Chronic Larval Exposure to Lambda-Cyhalothrin Alters Gene Expression in Both Larval and Adult Honey Bees (Apis mellifera)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Larval Exposure to LCY
2.1.1. In Vitro Rearing of First-Instar Honey Bee Larvae
2.1.2. Chronic Sublethal Larval LCY Exposure, and Collection of Larval and Adult Honey Bee Samples
2.2. Transcriptome Analysis
2.2.1. cDNA Library Construction
2.2.2. DEG Filtering
2.2.3. DEG Enrichment Analysis
2.3. DEG Validation
3. Results
3.1. DEGs in Honey Bee Larvae and Adults Exposed to LCY
3.2. Distinct and Overlapping DEGs in LCY-Exposed Honey Bee Larvae and Adult Groups
3.3. GO Enrichment of Significant DEGs
3.3.1. LLG vs. SLG
3.3.2. LAG vs. SAG
3.3.3. LAG vs. LLG and SAG vs. SLG
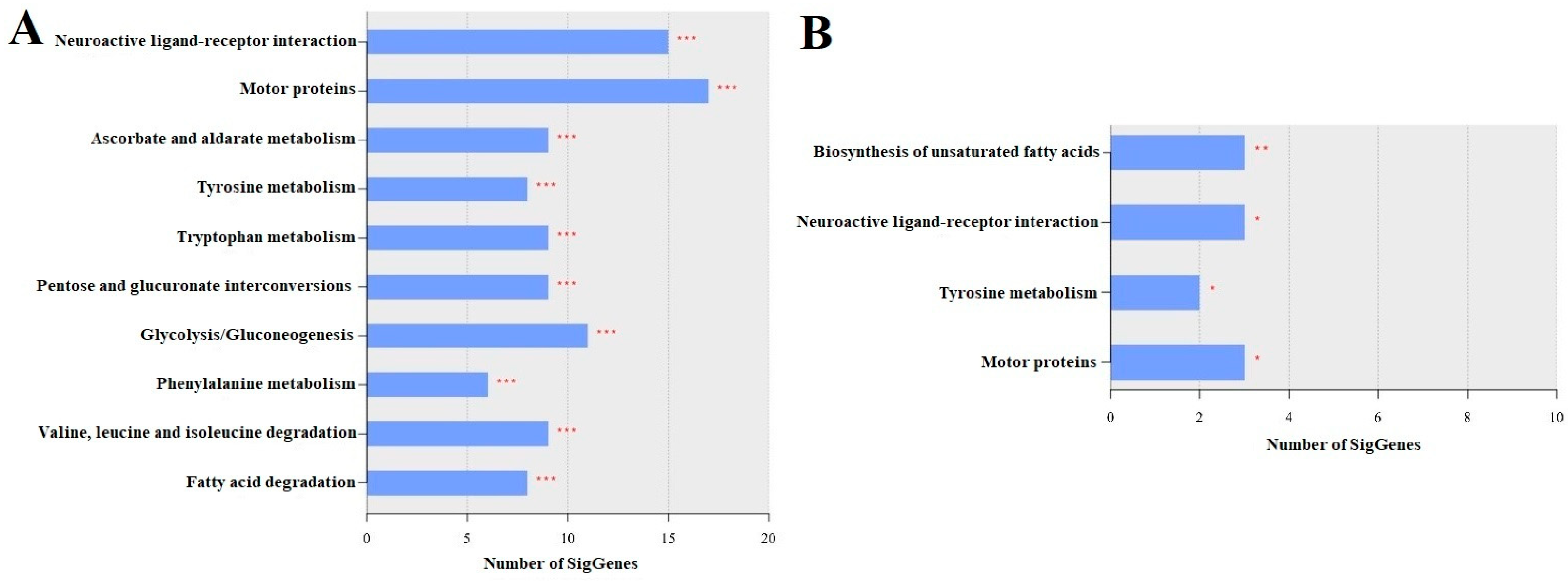
3.4. KEGG Enrichment Analysis
3.4.1. LLG vs. SLG
3.4.2. LAG vs. SAG
3.4.3. LAG vs. LLG and SAG vs. SLG
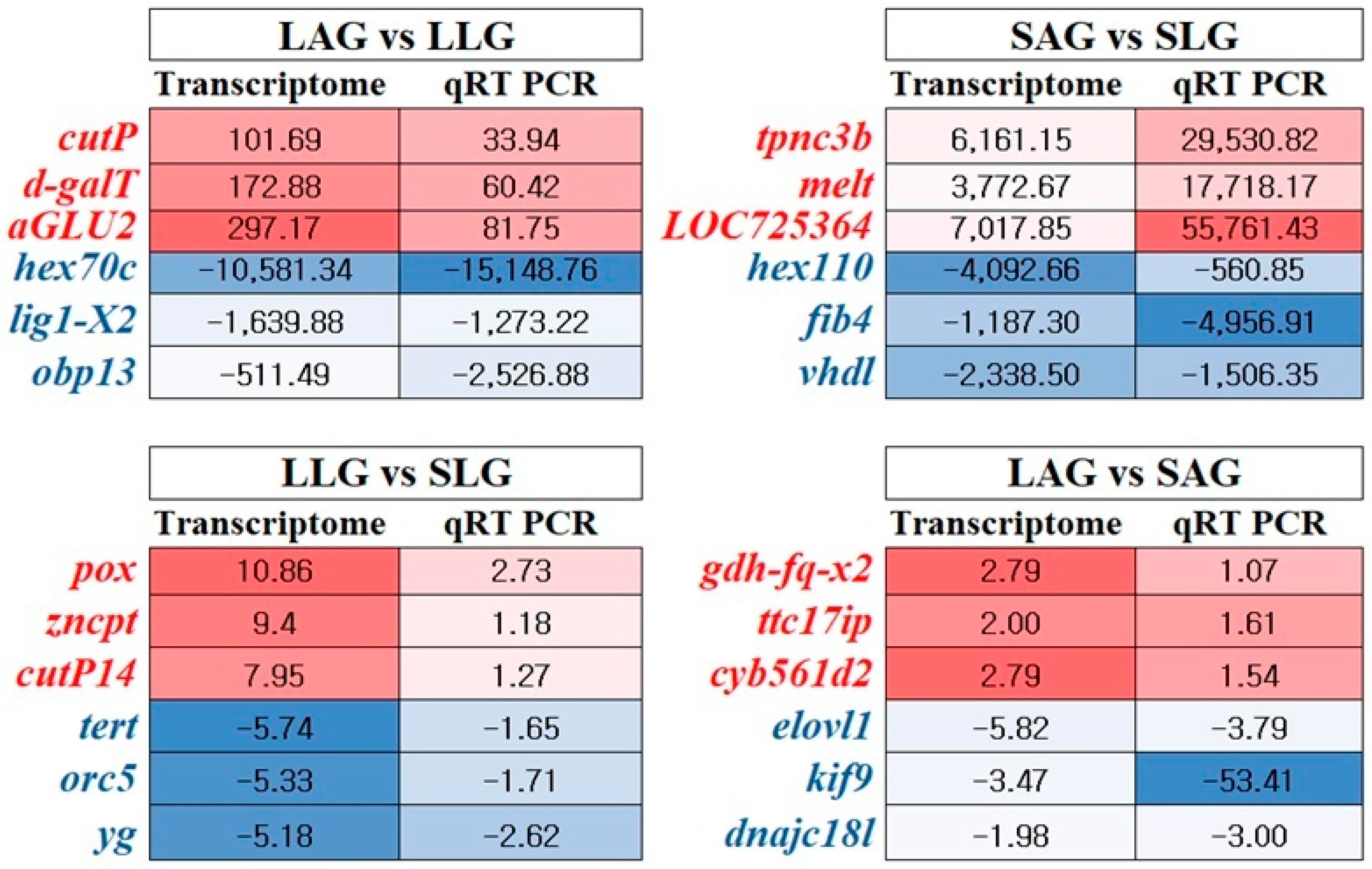
3.5. Transcriptome Data Validation
4. Discussion
4.1. Metabolic and Neurodevelopmental Impacts of LCY Exposure in Honey Bee Larvae
4.2. Persistent Neurobehavioral and Metabolic Dysfunctions in Adult Honey Bees Exposed to LCY
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| (NAD(P)H) | Nicotinamide Adenine Dinucleotide Phosphate |
| a.i. | Active Ingredient |
| acyl-CoA DeLCA | Acyl-Coenzyme A Dehydrogenase, Long Chain |
| akhr | Adipokine tic Hormone Receptor |
| ATP | Adenosine Triphosphate |
| BP | Biological Processes |
| CC | Cellular Components |
| cDNA | Complementary DNA |
| cpr | Cytochrome P450 Reductase |
| cyp | Cytochrome P450 |
| Cyp314a1 | Cytochrome P450 314a1 |
| cyp314A1 | Cytochrome P450 314A1 |
| cyp6a14 | Cytochrome P450 6a14 |
| cyp6k1 | Cytochrome P450 6k1 |
| cyp9e2 | Cytochrome P450 9e2 |
| DEGs | Differentially Expressed Genes |
| DNA | Deoxyribonucleic Acid |
| dop1 | Dopamine Receptor 1 |
| ELISA | Enzyme Linked Immunosorbent Assay |
| FAD | Flavin Adenine Dinucleotide |
| FC | Fold Change |
| FMN | Flavin Mononucleotide |
| FPKM | Fragments Per Kilobase of transcript per Million mapped reads |
| gapdh | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GC | Guanine and Cytosine |
| GO | Gene Ontology |
| HISAT | Hierarchical Indexing Spliced Alignment of Transcripts |
| hsp70 | Heat Shock Protein 70 |
| K5A | Kinesin Family Member 5A |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KIF9 | Kinesin Family Member 9 |
| KIN-7L | Kinesin-like protein KIN-7L |
| LAG | LCY-treated Adult Group |
| LCY | Lambda-cyhalothrin |
| LD50 | Median Lethal Dose |
| LLG | LCY-treated Larvae Group |
| LOC100578129 | Kinesin-like protein KIN-7I, transcript variant X2 |
| LOC100578936 | Methylenetetrahydrofolate reductase, transcript variant X1 |
| LOC408452 | Cytochrome P450 9e2 |
| LOC408559 | Retinal dehydrogenase 1 |
| LOC408650 | Inositol oxygenase |
| LOC410507 | Sodium-independent sulfate anion transporter, transcript variant X1 |
| LOC411140 | Putative aldehyde dehydrogenase family 7 member A1 homolog |
| LOC411672 | Neuropeptides capa receptor-like protein |
| LOC413816 | Sodium-independent sulfate anion transporter, transcript variant X2 |
| LOC551044 | Glucose dehydrogenase [FAD, quinone], transcript variant X2 |
| LOC551109 | Kinesin 5A, transcript variant X1 |
| LOC551179 | Methyl farnesoate epoxidase |
| LOC551465 | Homogentisate 1,2-dioxygenase |
| LOC551837 | Long-chain-fatty-acid–CoA ligase ACSBG2, transcript variant X1 |
| LOC727166 | Acyl-CoA Delta(11) desaturase, transcript variant X1 |
| map2 | Microtubule Associated Protein 2 |
| MDA | Malondialdehyde |
| MF | Molecular Functions |
| nAChR | Nicotinic Acetylcholine Receptor |
| nAChRa9 | Nicotinic Acetylcholine Receptor Alpha 9 |
| NCBI | National Center for Biotechnology Information |
| NLRP | Neuroactive Ligand–Receptor Interaction Pathway |
| nos | Nitric Oxide Synthase |
| NPC | Niemann-Pick C |
| Q20 | Quality Score of 20 |
| Q30 | Quality Score of 30 |
| qRT-PCR | Quantitative Real-time Polymerase Chain Reaction |
| RIN | RNA Integrity Number |
| Rpl13a | Ribosomal Protein L13a |
| rps5 | Ribosomal Protein S5 |
| SAG | Solvent-treated Adult Group |
| sifr | SIFamide Receptor |
| SLG | Solvent-treated Larvae Group |
| Sog | Short Gastrulation |
| TOX | Thymocyte Selection Associated High Mobility Group Box Protein |
| tpi | Triosephosphate Isomerase |
| TPM | Transcript Per Million |
| UDP | Uridine Diphosphate |
References
- Sillman, J.; Uusitalo, V.; Tapanen, T.; Salonen, A.; Soukka, R.; Kahiluoto, H. Contribution of honeybees towards the net environmental benefits of food. Sci. Total Environ. 2021, 756, 143880. [Google Scholar] [CrossRef]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.A.; Daisley, B.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Understanding the effects of sublethal pesticide exposure on honey bees: A role for probiotics as mediators of environmental stress. Front. Ecol. Evol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef]
- Wueppenhorst, K.; Eckert, J.H.; Steinert, M.; Erler, S. What about honey bee jelly? Pesticide residues in larval food jelly of the Western honey bee Apis mellifera. Sci. Total Environ. 2022, 850, 158095. [Google Scholar] [CrossRef]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef]
- Xiao, J.; He, Q.; Liu, Q.; Wang, Z.; Yin, F.; Chai, Y.; Yang, Q.; Jiang, X.; Liao, M.; Yu, L. Analysis of honey bee exposure to multiple pesticide residues in the hive environment. Sci. Total Environ. 2022, 805, 150292. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Chon, K.; Kim, J.; Vasamsetti, B.M.K.; Kim, B.-S.; Yoon, C.-Y.; Hwang, S.; Park, K.-H.; Lee, J.-H. Assessment of Lambda-Cyhalothrin and Spinetoram Toxicity and Their Effects on the Activities of Antioxidant Enzymes and Acetylcholinesterase in Honey Bee (Apis mellifera) Larvae. Insects 2024, 15, 587. [Google Scholar] [CrossRef]
- Kim, J.; Chon, K.; Kim, B.S.; Oh, J.A.; Yoon, C.Y.; Park, H.H. Assessment of acute and chronic toxicity of cyantraniliprole and sulfoxaflor on honey bee (Apis mellifera) larvae. Pest Manag. Sci. 2022, 78, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ke, L.; Li, A.-R.; Diao, Q.-Y.; Wang, Q.; Liu, Y.-J. Exposure of larvae to sublethal thiacloprid delays bee development and affects transcriptional responses of newly emerged honey bees. Front. Insect Sci. 2022, 2, 844957. [Google Scholar] [CrossRef]
- Waseem, M.A.; Thakur, M.; Singh, M.P.; Kasi, I.K. Evaluation of lambda cyhalothrin toxicity to Indian honeybees Apis Cerana in laboratory conditions. J. Entomol. Res. 2024, 48, 214–219. [Google Scholar] [CrossRef]
- Waseem, M.A.; Thakur, M.; Vallabuni, S.; Sharma, S.; Hashem, A.; Abd Allah, E.F. Toxicological Impact of Lambda-Cyhalothrin on Apis mellifera: Comparative Analysis Under Semi-Field and Field Conditions. J. Apic. Sci. 2024, 68, 119–131. [Google Scholar] [CrossRef]
- Furlan, L.; Kreutzweiser, D. Alternatives to neonicotinoid insecticides for pest control: Case studies in agriculture and forestry. Environ. Sci. Pollut. Res. 2015, 22, 135–147. [Google Scholar] [CrossRef]
- Grout, T.A.; Koenig, P.A.; Kapuvari, J.K.; McArt, S.H. Neonicotinoid insecticides in New York state: Economic benefits and risk to pollinators. Renew. Resour. J. 2021, 35, 18–22. [Google Scholar]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N.; Group, N.W. Alternatives to neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef]
- Liu, Q.; He, Q.; Zhang, S.; Chai, Y.; Gao, Q.; Xiao, J.; Fang, Q.; Yu, L.; Cao, H. Toxic effects of detected pyrethroid pesticides on honeybee (Apis mellifera ligustica Spin and Apis cerana cerana Fabricius). Sci. Rep. 2022, 12, 16695. [Google Scholar] [CrossRef]
- Drew, W.T.; Parmar A, H.K.; Lambda-Cyhalothrin, D.M. Human Health Risk Assessment for the Proposed Food/Feed Uses of the Insecticide on Cucurbit Vegetables (Group 9), Tuberous and Corm Vegetables (Subgroup 1C), Grass Forage, Fodder, and Hay (Group 17), Barley, Buckwheat, Oat, Rye, Wild Rice, and Pistachios; Petition Numbers 5F6994, 3E6593, and 6E7077EPA. Office of Prevention, Pesticides, and Toxic Substances Risk Assessment. 2007. Available online: https://www3.epa.gov/pesticides/endanger/litstatus/effects/redleg-frog/2012/lambda-cyha/appendix-j.pdf (accessed on 25 April 2024).
- Burr, S.A.; Ray, D.E. Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicol. Sci. 2004, 77, 341–346. [Google Scholar] [CrossRef]
- Naravaneni, R.; Jamil, K. Evaluation of cytogenetic effects of lambda-cyhalothrin on human lymphocytes. J. Biochem. Mol. Toxicol. 2005, 19, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Raza, I.; Muhammad, N.; Rahman, H.; ur Rehman, Z.; Azizullah, A.; Khattak, B.; Ullah, F.; Daud, M. Chlorpyrifos and lambda cyhalothrin-induced oxidative stress in human erythrocytes: In vitro studies. Toxicol. Ind. Health 2017, 33, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-S.; Jan, C.-R.; Liang, W.-Z. The investigation of the pyrethroid insecticide lambda-cyhalothrin (LCT)-affected Ca2+ homeostasis and-activated Ca2+-associated mitochondrial apoptotic pathway in normal human astrocytes: The evaluation of protective effects of BAPTA-AM (a selective Ca2+ chelator). Neurotoxicology 2018, 69, 97–107. [Google Scholar] [PubMed]
- Fetoui, H.; Garoui, E.M.; Zeghal, N. Lambda-cyhalothrin-induced biochemical and histopathological changes in the liver of rats: Ameliorative effect of ascorbic acid. Exp. Toxicol. Pathol. 2009, 61, 189–196. [Google Scholar] [CrossRef]
- Aouey, B.; Derbali, M.; Chtourou, Y.; Bouchard, M.; Khabir, A.; Fetoui, H. Pyrethroid insecticide lambda-cyhalothrin and its metabolites induce liver injury through the activation of oxidative stress and proinflammatory gene expression in rats following acute and subchronic exposure. Environ. Sci. Pollut. Res. 2017, 24, 5841–5856. [Google Scholar] [CrossRef]
- Fetoui, H.; Makni, M.; Garoui, E.M.; Zeghal, N. Toxic effects of lambda-cyhalothrin, a synthetic pyrethroid pesticide, on the rat kidney: Involvement of oxidative stress and protective role of ascorbic acid. Exp. Toxicol. Pathol. 2010, 62, 593–599. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Holden, L.; Sturgess, N.; Weiner, M.; Sheets, L.; Sargent, D.; Soderlund, D.M.; Choi, J.-S.; Symington, S.; Clark, J.M. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 2009, 30, S17–S31. [Google Scholar] [CrossRef]
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y.; Yu, L.; Cao, H. A survey of multiple pesticide residues in pollen and beebread collected in China. Sci. Total Environ. 2018, 640, 1578–1586. [Google Scholar] [CrossRef]
- Kaila, L.; Ketola, J.; Toivonen, M.; Loukola, O.; Hakala, K.; Raiskio, S.; Hurme, T.; Jalli, M. Pesticide residues in honeybee-collected pollen: Does the EU regulation protect honeybees from pesticides? Environ. Sci. Pollut. Res. 2022, 29, 18225–18244. [Google Scholar] [CrossRef]
- Waseem, M.A.; Sahoo, B.K.; Mohanty, P.; Thakur, M. Impact of Fipronil and Lambda-Cyhalothrin on Foraging Behaviour of Apis dorsata in Onion. Indian J. Entomol. 2024, 1–5. [Google Scholar] [CrossRef]
- Liao, C.-h.; He, X.-j.; Wang, Z.-l.; Barron, A.B.; Zhang, B.; Zeng, Z.-j.; Wu, X.-b. Short-term exposure to lambda-cyhalothrin negatively affects the survival and memory-related characteristics of worker bees Apis mellifera. Arch. Environ. Contam. Toxicol. 2018, 75, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Li, Z.; Zhang, Y.; Zhang, W.; Wang, C.; Zhang, D.-x.; Liu, F.; Gao, Z.; Xu, B.; Wang, N. The effect of lambda-cyhalothrin nanocapsules on the gut microbial communities and immune response of the bee elucidates the potential environmental impact of emerging nanopesticides. J. Hazard. Mater. 2024, 479, 135650. [Google Scholar] [CrossRef] [PubMed]
- Ingram, E.M.; Augustin, J.; Ellis, M.D.; Siegfried, B.D. Evaluating sub-lethal effects of orchard-applied pyrethroids using video-tracking software to quantify honey bee behaviors. Chemosphere 2015, 135, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razik, M.A.R.A.M. Toxicity and side effects of some insecticides applied in cotton fields on Apis mellifera. Environ. Sci. Pollut. Res. 2019, 26, 4987–4996. [Google Scholar] [CrossRef]
- PPDB: Pesticide Properties DataBase. Lambda-Cyhalothrin (Ref: OMS 3021). Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/415.htm (accessed on 20 March 2024).
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Yoon, C.-Y.; Kim, J.; Choi, J.-Y.; Hwang, S.; Park, K.-H. Transcriptome Profiling of Etridiazole-Exposed Zebrafish (Danio rerio) Embryos Reveals Pathways Associated with Cardiac and Ocular Toxicities. Int. J. Mol. Sci. 2023, 24, 15067. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Choi, J.-Y.; Kim, J.; Yoon, C.-Y. Transcriptome Analysis of Thiram-Treated Zebrafish (Danio rerio) Embryos Reveals Disruption of Reproduction Signaling Pathways. Biology 2023, 12, 156. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Kim, J.; Oh, J.-A.; Yoon, C.-Y.; Park, H.-H. Transcriptome-based identification of genes responding to the organophosphate pesticide phosmet in Danio rerio. Genes 2021, 12, 1738. [Google Scholar] [CrossRef]
- Park, M.Y.; Krishna Vasamsetti, B.M.; Kim, W.S.; Kang, H.J.; Kim, D.-Y.; Lim, B.; Cho, K.; Kim, J.S.; Chee, H.K.; Park, J.H. Comprehensive analysis of cardiac xeno-graft unveils rejection mechanisms. Int. J. Mol. Sci. 2021, 22, 751. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Kim, J.; Chon, K.; Kim, B.-S.; Yoon, C.-Y.; Hwang, S.; Park, K.-H. Molecular Impact of Sublethal Spinetoram Exposure on Honeybee (Apis mellifera) Larval and Adult Transcriptomes. Int. J. Mol. Sci. 2024, 25, 11923. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Guo, H.; Liu, X.; Wu, S.; Lv, L.; Tang, T. Uncovering hidden dangers: The combined toxicity of abamectin and lambda-cyhalothrin on honey bees. Sci. Total Environ. 2024, 933, 173126. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef]
- Nirupama, R.; Rajaraman, B.; Yajurvedi, H. Stress and glucose metabolism: A review. Imaging J. Clin. Med. Sci. 2018, 5, 008–012. [Google Scholar]
- Ghani, M.U.; Yang, Z.; Feng, T.; Chen, J.; Khosravi, Z.; Wu, Q.; Cui, H. Comprehensive review on glucose 6 phosphate dehydrogenase: A critical immunometabolic and redox switch in insects. Int. J. Biol. Macromol. 2024, 273, 132867. [Google Scholar] [CrossRef]
- Kunieda, T.; Fujiyuki, T.; Kucharski, R.; Foret, S.; Ament, S.; Toth, A.; Ohashi, K.; Takeuchi, H.; Kamikouchi, A.; Kage, E. Carbohydrate metabolism genes and pathways in insects: Insights from the honey bee genome. Insect Mol. Biol. 2006, 15, 563–576. [Google Scholar] [CrossRef]
- Torres, N.; Tobón-Cornejo, S.; Velazquez-Villegas, L.A.; Noriega, L.G.; Alemán-Escondrillas, G.; Tovar, A.R. Amino acid catabolism: An overlooked area of metabolism. Nutrients 2023, 15, 3378. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Pastore, A. The role of chaperones in iron–sulfur cluster biogenesis. FEBS Lett. 2018, 592, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Wedan, R.J.; Longenecker, J.Z.; Nowinski, S.M. Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism. Cell Metab. 2024, 36, 36–47. [Google Scholar] [CrossRef]
- Furse, S.; Koch, H.; Wright, G.A.; Stevenson, P.C. Sterol and lipid metabolism in bees. Metabolomics 2023, 19, 78. [Google Scholar] [CrossRef]
- Castaños, C.E.; Boyce, M.C.; Bates, T.; Millar, A.H.; Flematti, G.; Lawler, N.G.; Grassl, J. Lipidomic features of honey bee and colony health during limited supplementary feeding. Insect Mol. Biol. 2023, 32, 658–675. [Google Scholar] [CrossRef]
- du Rand, E.d.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.; Nicolson, S. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef]
- Cui, X.; Wang, C.; Wang, X.; Li, G.; Liu, Z.; Wang, H.; Guo, X.; Xu, B. Molecular Mechanism of the UDP-Glucuronosyltransferase 2B20-like Gene (AccUGT2B20-like) in Pesticide Resistance of Apis cerana cerana. Front. Genet. 2020, 11, 592595. [Google Scholar] [CrossRef]
- Yu, S.J. Detoxification Mechanisms in Insects. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1187–1201. [Google Scholar]
- Nazemi-Rafie, J.; Fatehi, F.; Hasrak, S. A comparative transcriptome analysis of the head of 1 and 9 days old worker honeybees (Apis mellifera). Bull. Entomol. Res. 2023, 113, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Choquet, K.; Lin, Z.Y.; Gingras, A.C.; Kleinman, C.L.; Shoubridge, E.A. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 2017, 18, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K. Nutrition and dopamine: An intake of tyrosine in royal jelly can affect the brain levels of dopamine in male honeybees (Apis mellifera L.). J. Insect Physiol. 2016, 87, 45–52. [Google Scholar] [CrossRef]
- Fan, W.; Li, G.; Zhang, X.; Wang, Y.; Wang, C.; Xu, B.; Guo, X.; Li, H. The role of melatonin and Tryptophan-5-hydroxylase-1 in different abiotic stressors in Apis cerana cerana. J. Insect Physiol. 2021, 128, 104180. [Google Scholar] [CrossRef]
- Harris, J.W.; Woodring, J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera L.) brain. J. Insect Physiol. 1992, 38, 29–35. [Google Scholar] [CrossRef]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Schroeder, A.; de Wit, J. Leucine-rich repeat-containing synaptic adhesion molecules as organizers of synaptic specificity and diversity. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Jones, A.K.; Raymond-Delpech, V.; Thany, S.H.; Gauthier, M.; Sattelle, D.B. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 2006, 16, 1422–1430. [Google Scholar] [CrossRef]
- Lu, W.; Liu, Z.; Fan, X.; Zhang, X.; Qiao, X.; Huang, J. Nicotinic acetylcholine receptor modulator insecticides act on diverse receptor subtypes with distinct subunit compositions. PLoS Genet. 2022, 18, e1009920. [Google Scholar] [CrossRef]
- Mustard, J.A.; Pham, P.M.; Smith, B.H. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J. Insect Physiol. 2010, 56, 422–430. [Google Scholar] [CrossRef]
- Gray, K.T.; Kostyukova, A.S.; Fath, T. Actin regulation by tropomodulin and tropomyosin in neuronal morphogenesis and function. Mol. Cell. Neurosci. 2017, 84, 48–57. [Google Scholar] [CrossRef]
- Kim, G.; Lujan, R.; Schwenk, J.; Kelley, M.H.; Aguado, C.; Watanabe, M.; Fakler, B.; Maylie, J.; Adelman, J.P. Membrane palmitoylated protein 2 is a synaptic scaffold protein required for synaptic SK2-containing channel function. eLife 2016, 5, e12637. [Google Scholar] [CrossRef]
- Martín, R.; Durroux, T.; Ciruela, F.; Torres, M.; Pin, J.-P.; Sánchez-Prieto, J. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J. Biol. Chem. 2010, 285, 17907–17917. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Fan, Y.; Wang, S.; Li, Z.; Deng, M.; Li, C.; Wang, J.; Ma, R.; Wang, X. Leucine-rich repeat neuronal protein-1 suppresses apoptosis of gastric cancer cells through regulation of Fas/FasL. Cancer Sci. 2019, 110, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, E.; Soustelle, L.; Parmentier, M.-L.; Verlinden, H.; Gherardi, M.-J.; Fourmy, D.; Mercer, A.R.; Devaud, J.-M.; Massou, I. Honey bee allatostatins target galanin/somatostatin-like receptors and modulate learning: A conserved function? PLoS ONE 2016, 11, e0146248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gou, X.; Qin, Z.; Li, D.; Wang, Y.; Ma, E.; Li, S.; Zhang, J. Identification and expression of cuticular protein genes based on Locusta migratoria transcriptome. Sci. Rep. 2017, 7, 45462. [Google Scholar] [CrossRef]
- Soares, M.P.; Elias-Neto, M.; Simões, Z.L.; Bitondi, M.M. A cuticle protein gene in the honeybee: Expression during development and in relation to the ecdysteroid titer. Insect Biochem. Mol. Biol. 2007, 37, 1272–1282. [Google Scholar] [CrossRef]
- Ramms, L.; Fabris, G.; Windoffer, R.; Schwarz, N.; Springer, R.; Zhou, C.; Lazar, J.; Stiefel, S.; Hersch, N.; Schnakenberg, U. Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 18513–18518. [Google Scholar] [CrossRef]
- Summers, J.A.; Yarbrough, M.; Liu, M.; McDonald, W.H.; Hudson, B.G.; Pastor-Pareja, J.C.; Boudko, S.P. Collagen IV of basement membranes: IV. Adaptive mechanism of collagen IV scaffold assembly in Drosophila. J. Biol. Chem. 2023, 299, 105394. [Google Scholar] [CrossRef]
- Huang, J.; Wang, T.; Qiu, Y.; Hassanyar, A.K.; Zhang, Z.; Sun, Q.; Ni, X.; Yu, K.; Guo, Y.; Yang, C. Differential brain expression patterns of microRNAs related to olfactory performance in honey bees (Apis mellifera). Genes 2023, 14, 1000. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Li, T.; Feng, X. G-protein coupled receptors (GPCRs): Signaling pathways, characterization, and functions in insect physiology and toxicology. Int. J. Mol. Sci. 2021, 22, 5260. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wan, K.; Lü, Y.; Ouyang, W.; Huang, J.; Zheng, L.; Miao, L.; Su, S.; Li, Z. Comparison of Brain Gene Expression Profiles Associated with Auto-Grooming Behavior between Apis cerana and Apis mellifera Infested by Varroa destructor. Genes 2024, 15, 763. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Habermehl, C.; Jiang, L. Metabolomic analysis of honey bee (Apis mellifera L.) response to glyphosate exposure. Mol. Omics 2022, 18, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Olgun, T.; Dayıoğlu, M.; Özsoy, N. Pesticide and pathogen induced oxidative stress in honey bees (Apis mellifera L.). Mellifera 2020, 20, 32–52. [Google Scholar]
- Zhang, V.; Kucharski, R.; Landers, C.; Richards, S.N.; Bröer, S.; Martin, R.E.; Maleszka, R. Characterization of a dopamine transporter and its splice variant reveals novel features of dopaminergic regulation in the honey bee. Front. Physiol. 2019, 10, 1375. [Google Scholar] [CrossRef]
- Wright, G. The role of dopamine and serotonin in conditioned food aversion learning in the honeybee. Commun. Integr. Biol. 2011, 4, 318–320. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Robinson, G.E.; Tobe, S.S.; Yagi, K.J.; Strambi, C.; Strambi, A.; Stay, B. Hormonal regulation of behavioural development in the honey bee is based on changes in the rate of juvenile hormone biosynthesis. J. Insect Physiol. 1991, 37, 733–741. [Google Scholar] [CrossRef]
- Robinson, G.E.; Strambi, C.; Strambi, A.; Huang, Z.-Y. Reproduction in worker honey bees is associated with low juvenile hormone titers and rates of biosynthesis. Gen. Comp. Endocrinol. 1992, 87, 471–480. [Google Scholar] [CrossRef]
- Friso, S.; Choi, S.-W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef]
- Cerone, M.; Smith, T.K. Desaturases: Structural and mechanistic insights into the biosynthesis of unsaturated fatty acids. Iubmb Life 2022, 74, 1036–1051. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Gao, R.; Liu, M.; Xie, W. A comprehensive review of the family of very-long-chain fatty acid elongases: Structure, function, and implications in physiology and pathology. Eur. J. Med. Res. 2023, 28, 532. [Google Scholar] [CrossRef]
- Fang, Y.; Feng, M.; Ma, C.; Rueppell, O.; Li, J. Major royal jelly proteins influence the neurobiological regulation of the division of labor among honey bee workers. Int. J. Biol. Macromol. 2023, 225, 848–860. [Google Scholar] [CrossRef]
- Anadon, A.; Martinez, M.; Martinez, M.; Diaz, M.; Martinez-Larranaga, M. Toxicokinetics of lambda-cyhalothrin in rats. Toxicol. Lett. 2006, 165, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Aziz, A.-T.; Saggu, S.; Abbas, Z.K.; Mohan, A.; Ansari, A.A. Systematic review on pyrethroid toxicity with special reference to deltamethrin. J. Entomol. Zool. Stud. 2014, 2, 60–70. [Google Scholar]
- Falcón, T.; Ferreira-Caliman, M.J.; Nunes, F.M.F.; Tanaka, É.D.; do Nascimento, F.S.; Bitondi, M.M.G. Exoskeleton formation in Apis mellifera: Cuticular hydrocarbons profiles and expression of desaturase and elongase genes during pupal and adult development. Insect Biochem. Mol. Biol. 2014, 50, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682–696. [Google Scholar] [CrossRef]
- Smykal, V.; Dolezel, D. Evolution of proteins involved in the final steps of juvenile hormone synthesis. J. Insect Physiol. 2023, 145, 104487. [Google Scholar] [CrossRef]
- Maestre, L.; García-García, J.F.; Jiménez, S.; Reyes-García, A.I.; García-González, Á.; Montes-Moreno, S.; Arribas, A.J.; González-García, P.; Caleiras, E.; Banham, A.H. High-mobility group box (TOX) antibody a useful tool for the identification of B and T cell subpopulations. PLoS ONE 2020, 15, e0229743. [Google Scholar] [CrossRef]
- Fouks, B.; Miller, K.J.; Ross, C.; Jones, C.; Rueppell, O. Alternative double strand break repair pathways shape the evolution of high recombination in the honey bee, Apis mellifera. Insect Mol. Biol. 2025, 34, 185–202. [Google Scholar] [CrossRef]



| Samples | Total Reads | Clean Reads | GC (%) | Q20 (%) | Q30 (%) | Mapped Reads | Unmapped Rate (%) | Mapped Rate (%) |
|---|---|---|---|---|---|---|---|---|
| LAG-1 | 58,406,928 | 56,777,182 | 38.40 | 98.48 | 97.65 | 55,333,933 | 2.54 | 97.46 |
| LAG-2 | 53,953,234 | 52,345,476 | 38.85 | 98.46 | 97.62 | 50,921,510 | 2.67 | 97.28 |
| LAG-3 | 54,812,774 | 53,237,224 | 38.06 | 98.49 | 97.64 | 51,798,939 | 2.70 | 97.30 |
| LLG-1 | 56,396,336 | 55,070,232 | 39.61 | 98.79 | 97.68 | 53,431,764 | 2.98 | 97.02 |
| LLG-2 | 50,705,676 | 49,305,624 | 39.42 | 98.55 | 97.73 | 47,920,635 | 2.81 | 97.19 |
| LLG-3 | 60,087,126 | 58,930,328 | 39.39 | 98.91 | 97.95 | 57,267,666 | 2.82 | 97.18 |
| SAG-1 | 62,326,854 | 60,905,516 | 37.71 | 98.75 | 97.73 | 59,163,527 | 2.86 | 97.14 |
| SAG-2 | 57,545,836 | 56,071,312 | 37.18 | 98.72 | 97.48 | 54,287,287 | 3.18 | 96.82 |
| SAG-3 | 55,289,460 | 54,121,612 | 37.86 | 98.83 | 97.78 | 52,478,090 | 3.04 | 96.96 |
| SLG-1 | 62,347,274 | 60,872,770 | 37.62 | 98.77 | 97.70 | 59,142,506 | 2.84 | 97.16 |
| SLG-2 | 50,206,390 | 48,908,502 | 38.75 | 98.53 | 97.73 | 47,621,910 | 2.63 | 97.37 |
| SLG-3 | 52,455,156 | 50,888,552 | 38.27 | 98.50 | 97.67 | 49,363,367 | 3.00 | 97.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasamsetti, B.M.K.; Chon, K.; Kim, J.; Choi, M.; Kim, B.-S.; Yoon, C.-Y.; Hwang, S.; Park, K.-H. Chronic Larval Exposure to Lambda-Cyhalothrin Alters Gene Expression in Both Larval and Adult Honey Bees (Apis mellifera). Insects 2025, 16, 833. https://doi.org/10.3390/insects16080833
Vasamsetti BMK, Chon K, Kim J, Choi M, Kim B-S, Yoon C-Y, Hwang S, Park K-H. Chronic Larval Exposure to Lambda-Cyhalothrin Alters Gene Expression in Both Larval and Adult Honey Bees (Apis mellifera). Insects. 2025; 16(8):833. https://doi.org/10.3390/insects16080833
Chicago/Turabian StyleVasamsetti, Bala Murali Krishna, Kyongmi Chon, Juyeong Kim, Minju Choi, Bo-Seon Kim, Chang-Young Yoon, Sojeong Hwang, and Kyeong-Hun Park. 2025. "Chronic Larval Exposure to Lambda-Cyhalothrin Alters Gene Expression in Both Larval and Adult Honey Bees (Apis mellifera)" Insects 16, no. 8: 833. https://doi.org/10.3390/insects16080833
APA StyleVasamsetti, B. M. K., Chon, K., Kim, J., Choi, M., Kim, B.-S., Yoon, C.-Y., Hwang, S., & Park, K.-H. (2025). Chronic Larval Exposure to Lambda-Cyhalothrin Alters Gene Expression in Both Larval and Adult Honey Bees (Apis mellifera). Insects, 16(8), 833. https://doi.org/10.3390/insects16080833








