Toxicity of Consecutive Treatments Combining Synthetic and Organic Miticides to Nurse Bees of Apis mellifera
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Samples
2.2. Determination of the Experimental Miticide Concentrations
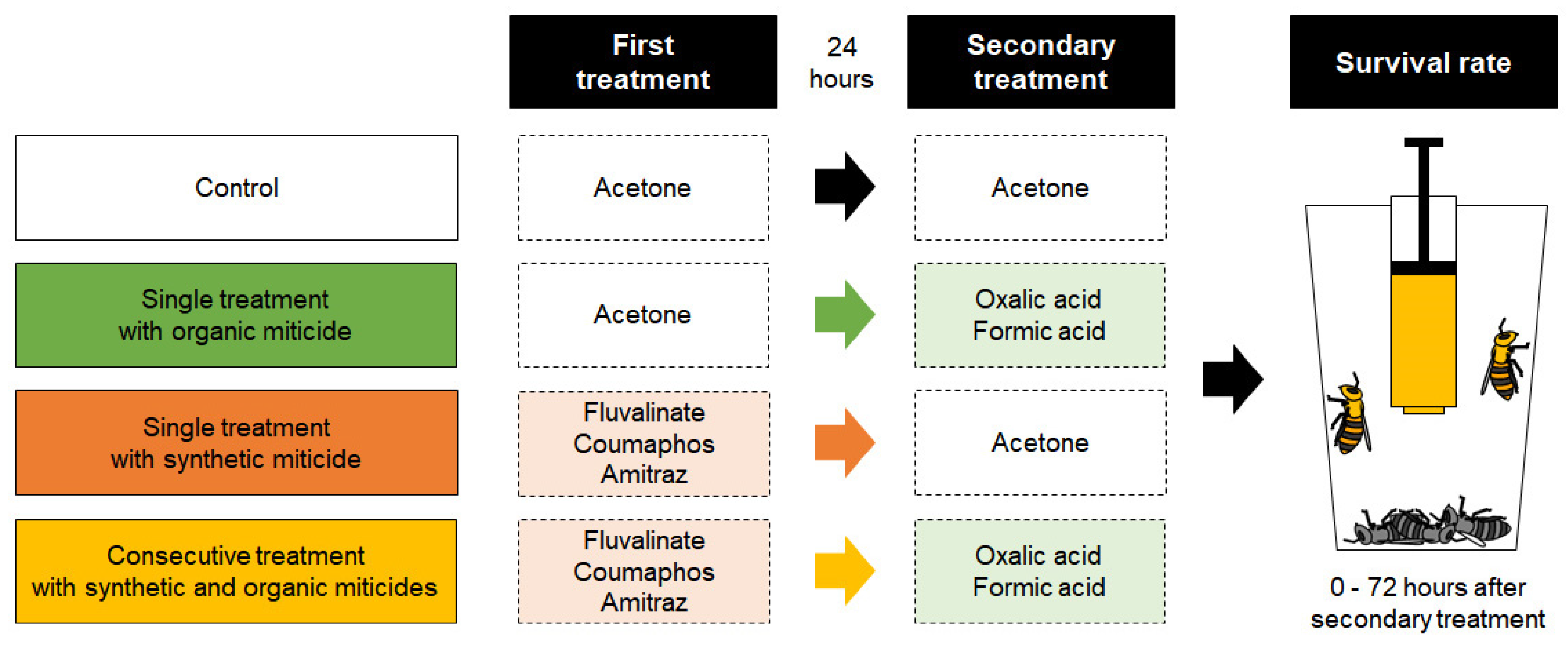
2.3. Bioassay
2.4. Data Analysis
3. Results
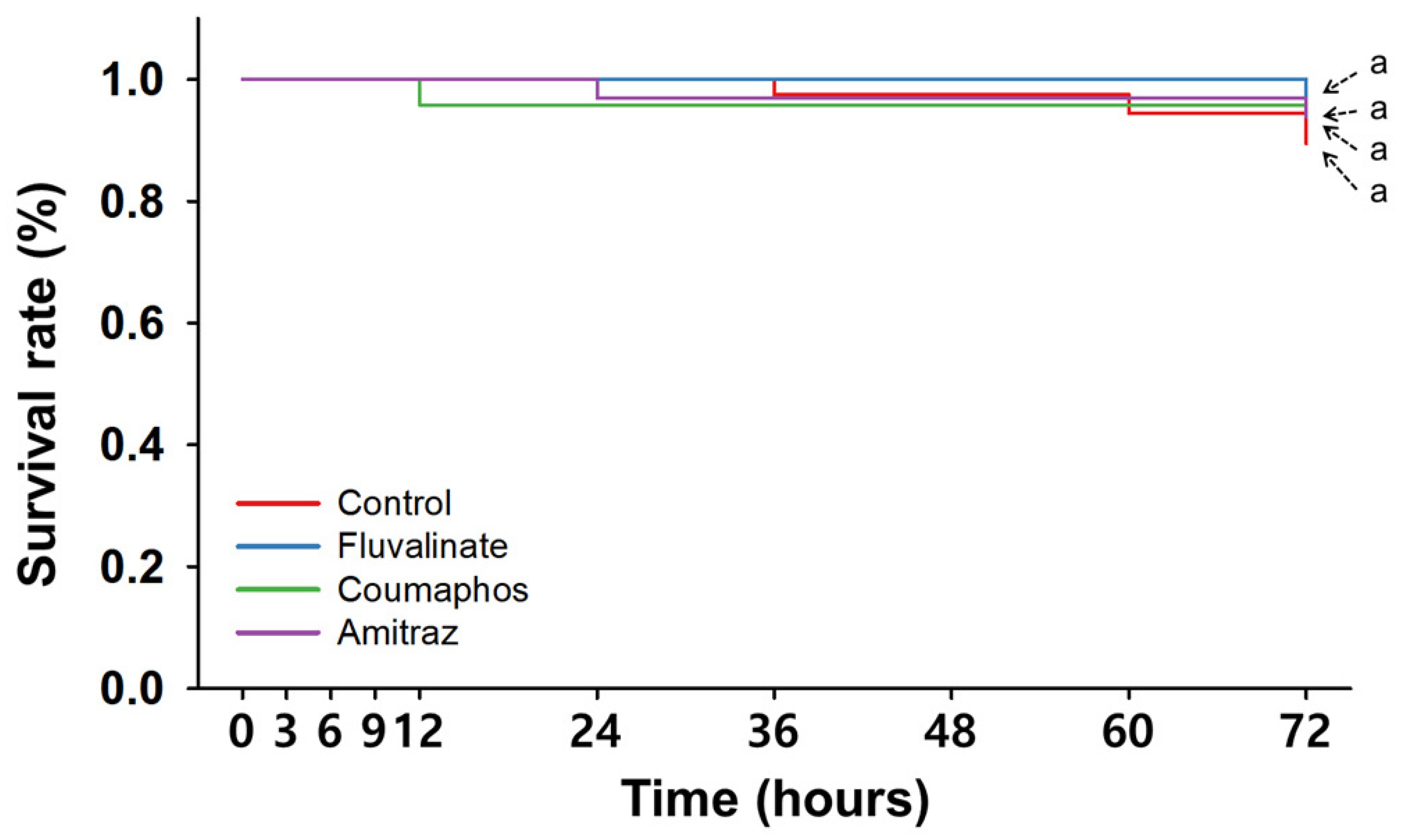
3.1. Single Treatments with Synthetic Miticides
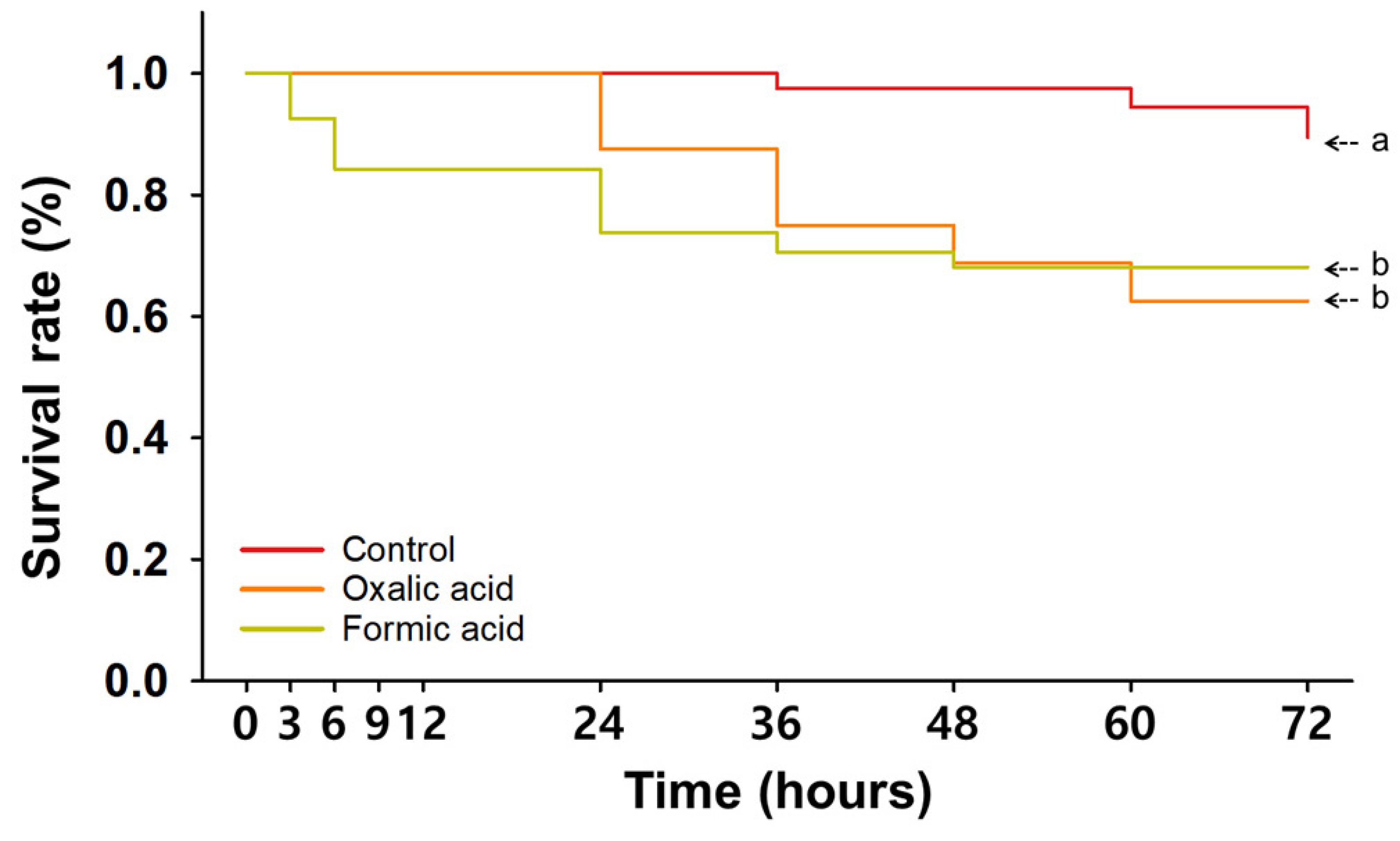
3.2. Single Treatments with Organic Miticides
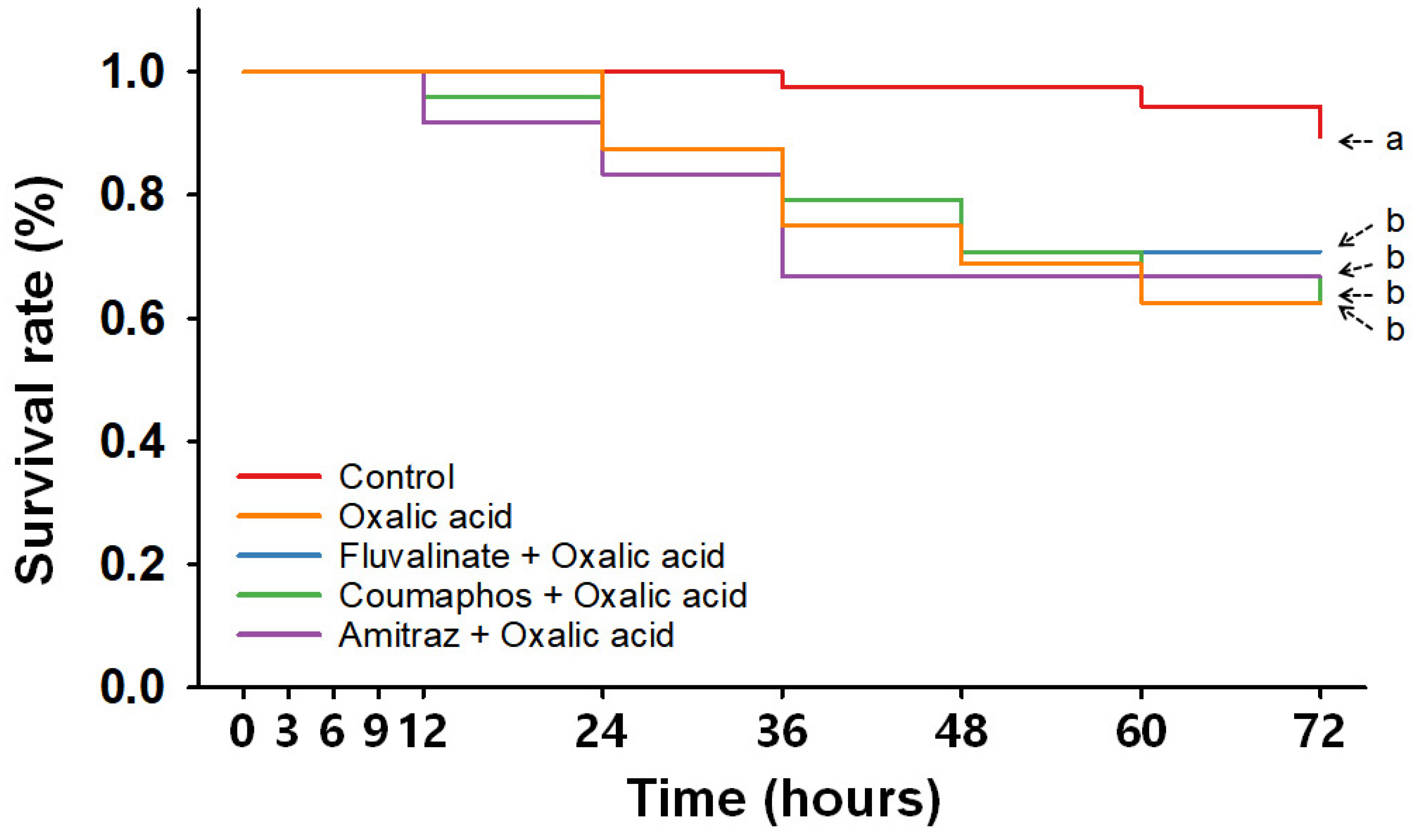
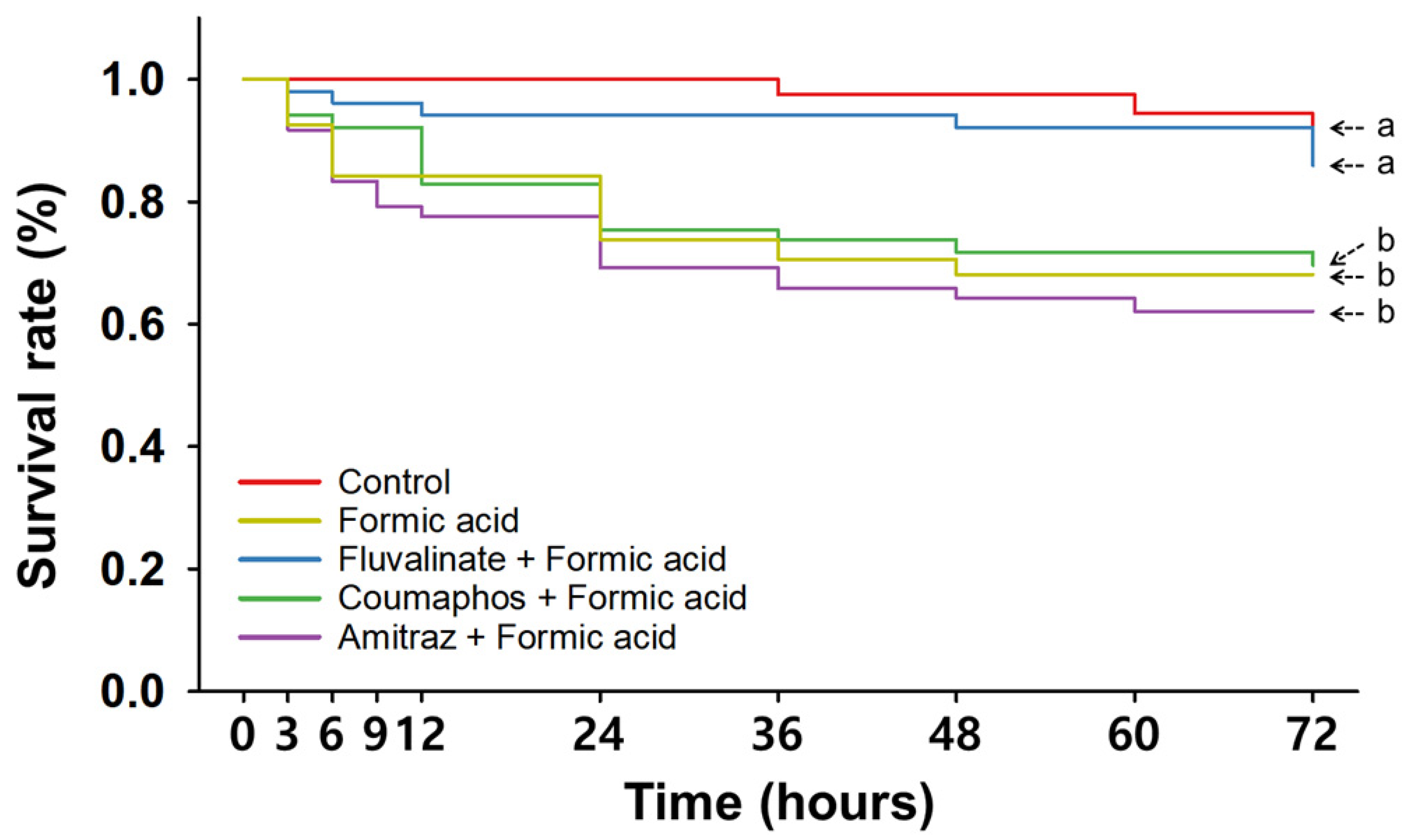
3.3. Consecutive Treatments with Synthetic and Organic Miticides
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruckner, S.; Wilson, M.; Aurell, D.; Rennich, K.; Vanengelsdorp, D.; Steinhauer, N.; Williams, G.R. A national survey of managed honey bee colony losses in the USA: Results from the Bee Informed Partnership for 2017–18, 2018–2019, and 2019–20. J. Apic. Res. 2023, 62, 429–443. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Bugeja Douglas, A.; Cadahía, L.; Charrière, J.D.; Chlebo, R.; Coffey, M.F. Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: The combined effects of operation size, migration and queen replacement. J. Apic. Res. 2023, 62, 204–210. [Google Scholar] [CrossRef]
- Kim, H.K. The Effect of Honey Bee Mites on the Winter Colony Losses. J. Apic. 2022, 37, 291–299. [Google Scholar]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.K. Current status of fluvalinate resistance in Varroa destructor in Korea and suggestion for possible solution. J. Apic. 2022, 37, 301–313. [Google Scholar]
- Pervez, M.; Manzoor, F.; Farooq, U. Differential expression of antimicrobial peptide encoding genes of honey bee (Apis mellifera) after Varroa mite (Varroa destructor) infestation. J. Apic. Res. 2024, 64, 1–8. [Google Scholar] [CrossRef]
- Rademacher, E.; Harz, M.; Schneider, S. Effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae). Insects 2017, 8, 84. [Google Scholar] [CrossRef]
- APHIS. Varroosis of Honey Bees; United States Department of Agriculture (USDA): Riverdale, MD, USA, 2023; pp. 1–2. [Google Scholar]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Sheppard, W.S. Honey bee exposure to pesticides: A four-year nationwide study. Insects 2019, 10, 13. [Google Scholar] [CrossRef]
- Perez-Cobo, I.; Fernández-Alba, A.R.; Hernando, M.D. First national survey of residues of active substances in honeybee apiaries across Spain between 2012 and 2016. Sci. Total Environ. 2022, 838, 155614. [Google Scholar] [CrossRef]
- Guo, L.; Fan, X.y.; Qiao, X.; Montell, C.; Huang, J. An octopamine receptor confers selective toxicity of amitraz on honeybees and Varroa mites. Elife 2021, 10, e68268. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc. Natl. Acad. Sci. USA 2011, 108, 12657–12662. [Google Scholar] [CrossRef] [PubMed]
- Liesivuori, J.; Savolainen, A.H. Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity–USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Locke, B.; Forsgren, E.; Fries, I.; de Miranda, J.R. Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 2012, 78, 227–235. [Google Scholar] [CrossRef]
- Frost, E.H.; Shutler, D.; Hillier, N.K. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exp. Biol. 2013, 216, 2931–2938. [Google Scholar] [CrossRef]
- Ilyasov, R.; Lim, S.; Lee, M.L.; Kwon, H.W.; Nikolenko, A. Effect of miticides amitraz and fluvalinate on reproduction and productivity of honey bee Apis mellifera. Uludag Bee J. 2021, 21, 21–30. [Google Scholar] [CrossRef]
- Sokół, R. Effects of long-term persistence of Fluwarol [fluvalinate] on honey bee colonies. Med. Weter 1996, 52, 718–720. [Google Scholar]
- Haarmann, T.; Spivak, M.; Weaver, D.; Weaver, B.; Glenn, T. Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. J. Econ. Entomol. 2002, 95, 28–35. [Google Scholar] [CrossRef]
- Bevk, D.; Kralj, J.; Čokl, A. Coumaphos affects food transfer between workers of honeybee Apis mellifera. Apidologie 2012, 43, 465–470. [Google Scholar] [CrossRef]
- Gregorc, A.; Bowen, I.D. Histochemical characterization of cell death in honeybee larvae midgut after treatment with Paenibacillus larvae, amitraz and oxytetracycline. Cell Biol. Int. 2000, 24, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Paleolog, J.; Olszewski, K.; Borsuk, G. Influence of amitraz and oxalic acid on the cuticle proteolytic system of Apis mellifera L. workers. Insects 2012, 3, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Bolli, H.; Bogdanov, S.; Imdorf, A.; Fluri, P. Action of formic acid on Varroa jacobsoni Oud and the honeybee (Apis mellifera L). Apidologie 1993, 24, 51–57. [Google Scholar] [CrossRef]
- Westcott, L.C.; Winston, M.L. Chemical acaricides in Apis mellifera (Hymenoptera: Apidae) colonies; do they cause nonlethal effects? Can. Entomol. 1999, 131, 363–371. [Google Scholar] [CrossRef]
- Gregorc, A.; Pogacnik, A.; Bowen, I.D. Cell death in honeybee (Apis mellifera) larvae treated with oxalic or formic acid. Apidologie 2004, 35, 453–460. [Google Scholar] [CrossRef]
- Underwood, R.M.; Currie, R.W. Effects of release pattern and room ventilation on survival of varroa mites and queens during indoor winter fumigation of honey bee colonies with formic acid. Can. Entomol. 2007, 139, 881–893. [Google Scholar] [CrossRef]
- Gregorc, A.; Škerl, M.I.S. Toxicological and immunohistochemical testing of honeybees after oxalic acid and rotenone treatments. Apidologie 2007, 38, 296–305. [Google Scholar] [CrossRef]
- Maggi, M.D.; Ruffinengo, S.R.; Damiani, N.; Sardella, N.H.; Eguaras, M.J. First detection of Varroa destructor resistance to coumaphos in Argentina. Exp. Appl. Acarol. 2009, 47, 317–320. [Google Scholar] [CrossRef]
- Maggi, M.D.; Ruffinengo, S.R.; Negri, P.; Eguaras, M.J. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol. Res. 2010, 107, 1189–1192. [Google Scholar] [CrossRef]
- Lee, J.; Moon, K.; Cho, S.; Lim, Y.; Kim, S.; Kim, S.-b.; Han, S.-M.; Kim, Y.H.; Lee, S.H. Establishment and application of bioassay-and molecular marker-based methods for monitoring fluvalinate resistance of Varroa mites. Pest. Biochem. Physiol. 2023, 197, 105655. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; VanEngelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed]
- Kast, C.; Kilchenmann, V.; Droz, B. Distribution of coumaphos in beeswax after treatment of honeybee colonies with CheckMite® against the parasitical mite Varroa destructor. Apidologie 2020, 51, 112–122. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Babnik, K.; Snoj, T.; Milčinski, L.; Pislak Ocepek, M.; Škof, M.; Jenčič, V.; Filazi, A.; Štajnbaher, D.; Kobal, S. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 2017, 48, 588–598. [Google Scholar] [CrossRef]
- Morales, M.M.; Ramos, M.J.G.; Vázquez, P.P.; Galiano, F.J.D.; Valverde, M.G.; López, V.G.; Flores, J.M.; Fernández-Alba, A.R. Distribution of chemical residues in the beehive compartments and their transfer to the honeybee brood. Sci. Total Environ. 2020, 710, 136288. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Díaz-Galiano, F.J.; Guitérrez-Tirado, I.; Flores, J.M.; Van der Steen, J.J.; Fernández-Alba, A.R. Dissipation and cross-contamination of miticides in apiculture. Evaluation by APIStrip-based sampling. Chemosphere 2021, 280, 130783. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Bernal, J.L.; del Nozal, M.J.; Martín, M.T. Residues of organic contaminants in beeswax. Eur. J. Lipid Sci. Techno. 2005, 107, 896–902. [Google Scholar] [CrossRef]
- Jeon, S. Relation Between the Honey Bee Mortality and the Pesticide Residue Detected During the Pear and Apple Blooming Season. Ph.D. Thesis, Andong National University, National University of Andong Graduate School, Andong-si, Republic of Korea, 2017. [Google Scholar]
- Shin, Y. Simultaneous Analysis of Pesticide Multiresidues in Human Serum, Urine, Apiculture Samples, and Representative Crops Using Tandem Mass Spectrometry. Ph.D. Thesis, Seoul National University, Seoul National University Graduate School, Seoul, Republic of Korea, 2018. [Google Scholar]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Aguilar, J.A.P.; Picó, Y. A two-year monitoring of pesticide hazard in-hive: High honey bee mortality rates during insecticide poisoning episodes in apiaries located near agricultural settings. Chemosphere 2019, 232, 471–480. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, C.; Kim, D.; Jung, C. Questionnaire study on the overwintering success and pest management of honeybee and damage assessment of Vespa hornets in Korea. Korean J. Apic. 2016, 31, 201–210. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of labor in honeybees: Form, function, and proximate mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef]
- Fakhimzadeh, K. Potential of super-fine ground, plain white sugar dusting as an ecological tool for the control of varroasis in the honey bee (Apis mellifera). Am. Bee J. 2000, 140, 487–491. [Google Scholar]
- Bogdanov, S.; Kilchenmann, V.; Imdorf, A. Acaricide residues in some bee products. J. Apic. Res. 1998, 37, 57–67. [Google Scholar] [CrossRef]
- Tremolada, P.; Bernardinelli, I.; Colombo, M.; Spreafico, M.; Vighi, M. Coumaphos distribution in the hive ecosystem: Case study for modeling applications. Ecotoxicology 2004, 13, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef]
- Oh, D.; Kim, K.W.; Kim, K.M.; Akongte, P.N.; Kim, J.K.; Lee, C.; Park, B.S.; Kim, S.B.; Jo, Y.Y.; Choi, Y.S.; et al. Evaluation of vaporization amount of formic acid in different conditions for controlling honeybee mites. J. Apic. 2023, 38, 11–20. [Google Scholar]
- Poquet, Y.; Bodin, L.; Tchamitchian, M.; Fusellier, M.; Giroud, B.; Lafay, F.; Buleté, A.; Tchamitchian, S.; Cousin, M.; Pelissier, M. A pragmatic approach to assess the exposure of the honey bee (Apis mellifera) when subjected to pesticide spray. PLoS ONE 2014, 9, e113728. [Google Scholar] [CrossRef]
- Mesa, M.G.; Weiland, L.K.; Maule, A.G. Progression and severity of gas bubble trauma in juvenile salmonids. Trans. Am. Fish. Soc. 2000, 129, 174–185. [Google Scholar] [CrossRef]
- Hillier, N.K.; Frost, E.H.; Shutler, D. Fate of dermally applied miticides fluvalinate and amitraz within honey bee (Hymenoptera: Apidae) bodies. J. Econ. Entomol. 2013, 106, 558–565. [Google Scholar] [CrossRef]
- Santiago, G.P.; Otero-Colina, G.; Sánchez, D.M.; Guzmán, M.E.R.; Vandame, R. Comparing effects of three acaricides on Varroa jacobsoni (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae) using two application techniques. Fla. Entomol. 2000, 83, 468–476. [Google Scholar] [CrossRef]
- Nielsen, S.A.; Brødsgaard, C.J.; Hansen, H. Effects on detoxification enzymes in different life stages of honey bees (Apis mellifera L., Hymenoptera: Apidae) treated with a synthetic pyrethroid (flumethrin). Altern. Lab. Anim. 2000, 28, 437–443. [Google Scholar] [CrossRef]
- Vannette, R.L.; Mohamed, A.; Johnson, B.R. Forager bees (Apis mellifera) highly express immune and detoxification genes in tissues associated with nectar processing. Sci. Rep. 2015, 5, 16224. [Google Scholar] [CrossRef]
- Kraig, R.P.; Wagner, R.J. Acid-induced changes of brain protein buffering. Brain Res. 1987, 410, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Mckee, M.J.; Knowles, C.O. Effects of pyrethroids on respiration in the twospotted spider mite (Acari: Tetranychidae). J. Econ. Entomol. 1984, 77, 1376–1380. [Google Scholar] [CrossRef]
- Kankaya, E. Susceptibility of fluvalinate and esfenvalerate on adult tarek Alburnus tarichi (Güldenstädt 1814). Croat. J. Fish. 2023, 81, 49–53. [Google Scholar] [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [PubMed]
- Corta, E.; Bakkali, A.; Berrueta, L.; Gallo, B.; Vicente, F. Kinetics and mechanism of amitraz hydrolysis in aqueous media by HPLC and GC-MS. Talanta 1999, 48, 189–199. [Google Scholar] [CrossRef]
| Chemical | Product (A.I. %) a | Estimated Exposure Concentration | References | |
|---|---|---|---|---|
| Synthetic miticide | Fluvalinate | Apistan (824 mg/strip) | 41.67 ppm b | [44,45,46] |
| Coumaphos | Coumaking (3.2%) | 626.67 ppm c | Product label registered at the APQA | |
| Couma-H (3.2%) | 626.67 ppm c | |||
| Amitraz | Soksal-gold Solution (12.5%) | 125 ppm c | ||
| Dr+ Bee Solution (12.5%) | 125 ppm c | |||
| Organic miticide | Formic acid | Handmade solution | 600,000 ppm b | [47] |
| Oxalic acid | Handmade solution | 35,000 ppm c | Product label registered at the EPA | |
| Time (hpt a) | Control | Single Treatment | Consecutive Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluvalinate | Coumaphos | Amitraz | Oxalic Acid | Formic Acid | Fluvalinate + Oxalic Acid | Coumaphos + Oxalic Acid | Amitraz + Oxalic Acid | Fluvalinate + Formic Acid | Coumaphos + Formic Acid | Amitraz + Formic Acid | ||
| 3 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 92.5 ± 5.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 97.9 ± 2.1 | 94.2 ± 4.2 | 91.7 ± 5.3 |
| 6 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 84.2 ± 7.3 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 96.1 ± 2.5 | 92.1 ± 4.1 | 83.3 ± 7.7 |
| 9 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 84.2 ± 7.3 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 96.1 ± 2.5 | 92.1 ± 4.1 | 79.2 ± 9.5 |
| 12 | 100 ± 0.0 | 100 ± 0.0 | 95.8 ± 4.2 | 100 ± 0.0 | 100 ± 0.0 | 84.2 ± 7.3 | 100 ± 0.0 | 95.8 ± 4.2 | 91.7 ± 4.2 | 94.2 ± 2.6 | 82.9 ± 6.5 | 77.5 ± 8.9 |
| 24 | 100 ± 0.0 | 100 ± 0.0 | 95.8 ± 4.2 | 96.9 ± 3.1 | 87.5 ± 12.5 | 73.8 ± 8.9 | 83.3 ± 8.3 | 87.5 ± 7.2 | 83.3 ± 8.3 | 94.2 ± 2.6 | 75.4 ± 5.3 ** | 69.2 ± 5.8 *** |
| 36 | 97.5 ± 2.5 | 100 ± 0.0 | 95.8 ± 4.2 | 96.9 ± 3.1 | 75 ± 12.5 | 70.6 ± 10.6 * | 75.0 ± 7.2 * | 79.2 ± 11.0 | 66.7 ± 4.2 *** | 94.2 ± 2.6 | 73.8 ± 6.4 * | 65.8 ± 6.6 ** |
| 48 | 97.5 ± 2.5 | 100 ± 0.0 | 95.8 ± 4.2 | 96.9 ± 3.1 | 68.8 ± 18.8 | 68.1 ± 8.4 * | 70.8 ± 11.0 * | 70.8 ± 4.2 *** | 66.7 ± 4.2 *** | 92.1 ± 2.5 | 71.7 ± 7.4 * | 64.2 ± 7.5 ** |
| 60 | 94.4 ± 3.3 | 100 ± 0.0 | 95.8 ± 4.2 | 96.9 ± 3.1 | 62.5 ± 12.5 * | 68.1 ± 8.4 * | 70.8 ± 11.0 | 66.7 ± 4.2 *** | 66.7 ± 4.2 *** | 92.1 ± 2.5 | 71.7 ± 7.4 * | 62.1 ± 7.9 * |
| 72 | 89.4 ± 4.1 | 96.9 ± 3.1 | 95.8 ± 4.2 | 93.8 ± 3.6 | 62.5 ± 12.5 | 68.1 ± 8.4 | 70.8 ± 11.0 | 62.5 ± 7.2 * | 66.7 ± 4.2 * | 85.9 ± 3.9 | 69.6 ± 7.5 | 62.1 ± 7.9 * |
| Treatment | Estimated Lethal Times (h) | |||
|---|---|---|---|---|
| LT5 | LT10 | LT30 | ||
| Single treatment | Fluvalinate | >72 * | >72 * | >72 * |
| Coumaphos | >72 * | >72 * | >72 * | |
| Amitraz | >72 * | >72 * | >72 * | |
| Oxalic acid | 16.04 | 22.46 | 44.09 | |
| Formic acid | 1.49 | 4.92 | 29.41 | |
| Consecutive treatment | Fluvalinate + Oxalic acid | 12.62 | 18.67 | 38.99 |
| Coumaphos + Oxalic acid | 13.18 | 19.98 | 42.74 | |
| Amitraz + Oxalic acid | 9.93 | 15.77 | 43.11 | |
| Fluvalinate + Formic acid | 15.71 | 70.54 | >72 * | |
| Coumaphos + Formic acid | 3.45 | 7.35 | 56.50 | |
| Amitraz + Formic acid | 1.15 | 3.74 | 24.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; You, E.; Cha, J.; Lee, S.H.; Kim, Y.H. Toxicity of Consecutive Treatments Combining Synthetic and Organic Miticides to Nurse Bees of Apis mellifera. Insects 2025, 16, 657. https://doi.org/10.3390/insects16070657
Kim H, You E, Cha J, Lee SH, Kim YH. Toxicity of Consecutive Treatments Combining Synthetic and Organic Miticides to Nurse Bees of Apis mellifera. Insects. 2025; 16(7):657. https://doi.org/10.3390/insects16070657
Chicago/Turabian StyleKim, HeeJin, Euijin You, JooHeon Cha, Si Hyeock Lee, and Young Ho Kim. 2025. "Toxicity of Consecutive Treatments Combining Synthetic and Organic Miticides to Nurse Bees of Apis mellifera" Insects 16, no. 7: 657. https://doi.org/10.3390/insects16070657
APA StyleKim, H., You, E., Cha, J., Lee, S. H., & Kim, Y. H. (2025). Toxicity of Consecutive Treatments Combining Synthetic and Organic Miticides to Nurse Bees of Apis mellifera. Insects, 16(7), 657. https://doi.org/10.3390/insects16070657







