Nesting and Foraging Preferences of Four Sympatric Species of Cavity-Nesting Leafcutting Bees (Hymenoptera: Megachilidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
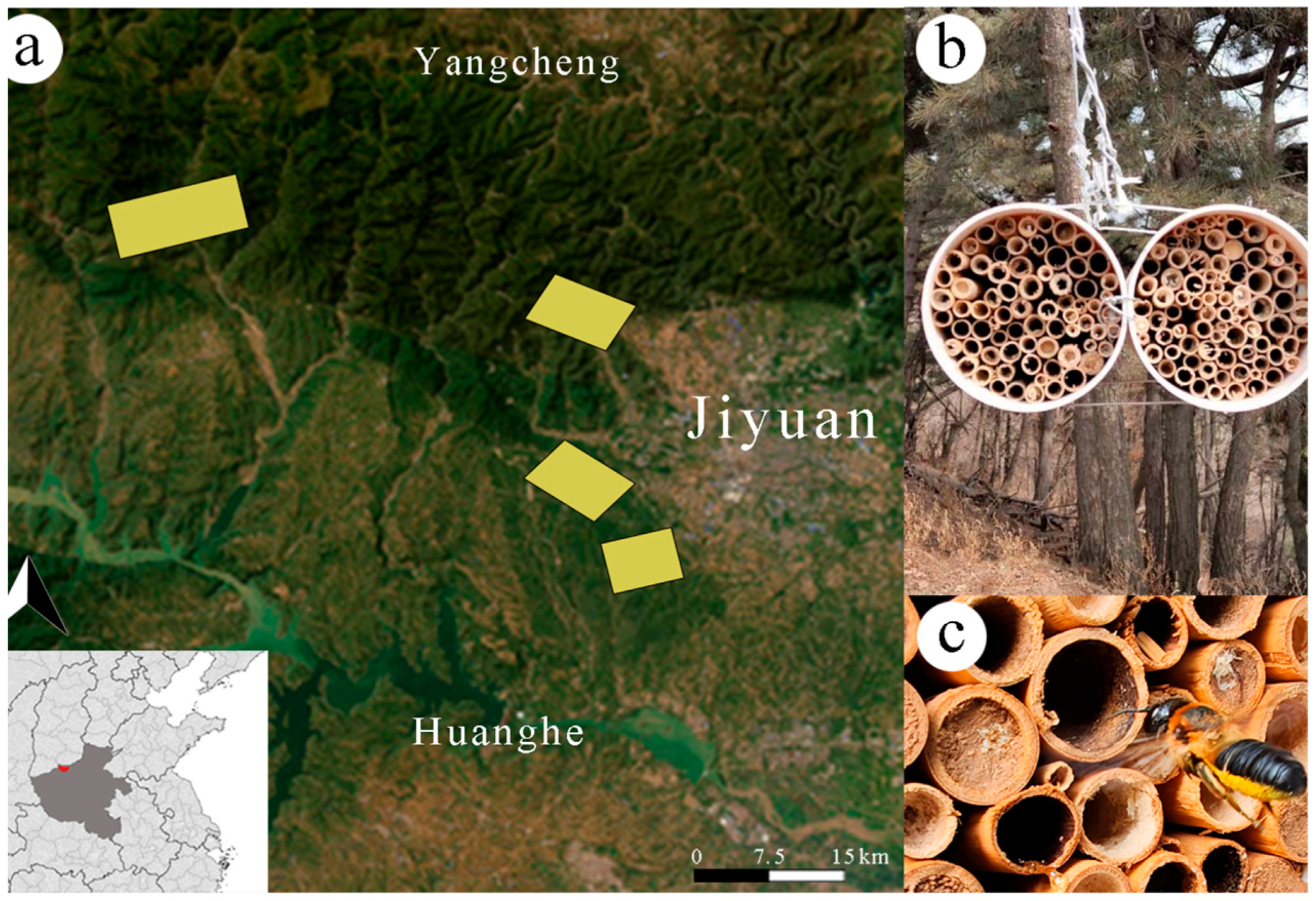
2.1. Study Area
2.2. Bee Capture
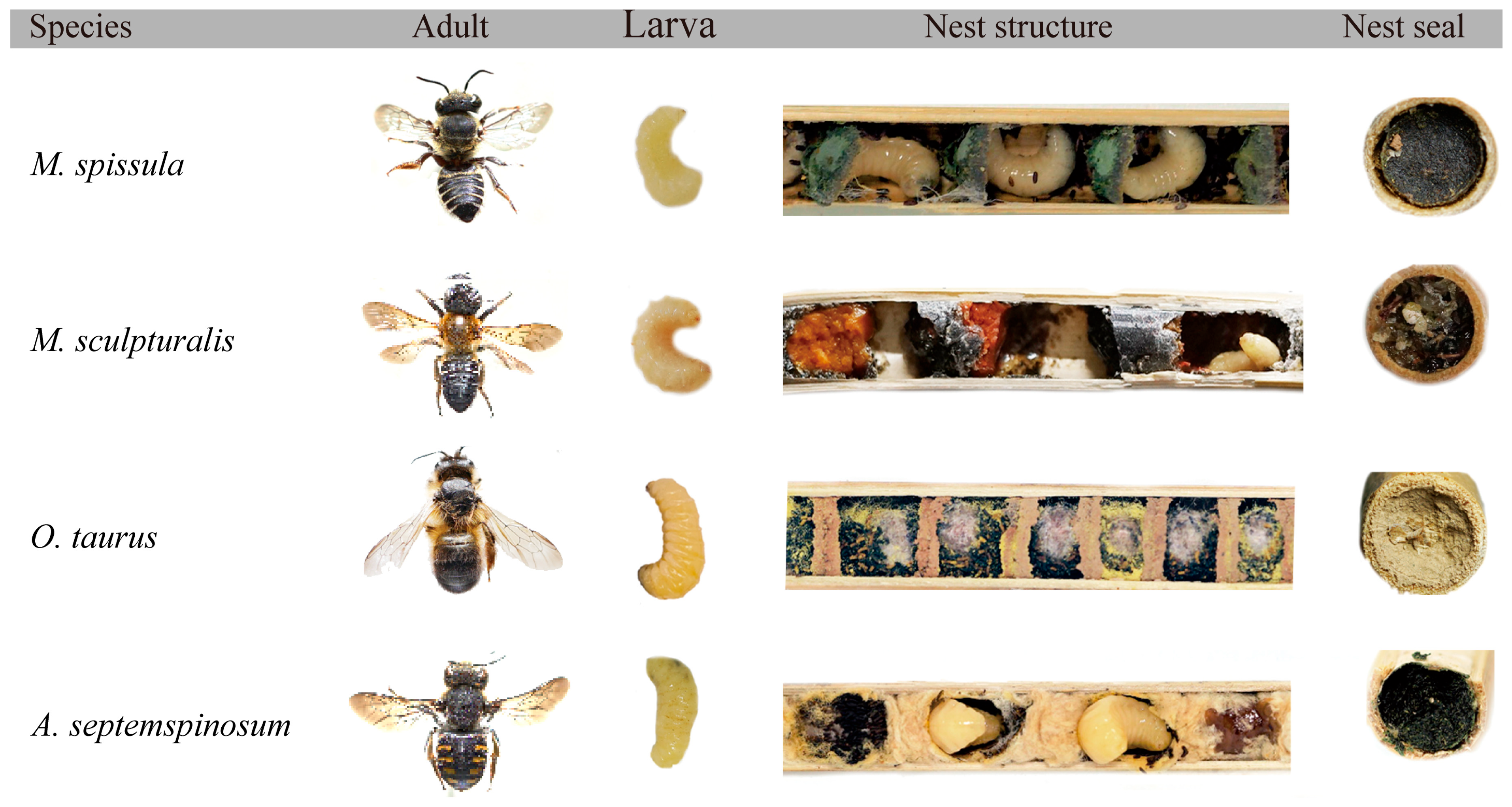
2.3. Nest Structure
2.4. Measurement of Morphological Characteristics
2.5. Identification of the Pollen Spectrum Within Bee Nests
2.6. Data Analysis
3. Results
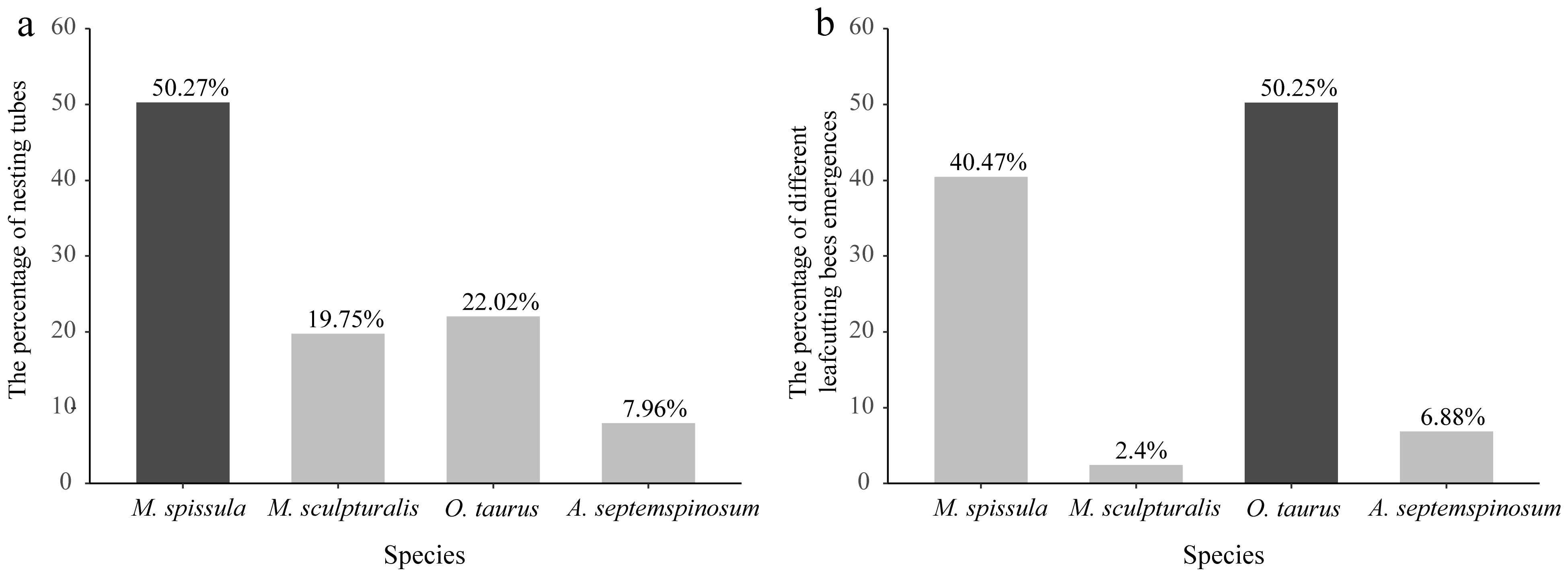
3.1. The Number of Leafcutting Bees
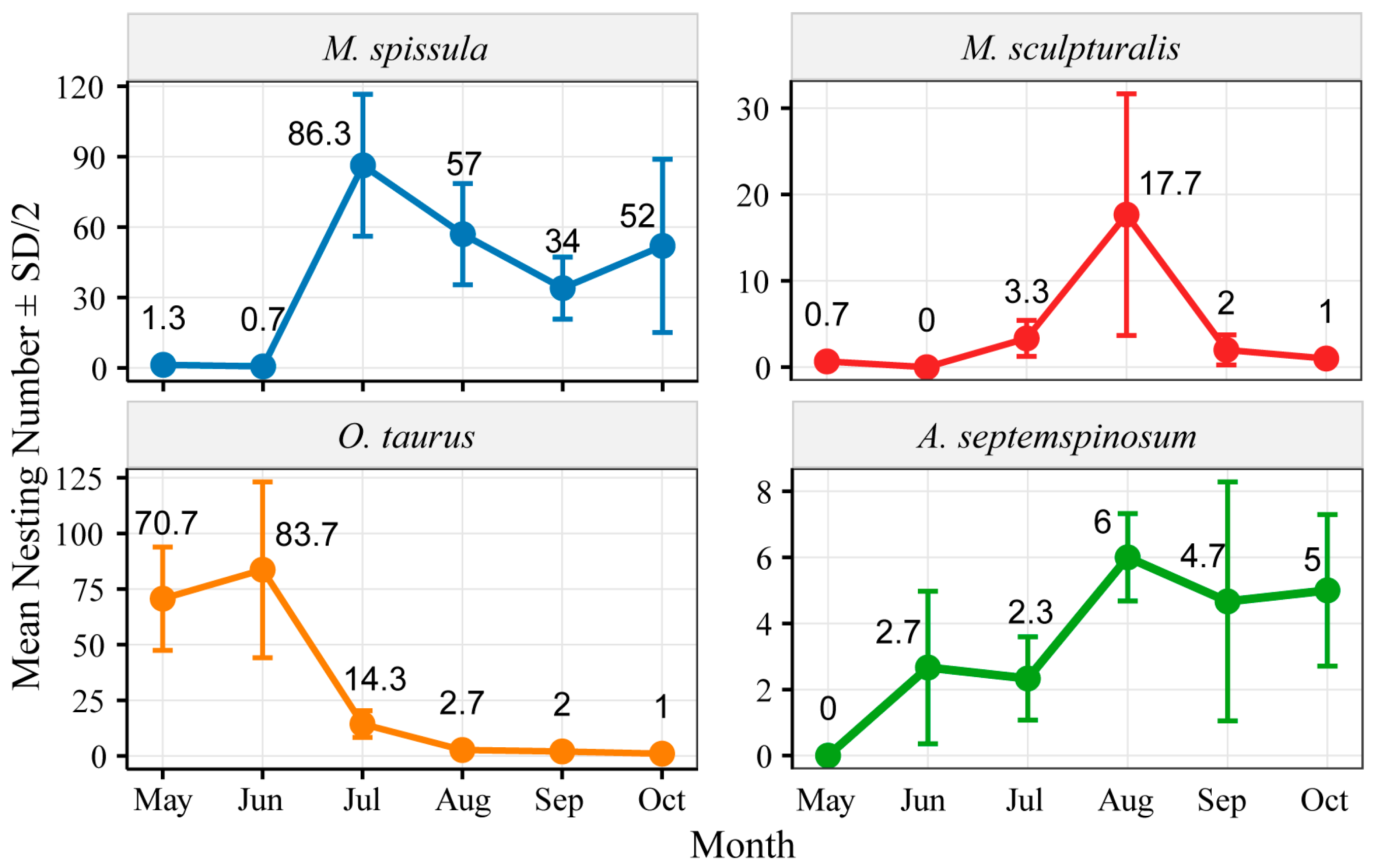
3.2. Nesting Activity Duration
3.3. Correlation Between Bee Abundance and Temperature, Rainfall, and Wind Speed
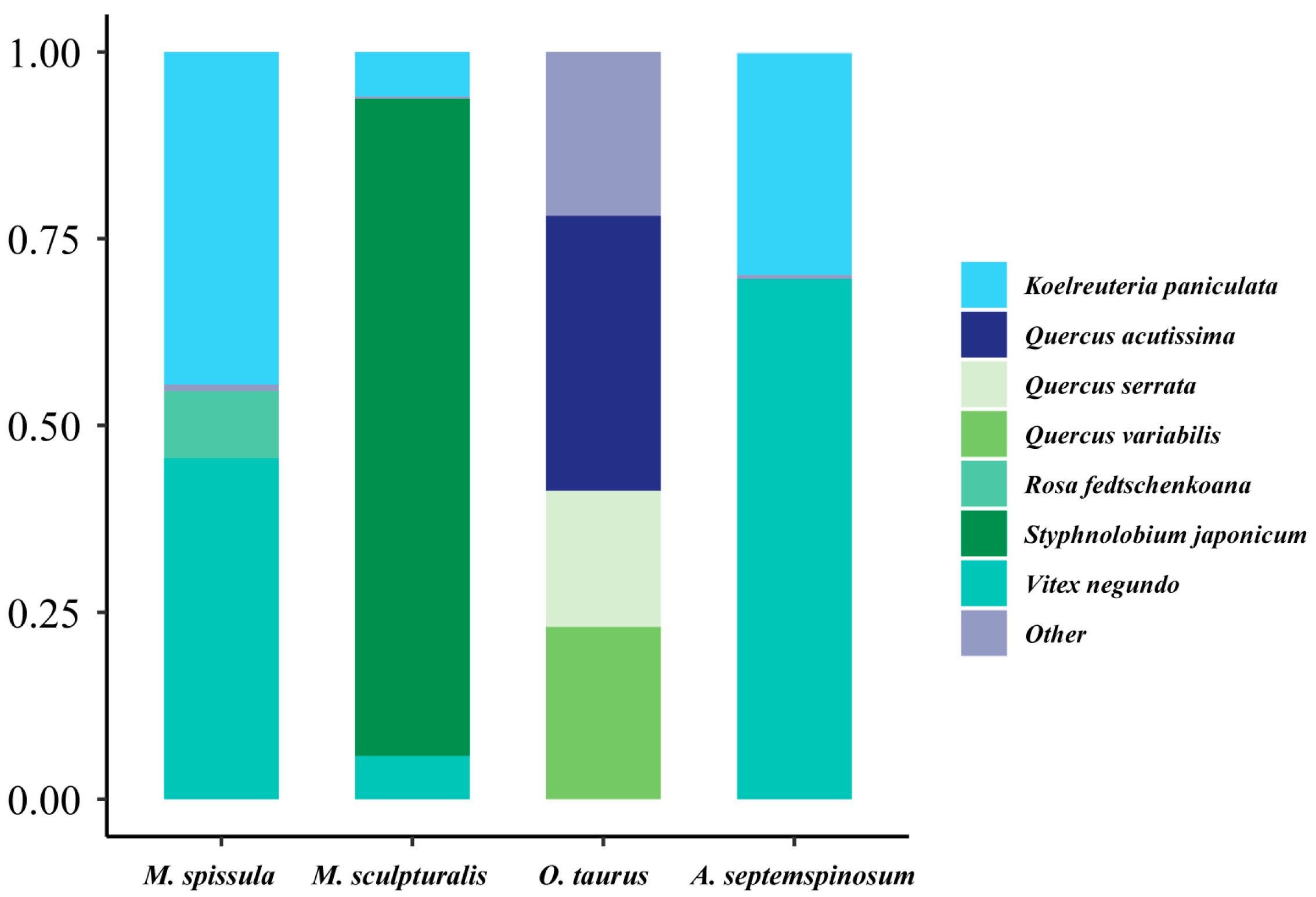
3.4. Nesting Material
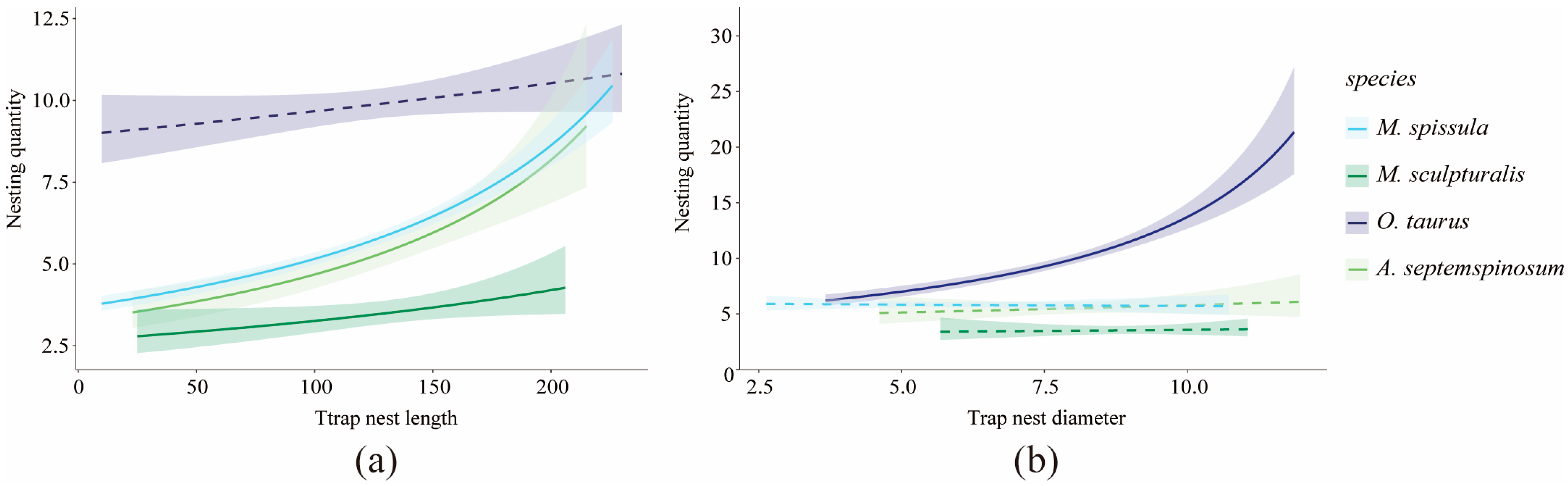
3.5. Nest Inner Diameter and Length
3.6. Brood Cell
3.7. Pollen Spectrum of Bee Bread
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2006, 79, 674–681. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Kline, O.; Joshi, N.K. Mitigating the Effects of Habitat Loss on Solitary Bees in Agricultural Ecosystems. Agriculture 2020, 10, 115. [Google Scholar] [CrossRef]
- Muniz, V.I.M.d.S.; Santos, L.F.d.; Oliveira, P.d.A.d.; Silveira, D.R.d.; Freitas, B.M. Nesting and foraging behaviour of the solitary bee Epanthidium tigrinum (Schrottky, 1905) bred in trap nests. Rev. CiÊncia AgronÔmica 2023, 54, e20228348. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Klein, A.-M.; Boreux, V.; Fornoff, F.; Mupepele, A.-C.; Pufal, G. Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 2018, 26, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.M.A.; Zanette, L.R.S.; Araujo, F.S. Effects of habitat simplification on assemblages of cavity nesting bees and wasps in a semiarid neotropical conservation area. Biodivers. Conserv. 2018, 27, 311–328. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Kunjwal, N.; Khan, M.S.; Kumar, G.; Srivastava, P. Notes on the nesting ecology of the Megachile bees from North India. J. Apic. Res. 2020, 60, 807–816. [Google Scholar] [CrossRef]
- Rauf, A.; Saeed, S.; Ali, M.; Tahir, M.H.N. Nest preference and ecology of cavity-nesting bees (Hymenoptera: Apoidea) in Punjab, Pakistan. J. Asia-Pac. Entomol. 2022, 25, 101907. [Google Scholar] [CrossRef]
- Sinu, P.A.; Aiswarya, V. Leafcutter bee preference of plant saplings in plant nurseries: Context for future research and conservation. Apidologie 2023, 54, 55. [Google Scholar] [CrossRef]
- Akram, W.; Sajjad, A.; Ghramh, H.A.; Ali, M.; Khan, K.A. Nesting Biology and Ecology of a Resin Bee, Megachile cephalotes (Megachilidae: Hymenoptera). Insects 2022, 13, 1058. [Google Scholar] [CrossRef]
- Mello, B.N.d.S.; Gaglianone, M.C. Nesting Biology of Sympatric Species of Megachilidae Bees in a Conservation Area in Brazilian Atlantic Forest. Sociobiology 2019, 66, 52. [Google Scholar] [CrossRef]
- Lu, H.; He, B.; Hao, Y.; Zhou, Z.; Su, C.; Huang, D. Comparative Mitogenomic Analysis of Two Cuckoo Bees (Apoidea: Anthophila: Megachilidae) with Phylogenetic Implications. Insects 2021, 12, 29. [Google Scholar] [CrossRef]
- Raina, R.H.; Pathak, P.; Kumar, K.; Jangid, T. The Family Megachilidae (Hymenoptera: Apoidea) in Pollination Ecology—A Review. Indian J. Entomol. 2023, 86, 997–1003. [Google Scholar] [CrossRef]
- Pramanik, K.; Layek, A.; Visakh, N.U.; Jha, S. Comparative seasonal plant diversity and leaf foraging pattern of leafcutter bees (Megachilidae: Hymenoptera) in urban, semi-urban and agricultural areas of Eastern India. Arthropod-Plant Interact. 2025, 19, 26. [Google Scholar] [CrossRef]
- Barda, M.; Karamaouna, F.; Kati, V.; Perdikis, D. Do Patches of Flowering Plants Enhance Insect Pollinators in Apple Orchards? Insects 2023, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.W. Effectiveness of the alfalfa leafcutter bee Megachile rotundata Fab. to pollinate perennial clovers. J. Apic. Res. 2016, 55, 259–267. [Google Scholar] [CrossRef]
- Richards, K.W. Effectiveness of the alfalfa leafcutter bee Megachile rotundata Fab. to pollinate four perennial legumes. J. Apic. Res. 2019, 59, 69–76. [Google Scholar] [CrossRef]
- Kraemer, M.E.; Favi, F.D.; Niedziela, C.E. Nesting and Pollen Preference of Osmia lignaria (Hymenoptera: Megachilidae) in Virginia and North Carolina Orchards. Environ. Entomol. 2014, 43, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Parker, F.D.; Frohlich, D.R. Studies on Management of the Sunflower Leafcutter Bee Eumegachile pugnata (Say) (Hymenoptera: Megachilidae). J. Apic. Res. 1985, 24, 125–131. [Google Scholar] [CrossRef]
- Matsumoto, S.; Soejima, J.; Maejima, T. Influence of repeated pollination on seed number and fruit shape of ‘Fuji’ apples. Sci. Hortic. 2012, 137, 131–137. [Google Scholar] [CrossRef]
- dos Santos, A.A.; Parizotto, D.; Schlindwein, C.; Martins, C.F. Nesting biology and flower preferences of Megachile (Sayapis) zaptlana. J. Apic. Res. 2020, 59, 609–625. [Google Scholar] [CrossRef]
- Sinu, P.A.; Bronstein, J.L.; Lowe, A. Foraging preferences of leafcutter bees in three contrasting geographical zones. Divers. Distrib. 2018, 24, 621–628. [Google Scholar] [CrossRef]
- Sgolastra, F.; Hinarejos, S.; Pitts-Singer, T.L.; Boyle, N.K.; Joseph, T.; Lūckmann, J.; Raine, N.E.; Singh, R.; Williams, N.M.; Bosch, J. Pesticide Exposure Assessment Paradigm for Solitary Bees. Environ. Entomol. 2019, 48, 22–35. [Google Scholar] [CrossRef]
- von Königslöw, V.; Klein, A.-M.; Staab, M.; Pufal, G. Benchmarking nesting aids for cavity-nesting bees and wasps. Biodivers. Conserv. 2019, 28, 3831–3849. [Google Scholar] [CrossRef]
- Nascimento, F.S.; MacIvor, J.S.; Packer, L. ‘Bee Hotels’ as Tools for Native Pollinator Conservation: A Premature Verdict? PLoS ONE 2015, 10, e0122126. [Google Scholar] [CrossRef]
- Mirwan, H.B. Nesting Traps to Collect Solitary Cavity-Nesting Hymenoptera. Al-Mukhtar J. Sci. 2023, 38, 160–172. [Google Scholar] [CrossRef]
- Mahmoud, K.M.; Abdo, E.S.; Kamel, S.M.; El-Shafy, A.S.; Shebl, M.A. Nesting Preference, Nesting Biology and Associated Natural Enemies of Chalichadoma flavipes Spinola, 1838 (hymenoptera: Megachilidae). Sociobiology 2025, 72, e11251. [Google Scholar] [CrossRef]
- Rahimi, E.; Barghjelveh, S.; Dong, P. How effective are artificial nests in attracting bees? A review. J. Ecol. Environ. 2021, 45, 16. [Google Scholar] [CrossRef]
- Seidelmann, K.; Bienasch, A.; Pröhl, F. The impact of nest tube dimensions on reproduction parameters in a cavity nesting solitary bee, Osmia bicornis (Hymenoptera: Megachilidae). Apidologie 2015, 47, 114–122. [Google Scholar] [CrossRef]
- Ivanov, S.P.; Fateryga, A.V.; Kobetskaya, M.A. The nesting biology of the bee, Osmia dimidiata Morawitz, 1870 (Hymenoptera, Megachilidae) in the Crimea. Entomol. Rev. 2013, 93, 675–694. [Google Scholar] [CrossRef]
- Payne, A.; Schildroth, D.A.; Starks, P.T. Nest site selection in the European wool-carder bee, Anthidium manicatum, with methods for an emerging model species. Apidologie 2011, 42, 181–191. [Google Scholar] [CrossRef]
- Vitale, N.; Gonzalez, V.H.; Vázquez, D.P. Nesting ecology of sympatric species of wool carder bees (Hymenoptera: Megachilidae: Anthidium) in South America. J. Apic. Res. 2017, 56, 497–509. [Google Scholar] [CrossRef]
- Okely, M.; Engel, M.S.; Shebl, M.A. Climate Change Influence on the Potential Distribution of Some Cavity-Nesting Bees (Hymenoptera: Megachilidae). Diversity 2023, 15, 1172. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Wilson, R.S. Bee Hotels as a Tool for Post-Fire Recovery of Cavity-Nesting Native Bees. Insects 2025, 16, 659. [Google Scholar] [CrossRef] [PubMed]
- HU, J.; Fang, Q.; Wang, X.; Sun, K.; Liu, X.; He, C. Morphological characteristics and nesting behavior of Trypoxylon melanocorne (Hymenoptera: Sphecidae). Acta Entomol. Sin. 2023, 66, 805–815. [Google Scholar]
- Marinho, D.; Muniz, D.B.; Azevedo, G.G. Nesting biology of three Megachile (Hymenoptera: Megachilidae) species from Eastern Amazonia, Brazil. Rev. Bras. Entomol. 2018, 62, 97–106. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World; JHU Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Chole, H.; Woodard, S.H.; Bloch, G. Body size variation in bees regulation, mechanisms, and relationship to social organization. Curr. Opin. Insect Sci. 2019, 35, 77–87. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Vitale, N.; Dorado, J.; Amico, G.; Stevani, E.L. Phenological mismatches and the demography of solitary bees. Proc. R. Soc. B Biol. Sci. 2023, 290, 20221847. [Google Scholar] [CrossRef]
- Flórez-Gómez, N.A.; Maldonado-Cepeda, J.D.; Ospina-Torres, R. Bee-Plant Interaction Networks in a Seasonal Dry Tropical Forest of the Colombian Caribbean. Neotrop. Entomol. 2020, 49, 533–544. [Google Scholar] [CrossRef]
- Aleixo, K.P.; Menezes, C.; Imperatriz Fonseca, V.L.; da Silva, C.I. Seasonal availability of floral resources and ambient temperature shape stingless bee foraging behavior (Scaptotrigona aff. depilis). Apidologie 2016, 48, 117–127. [Google Scholar] [CrossRef]
- Roulston, T.a.H.; Goodell, K. The Role of Resources and Risks in Regulating Wild Bee Populations. Annu. Rev. Entomol. 2011, 56, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Vitale, N.; Torretta, J.P.; Durante, S.; Basilio, A.; Vázquez, D.P. Similarities and differences in the realized niche of two allopatric populations of a solitary bee under environmental variability. Apidologie 2020, 51, 439–454. [Google Scholar] [CrossRef]
- da Costa, D.M.; Batista, M.C.; de Brito, A.S.; de Barros, I.; Teodoro, A.V. Rainfall, temperature, and vegetation type influence nesting by the oil-collecting bee Centris (Hemisiella) tarsata in Brazilian restinga. Apidologie 2019, 50, 811–820. [Google Scholar] [CrossRef]
- Kunjwal, N.; Khan, M.S.; Srivastava, P. Species Richness and Seasonal Activity of the Leaf Cutter and Resin Bees (Hymenoptera: Megachilidae) at Pantnagar. Int. J. Sci. Res. (IJSR) 2016, 5, 972–977. [Google Scholar] [CrossRef]
- Stupski, S.D.; Schilder, R.J. Operative temperature analysis of the honey bee Apis mellifera. J. Exp. Biol. 2021, 224, jeb231134. [Google Scholar] [CrossRef]
- Rehan, S.M.; Richards, M.H. Nesting biology and subsociality in Ceratina calcarata (Hymenoptera: Apidae). Can. Entomol. 2010, 142, 65–74. [Google Scholar] [CrossRef]
- Orr, M.C.; Jakob, M.; Harmon-Threatt, A.; Mupepele, A.-C. A review of global trends in the study types used to investigate bee nesting biology. Basic Appl. Ecol. 2022, 62, 12–21. [Google Scholar] [CrossRef]
- Gomes, A.M.S.; Silva, C.I.d.; Cavalcante, A.M.; Rocha, E.E.M.; Freitas, B.M. Bionomy and Nesting Behavior of the Bee Epanthidium tigrinum (Schrottky, 1905) (Hymenoptera: Megachilidae) in Trap-Nests. Sociobiology 2020, 67, 247. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Cheesman, S.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Long foraging distances impose high costs on offspring production in solitary bees. J. Anim. Ecol. 2010, 79, 674–681. [Google Scholar] [CrossRef]
- Kim, J.Y.; Thorp, R.W. Maternal investment and size-number trade-off in a bee, Megachile apicalis, in seasonal environments. Oecologia 2001, 126, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.H.; Roitberg, B.D.; Peterson, J.H. Impacts of flight distance on sex ratio and resource allocation to offspring in the leafcutter bee, Megachile rotundata. Behav. Ecol. Sociobiol. 2005, 59, 589–596. [Google Scholar] [CrossRef]
- Bosch, J.; Kemp, W.P. Effect of pre-wintering and wintering temperature regimes on weight loss, survival, and emergence time in the mason bee Osmia cornuta (Hymenoptera: Megachilidae). Apidologie 2004, 35, 469–479. [Google Scholar] [CrossRef]
- Goodell, K. Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecologia 2003, 134, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, K. Open-cell parasitism shapes maternal investment patterns in the Red Mason bee Osmia rufa. Behav. Ecol. 2006, 17, 839–848. [Google Scholar] [CrossRef]
- MacIvor, J.S. Cavity-nest boxes for solitary bees: A century of design and research. Apidologie 2016, 48, 311–327. [Google Scholar] [CrossRef]
- Bogo, G.; Fisogni, A.; Iannone, A.; Grillenzoni, F.-V.; Corvucci, F.; Bortolotti, L. Nesting biology and nest structure of the exotic bee Megachile sculpturalis. Bull. Entomol. Res. 2024, 114, 67–76. [Google Scholar] [CrossRef]
- Quaranta, M.; Sommaruga, A.; Balzarini, P.; Felicioli, A. A new species for the bee fauna of Italy: Megachile sculpturalis continues its colonization of Europe. Bull. Insectology 2014, 67, 287–293. [Google Scholar]
- Dubaic’, J.B.; Lanner, J. Megachile sculpturalis (Hymenoptera: Megachilidae): A Valuable Study Organism for Invasive Pollinators and the Role of Beekeepers in Ongoing Monitoring Programs. Bee World 2021, 98, 78–82. [Google Scholar] [CrossRef]
- Bosch, J.; Vicens, N. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecol. Sociobiol. 2005, 60, 26–33. [Google Scholar] [CrossRef]
- Polidori, C.; Boesi, R.; Borsato, W. Few, small, and male: Multiple effects of reduced nest space on the offspring of the solitary wasp, Euodynerus (Pareuodynerus) posticus (Hymenoptera: Vespidae). Comptes Rendus Biol. 2010, 334, 50–60. [Google Scholar] [CrossRef]
- Khan, D.H.; Ali, M.; Khan, F.Z.A.; Mehmood, M.A.; Saeed, S. Effect of landscape complexity, nesting substrate, and nest orientation on cavity-nesting solitary bees in southern Punjab, Pakistan. Int. J. Trop. Insect Sci. 2024, 44, 339–349. [Google Scholar] [CrossRef]
- Ivanov, S.P. The nesting of Osmia rufa (L.) (Hymenoptera, Megachilidae) in the Crimea: Structure and composition of nests. Entomol. Rev. 2006, 86, 524–533. [Google Scholar] [CrossRef]
- Gruber, B.; Eckel, K.; Everaars, J.; Dormann, C.F. On managing the red mason bee (Osmia bicornis) in apple orchards. Apidologie 2011, 42, 564–576. [Google Scholar] [CrossRef]
- Torretta, J.P.; Durante, S.P. Nesting ecology of Megachile (Sayapis) mendozana Cockerell and its synonymy with Megachile (Sayapis) santiaguensis Durante (Hymenoptera Megachilidae). Zootaxa 2011, 3008, 63–68. [Google Scholar] [CrossRef]
- Rauf, A.; Saeed, S.; Ali, M.; Nadeem Tahir, M.H. Comparative Efficiency of Native Insect Pollinators in Reproductive Performance of Medicago sativa L. in Pakistan. Insects 2021, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.; Bar-Shai, N.; Cytter, Y.; Green, R. Time is honey: Circadian clocks of bees and flowers and how their interactions may influence ecological communities. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160256. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Gutiérrez, R.; Roubik, D.W.; Porter-Bolland, L. Bee–Plant Interactions: Competition and Phenology of Flowers Visited by Bees. In Biodiversity and Conservation of the Yucatán Peninsula; Springer: Cham, Switzerland, 2015; pp. 131–152. [Google Scholar]


| Years | Temperature (°C) | Precipitation (mm) | Wind Speed (km/h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | sd | r | p-Value | Average | sd | r | p-Value | Average | sd | r | p-Value | |
| 2019 | 15.47 | 9.99 | 0.58 | 0.04 | 1.34 | 1.31 | 0.49 | 0.10 | 1.41 | 0.17 | −0.20 | 0.51 |
| 2020 | 15.56 | 9.48 | 0.88 | 0.001 | 1.59 | 1.81 | 0.76 | 0.003 | 1.52 | 0.21 | 0.13 | 0.67 |
| 2021 | 15.66 | 9.38 | 0.88 | 0.001 | 2.56 | 3.33 | 0.68 | 0.01 | 1.68 | 0.18 | −0.08 | 0.79 |
| 2022 | 15.96 | 10.28 | 0.82 | 0.001 | 1.57 | 2.06 | 0.45 | 0.13 | 1.54 | 0.25 | 0.15 | 0.62 |
| Species | Partition | Sealing | Nesting Period |
|---|---|---|---|
| M. spissula | Leaves | Leaves | 7–8 months |
| M. sculpturalis | Resin, mud | Resin, pebbles, mud | 7–8 months |
| O. taurus | Mud | Mud | 5–6 months |
| A. septemspinosum | Cotton fluff | Leaf mix | 7–9 months |
| Species | Thorax Width (mm) ± SE (n = 10) | The Number of Nest-Building Inner Diameters (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | 2–4 mm | 4–6 mm | 6–8 mm | 8–10 mm | 10–12 mm | 12–14 mm | |
| M. spissula | 2.85 ± 0.22 | 2.49 ± 0.25 | 22 | 262 | 387 | 59 | 2 | |
| M. sculpturalis | 6.81 ± 0.49 | 5.62 ± 0.73 | 8 | 45 | 24 | |||
| O. taurus | 5.10 ± 0.66 | 3.32 ± 0.36 | 53 | 201 | 219 | 54 | 4 | |
| A. septemspinosum | 4.35 ± 0.34 | 4.8 ± 0.53 | 2 | 42 | 51 | 19 | 1 | |
| Species | Body Length (mm) ± SE (n = 10) | The Quantity of Nest-Building Lengths (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | 10–50 mm | 51–90 mm | 91–130 mm | 131–170 mm | 171–210 mm | 211–250 mm | |
| M. spissula | 9.70 ± 0.73 | 8.30 ± 0.74 | 72 | 155 | 209 | 194 | 79 | 19 |
| M. sculpturalis | 23.02 ± 1.93 | 17.22 ± 1.92 | 4 | 9 | 25 | 28 | 12 | 0 |
| O. taurus | 11.96 ± 1.03 | 9.63 ± 1.64 | 45 | 91 | 131 | 172 | 87 | 3 |
| A. septemspinosum | 13.29 ± 0.94 | 14.06 ± 1.79 | 5 | 21 | 34 | 40 | 12 | 3 |
| Species | Sample Size (n) | Total Chambers | Nursery Chambers | ||
|---|---|---|---|---|---|
| Range | Mean ± SE | Range | Mean ± SE | ||
| M. spissula | 728 | 1–22 | 5.82 (0.11) | 1–15 | 4.50 (0.09) |
| M. sculpturalis | 78 | 1–9 | 3.53 (0.18) | 1–9 | 2.62 (0.18) |
| O. taurus | 529 | 1–27 | 9.88 (0.23) | 1–27 | 8.93 (0.22) |
| A. septemspinosum | 116 | 1–12 | 5.53 (0.25) | 1–12 | 5.32 (0.0.25) |
| Species | Number of Interacting Species | Species Strength | Specialization (d’) | Interspecific Asymmetry |
|---|---|---|---|---|
| M. spissula | 36 | 17.91 | 0.06 | 0.46 |
| M. sculpturalis | 19 | 5.75 | 0.04 | 0.25 |
| O. taurus | 31 | 14.75 | 0.14 | 0.44 |
| A. septemspinosum | 27 | 9.58 | 0.03 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Hu, J.; Liu, X.; Wan, J.; Wei, J.; Yang, D.; He, C. Nesting and Foraging Preferences of Four Sympatric Species of Cavity-Nesting Leafcutting Bees (Hymenoptera: Megachilidae). Insects 2025, 16, 831. https://doi.org/10.3390/insects16080831
Dai Q, Hu J, Liu X, Wan J, Wei J, Yang D, He C. Nesting and Foraging Preferences of Four Sympatric Species of Cavity-Nesting Leafcutting Bees (Hymenoptera: Megachilidae). Insects. 2025; 16(8):831. https://doi.org/10.3390/insects16080831
Chicago/Turabian StyleDai, Qianlei, Junjie Hu, Xuan Liu, Jia Wan, Jiabao Wei, Dongshuo Yang, and Chunling He. 2025. "Nesting and Foraging Preferences of Four Sympatric Species of Cavity-Nesting Leafcutting Bees (Hymenoptera: Megachilidae)" Insects 16, no. 8: 831. https://doi.org/10.3390/insects16080831
APA StyleDai, Q., Hu, J., Liu, X., Wan, J., Wei, J., Yang, D., & He, C. (2025). Nesting and Foraging Preferences of Four Sympatric Species of Cavity-Nesting Leafcutting Bees (Hymenoptera: Megachilidae). Insects, 16(8), 831. https://doi.org/10.3390/insects16080831




