefly Adulticides That Are Safe Against Non-Target Species. Insects, 16(8), 855. https://doi.org/10.3390/insects16080855






