The Heat Shock Response Under Natural Conditions in Two Paper Wasp Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
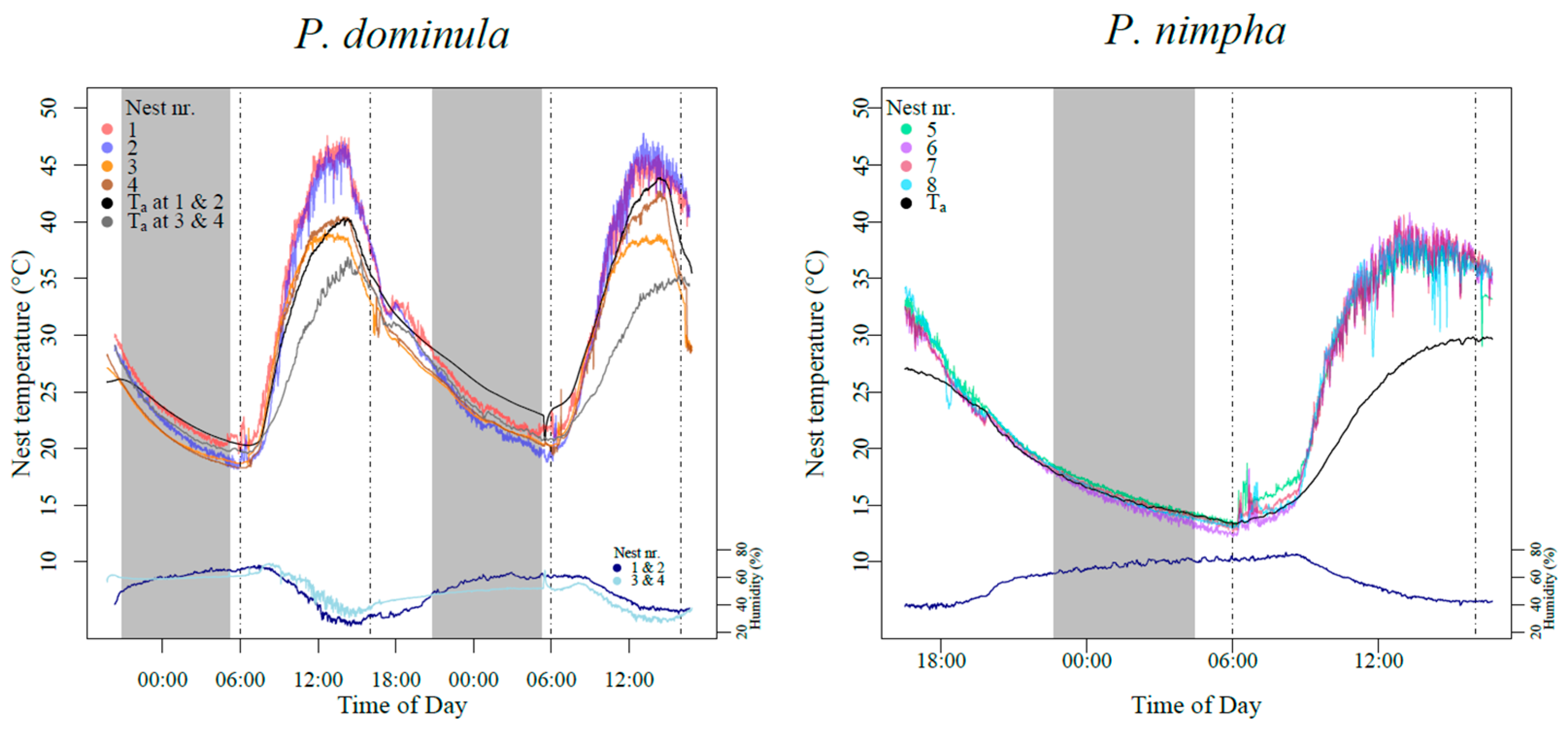
2.1. Sample Collection
2.2. qPCR Procedure
2.3. Data Analysis
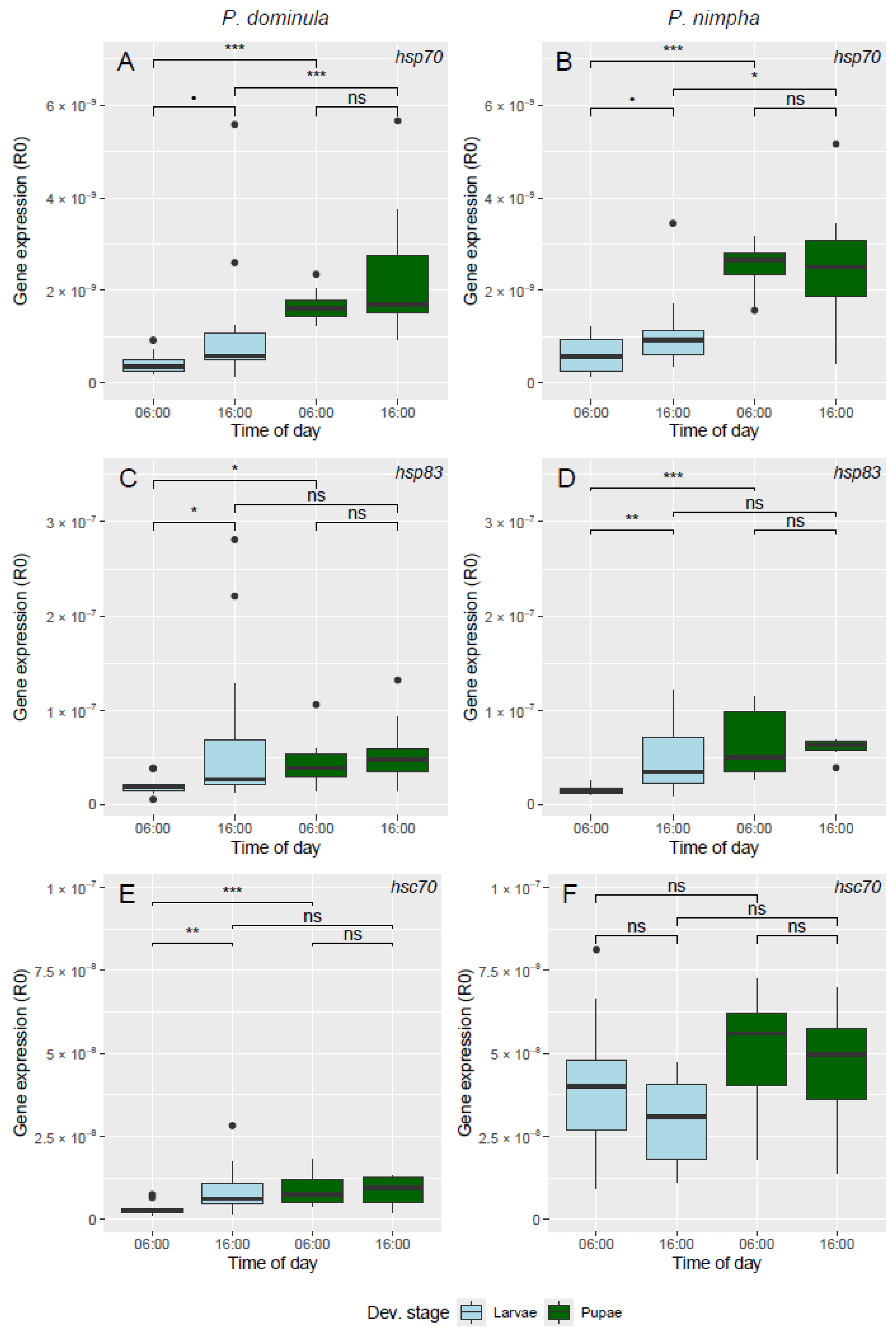
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSR | Heat shock response |
| Hsp | Heat shock protein |
| hsp | Gene encoding a heat shock protein |
References
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009; ISBN 9780191718748. [Google Scholar]
- Kellermann, V.; Overgaard, J.; Hoffmann, A.A.; Flojgaard, C.; Svenning, J.C.; Loeschcke, V. Upper Thermal Limits of Drosophila Are Linked to Species Distributions and Strongly Constrained Phylogenetically. Proc. Natl. Acad. Sci. USA 2012, 109, 16228–16233. [Google Scholar] [CrossRef] [PubMed]
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Thermal Tolerance, Climatic Variability and Latitude. Proc. R. Soc. B 2000, 267, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Blows, M.W. Species Borders: Ecological and evolutionary Perspectives. Trends Ecol. Evol. 1992, 9, 223–227. [Google Scholar] [CrossRef] [PubMed]
- IPCC; Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Pathak, P.; et al. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2022: Mitigation of Climate Change; IPCC: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Tett, S.F.B.; Stott, P.A.; Allen, M.R.; Ingram, W.J.; Mitchell, J.F.B. Causes of Twentieth-Century Temperature Change near the Earth’s Surface. Nature 1999, 399, 569–572. [Google Scholar] [CrossRef]
- Dewitt, T.J.; Scheiner, S.M. Phenotypic Plasticity; Oxford University Press: Oxford, UK, 2004; ISBN 0195138961. [Google Scholar]
- Levins, R. Thermal Acclimation and Heat Resistance in Drosophila Species. Am. Nat. 1969, 103, 483–499. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Nagy, J.M.; Kovac, H.; Käfer, H.; Petrocelli, I.; Turillazzi, S. Effect of Climate on Strategies of Nest and Body Temperature Regulation in Paper Wasps, Polistes biglumis and Polistes gallicus. Sci. Rep. 2022, 12, 3372. [Google Scholar] [CrossRef]
- Little, C.M.; Little, C.M.; Chapman, T.W.; Hillier, N.K. Plasticity Is Key to Success of Drosophila suzukii (Diptera: Drosophilidae) Invasion. J. Insect Sci. 2020, 20, 3. [Google Scholar] [CrossRef]
- Kovac, H.; Nagy, J.M.; Käfer, H.; Stabentheiner, A. Relationship between Nest and Body Temperature and Microclimate in the Paper Wasp Polistes dominula. Insects 2023, 14, 886. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Woods, A.H.; Buckley, L.B.; Potter, K.A.; MacLean, H.J.; Higgins, J.K. Complex Life Cycles and the Responses of Insects to Climate Change. Integr. Comp. Biol. 2011, 51, 719–732. [Google Scholar] [CrossRef]
- Chou, C.C.; Perez, D.M.; Johns, S.; Gardner, R.; Kerr, K.A.; Head, M.L.; McCullough, E.L.; Backwell, P.R.Y. Staying Cool: The Importance of Shade Availability for Tropical Ectotherms. Behav. Ecol. Sociobiol. 2019, 73, 106. [Google Scholar] [CrossRef]
- Klose, M.K.; Chu, D.; Xiao, C.; Seroude, L.; Robertson, R.M. Heat Shock-Mediated Thermoprotection of Larval Locomotion Compromised by Ubiquitous Overexpression of Hsp70 in Drosophila melanogaster. J. Neurophysiol. 2005, 94, 3563–3572. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The Evolutionary and Ecological Role of Heat Shock Proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Parsell, D.A.; Lindquist, S. The Function of Heat-Shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.B.; Bæk, I.; Loeschcke, V.; Sørensen, J.G. A Functional Study of the Role of Turandot Genes in Drosophila melanogaster: An Emerging Candidate Mechanism for Inducible Heat Tolerance. J. Insect Physiol. 2022, 143, 104456. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Li, Q.; Lu, Y. Heat Shock Protein 70 and Cathepsin B Genes Are Involved in the Thermal Tolerance of Aphis gossypii. Pest. Manag. Sci. 2023, 79, 2075–2086. [Google Scholar] [CrossRef]
- Niu, D.L.; Zhao, Y.E.; Gong, X.J.; Yang, R.; Hu, L.; Zhang, W.Y. Stress Response and Silencing Verification of Heat Shock Proteins in Dermatophagoides farinae under Temperature Stress. Int. J. Biol. Macromol. 2020, 144, 351–361. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Somero, G.N. Evidence for Protein Damage at Environmental Temperatures: Seasonal Changes in Levels of Ubiquitin Conjugates and Hsp70 in the Intertidal Mussel Mytilus trossulus. J. Exp. Biol. 1995, 198, 1509–1518. [Google Scholar] [CrossRef]
- Roberts, D.A.; Hofmann, G.E.; Somero, G.N. Heat-Shock Protein Expression in Mytilus californianus: Acclimatization (Seasonal and Tidal-Height Comparisons) and Acclimation Effects. Bulletin 1997, 192, 309–320. [Google Scholar] [CrossRef]
- Sørensen, J.G. Application of Heat Shock Protein Expression for Detecting Natural Adaptation and Exposure to Stress in Natural Populations. Curr. Zool. 2010, 56, 703–713. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Hoffmann, A.A.; Overgaard, J.; Sørensen, J.G.; Hallas, R.; Loeschcke, V. Costs and Benefits of Cold Acclimation in Field-Released Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 216–221. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Loeschcke, V.; Kristensen, T.N. Lessons from the Use of Genetically Modified Drosophila melanogaster in Ecological Studies: Hsf Mutant Lines Show Highly Trait-Specific Performance in Field and Laboratory Thermal Assays. Funct. Ecol. 2009, 23, 240–247. [Google Scholar] [CrossRef]
- McMillan, D.M.; Fearnley, S.L.; Rank, N.E.; Dahlhoff, E.P. Natural Temperature Variation Affects Larval Survival, Development and Hsp70 Expression in a Leaf Beetle. Funct. Ecol. 2005, 19, 844–852. [Google Scholar] [CrossRef]
- Miller, S.E.; Bluher, S.E.; Bell, E.; Cini, A.; Silva, R.C.d.; de Souza, A.R.; Gandia, K.M.; Jandt, J.; Loope, K.; Prato, A.; et al. WASPnest: A Worldwide Assessment of Social Polistine Nesting Behavior. Ecology 2018, 99, 2405. [Google Scholar] [CrossRef]
- Santos, B.F.; Payne, A.; Pickett, K.M.; Carpenter, J.M. Phylogeny and Historical Biogeography of the Paper Wasp Genus Polistes (Hymenoptera: Vespidae): Implications for the Overwintering Hypothesis of Social Evolution. Cladistics 2015, 31, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Egger, C.; van Achterberg, K.; Neumeyer, R.; Morinière, J.; Schmidt, S. Revision of the West Palaearctic Polistes latreille, with the Descriptions of Two Species—An Integrative Approach Using Morphology and DNA Barcodes (Hymenoptera, Vespidae). Zookeys 2017, 2017, 53–112. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.B.; Kovac, H.; Käfer, H.; Stabentheiner, A.; Sørensen, J.G. The Heat Shock Response in Polistes Spp. Brood from Differing Climates Following Heat Stress. J. Insect Physiol. 2024, 156, 104667. [Google Scholar] [CrossRef]
- Amstrup, A.B.; Kovac, H.; Käfer, H.; Stabentheiner, A.; Sørensen, J.G. The Effect of Heat Stress on Paper Wasps from Different Climates. J. Therm. Biol. 2025, accepted. [Google Scholar] [CrossRef]
- Höcherl, N.; Kennedy, S.; Tautz, J. Nest Thermoregulation of the Paper Wasp Polistes dominula. J. Therm. Biol. 2016, 60, 171–179. [Google Scholar] [CrossRef]
- Höcherl, N.; Tautz, J. Thermoregulation of Individual Paper Wasps (Polistes dominula) Plays an Important Role in Nest Defence and Dominance Battles. Sci. Nat. 2015, 102, 32. [Google Scholar] [CrossRef]
- Kovac, H.; Käfer, H.; Petrocelli, I.; Stabentheiner, A. Comparison of Thermal Traits of Polistes Dominula and Polistes Gallicus, Two European Paper Wasps with Strongly Differing Distribution Ranges. J. Comp. Physiol. B 2017, 187, 277–290. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, R.; Barrett, T.; Beck, J.; Benson, D.A.; Bollin, C.; Bolton, E.; Bourexis, D.; Brister, J.R.; Bryant, S.H.; Canese, K.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Peirson, S.N.; Butler, J.N.; Foster, R.G. Experimental Validation of Novel and Conventional Approaches to Quantitative Real-Time PCR Data Analysis. Nucleic Acids Res. 2003, 31, 14. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, L.-H.; Sørensen, P.B.; Krogh, P.H.; Sørensen, J.G. NORMA-Gene: A Simple and Robust Method for QPCR Normalization Based on Target Gene Data. BMC Bioinform. 2011, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.J.; Bates, D.M. Nonlinear Mixed Effects Models for Repeated Measures Data. Biometrics 1990, 46, 673–687. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.11.0. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 4 August 2025).
- Käfer, H.; Kovac, H.; Amstrup, A.B.; Sørensen, J.G.; Stabentheiner, A. Critical Thermal Maxima of Polistes Life Stages from Different Climates, with a Critical Evaluation of Methods. J. Therm. Biol. 2025, 129, 104111. [Google Scholar] [CrossRef]
- Benoit, J.B.; Lopez-Martinez, G.; Phillips, Z.P.; Patrick, K.R.; Denlinger, D.L. Heat Shock Proteins Contribute to Mosquito Dehydration Tolerance. J. Insect Physiol. 2010, 56, 151–156. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Rinehart, J.P.; Denlinger, D.L. Desiccation and Rehydration Elicit Distinct Heat Shock Protein Transcript Responses in Flesh Fly Pupae. J. Exp. Biol. 2004, 207, 963–971. [Google Scholar] [CrossRef]
- Nguyen, A.D.; DeNovellis, K.; Resendez, S.; Pustilnik, J.D.; Gotelli, N.J.; Parker, J.D.; Cahan, S.H. Effects of Desiccation and Starvation on Thermal Tolerance and the Heat-Shock Response in Forest Ants. J. Comp. Physiol. B 2017, 187, 1107–1116. [Google Scholar] [CrossRef]
- Tammariello, S.P.; Rinehart, J.P.; Denlinger, D.L. Desiccation Elicits Heat Shock Protein Transcription in the Flesh Fly, Sarcophaga crassipalpis, but Does Not Enhance Tolerance to High or Low Temperatures. J. Insect Physiol. 1999, 45, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, L.A.; Montooth, K.L. Inducing Extra Copies of the Hsp70 Gene in Drosophila melanogaster Increases Energetic Demand. BMC Evol. Biol. 2013, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.H.; Rossi, J.M.; Solomon, J.; Solomon, N.; Lindquist, S. The Consequences of Expressing Hsp70 in Drosophila Cells at Normal Temperatures. Genes. Dev. 1992, 6, 1402–1413. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Michalak, P.; Justesen, J.; Loeschcke, V. Expression of the Heat-Shock Protein HSP70 in Drosophila buzzatii Lines Selected for Thermal Resistance. Hereditas 1999, 131, 155–164. [Google Scholar] [CrossRef]
- Manenti, T.; Sørensen, J.G.; Moghadam, N.N.; Loeschcke, V. Predictability Rather than Amplitude of Temperature Fluctuations Determines Stress Resistance in a Natural Population of Drosophila simulans. J. Evol. Biol. 2014, 27, 2113–2122. [Google Scholar] [CrossRef]
- Overgaard, J.; Sørensen, J.G. Rapid Thermal Adaptation during Field Temperature Variations in Drosophila melanogaster. Cryobiology 2008, 56, 159–162. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Nyamukondiwa, C.; Kleynhans, E. Thermal Variability Alters Climatic Stress Resistance and Plastic Responses in a Globally Invasive Pest, the Mediterranean Fruit Fly (Ceratitis capitata). Entomol. Exp. Appl. 2010, 137, 304–315. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Alattal, Y.Z. Expression Levels of Heat-Shock Proteins in Apis mellifera jemenetica and Apis mellifera carnica Foragers in the Desert Climate of Saudi Arabia. Insects 2023, 14, 432. [Google Scholar] [CrossRef]
- Huang, L.H.; Kang, L. Cloning and Interspecific Altered Expression of Heat Shock Protein Genes in Two Leafminer Species in Response to Thermal Stress. Insect Mol. Biol. 2007, 16, 491–500. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, C.S.; Yan, Y.; Renault, D.; Colinet, H. The Interspecific Variations in Molecular Responses to Various Doses of Heat and Cold Stress: The Case of Cereal Aphids. J. Insect Physiol. 2023, 147, 104520. [Google Scholar] [CrossRef]
- Klockmann, M.; Günter, F.; Fischer, K. Heat Resistance throughout Ontogeny: Body Size Constrains Thermal Tolerance. Glob. Change Biol. 2017, 23, 686–696. [Google Scholar] [CrossRef]
- MacLean, H.J.; Overgaard, J.; Kristensen, T.N.; Lyster, C.; Hessner, L.; Olsvig, E.; Sørensen, J.G. Temperature Preference across Life Stages and Acclimation Temperatures Investigated in Four Species of Drosophila. J. Therm. Biol. 2019, 86, 102428. [Google Scholar] [CrossRef] [PubMed]
- Dahlgaard, J.; Loeschcke, V.; Michalak, P.; Justesen, J. Induced Thermotolerance and Associated Expression of the Heat-Shock Protein Hsp70 in Adult Drosophila melanogaster. Funct. Ecol. 1998, 12, 786–793. [Google Scholar] [CrossRef]
- Ekengren, S.; Hultmark, D. A Family of Turandot-Related Genes in the Humoral Stress Response of Drosophila. Biochem. Biophys. Res. Commun. 2001, 284, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Manenti, T.; Loeschcke, V.; Sørensen, J.G. Constitutive Up-Regulation of Turandot Genes Rather than Changes in Acclimation Ability Is Associated with the Evolutionary Adaptation to Temperature Fluctuations in Drosophila simulans. J. Insect Physiol. 2018, 104, 40–47. [Google Scholar] [CrossRef]
- Ekengren, S.; Tryselius, Y.; Dushay, M.S.; Liu, G.; Steiner, H.; Hultmark, D. A Humoral Stress Response in Drosophila. Curr. Biol. 2001, 11, 714–718. [Google Scholar] [CrossRef]
| Target Gene | Target NCBI Reference Sequence | Primer Sequence (5′→3′) |
|---|---|---|
| hsp70 | XM_015333810.1 | Fw: ACCCTTGCTGAAACCGAAGA Rv: TGCTCCGCCCTGATGAATTT |
| hsp83 | XM_015329297.1 | Fw: GCACAGGCACTTCGTGATAC Rv: GCTTCAGCTTTTTGGCGTAGA |
| hsc70 | XM_015321430.1 | Fw: AGCGGATGGTCAAACCCAAG Rv: CGGGAGGAATTCCGACCAAT |
| Variable | Gene | ||
|---|---|---|---|
| hsp70 | hsp83 | hsc70 | |
| ToD | F(1,66) = 4.45 p = 0.039 | F(1,66) = 10.21 p = 0.0021 | F(1,66) = 1.40 p = 0.24 |
| Species | F(1,6) = 0.61 p = 0.46 | F(1,6) = 0.00 p = 0.99 | F(1,6) = 49.55 p = 0.0004 |
| Dev.group | F(1,66) = 84.14 p = < 0.0001 | F(1,66) = 21.02 p < 0.0001 | F(1,66) = 16.69 p = 0.0001 |
| ToD × Species | F(1,66) = 0.37 p = 0.54 | F(1,66) = 0.002 p = 0.97 | F(1,66) = 5.65 p = 0.020 |
| ToD × dev.group | F(1,66) = 4.86 p = 0.031 | F(1,66) = 8.09 p = 0.0059 | F(1,66) = 5.00 p = 0.029 |
| Species × dev.group | F(1,66) = 0.00 p = 0.98 | F(1,66) = 1.82 p = 0.18 | F(1,66) = 0.47 p = 0.50 |
| ToD × species × dev.group | F(1,66) = 0.43 p = 0.51 | F(1,66) = 0.41 p = 0.52 | F(1,66) = 3.53 p = 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amstrup, A.B.; Kovac, H.; Käfer, H.; Stabentheiner, A.; Sørensen, J.G. The Heat Shock Response Under Natural Conditions in Two Paper Wasp Species. Insects 2025, 16, 849. https://doi.org/10.3390/insects16080849
Amstrup AB, Kovac H, Käfer H, Stabentheiner A, Sørensen JG. The Heat Shock Response Under Natural Conditions in Two Paper Wasp Species. Insects. 2025; 16(8):849. https://doi.org/10.3390/insects16080849
Chicago/Turabian StyleAmstrup, Astrid Bay, Helmut Kovac, Helmut Käfer, Anton Stabentheiner, and Jesper Givskov Sørensen. 2025. "The Heat Shock Response Under Natural Conditions in Two Paper Wasp Species" Insects 16, no. 8: 849. https://doi.org/10.3390/insects16080849
APA StyleAmstrup, A. B., Kovac, H., Käfer, H., Stabentheiner, A., & Sørensen, J. G. (2025). The Heat Shock Response Under Natural Conditions in Two Paper Wasp Species. Insects, 16(8), 849. https://doi.org/10.3390/insects16080849







