Evaluation of Spatial Distribution of Pulse Blue Butterfly (Lampides boeticus), Pest of Legume Crops, in Response to Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
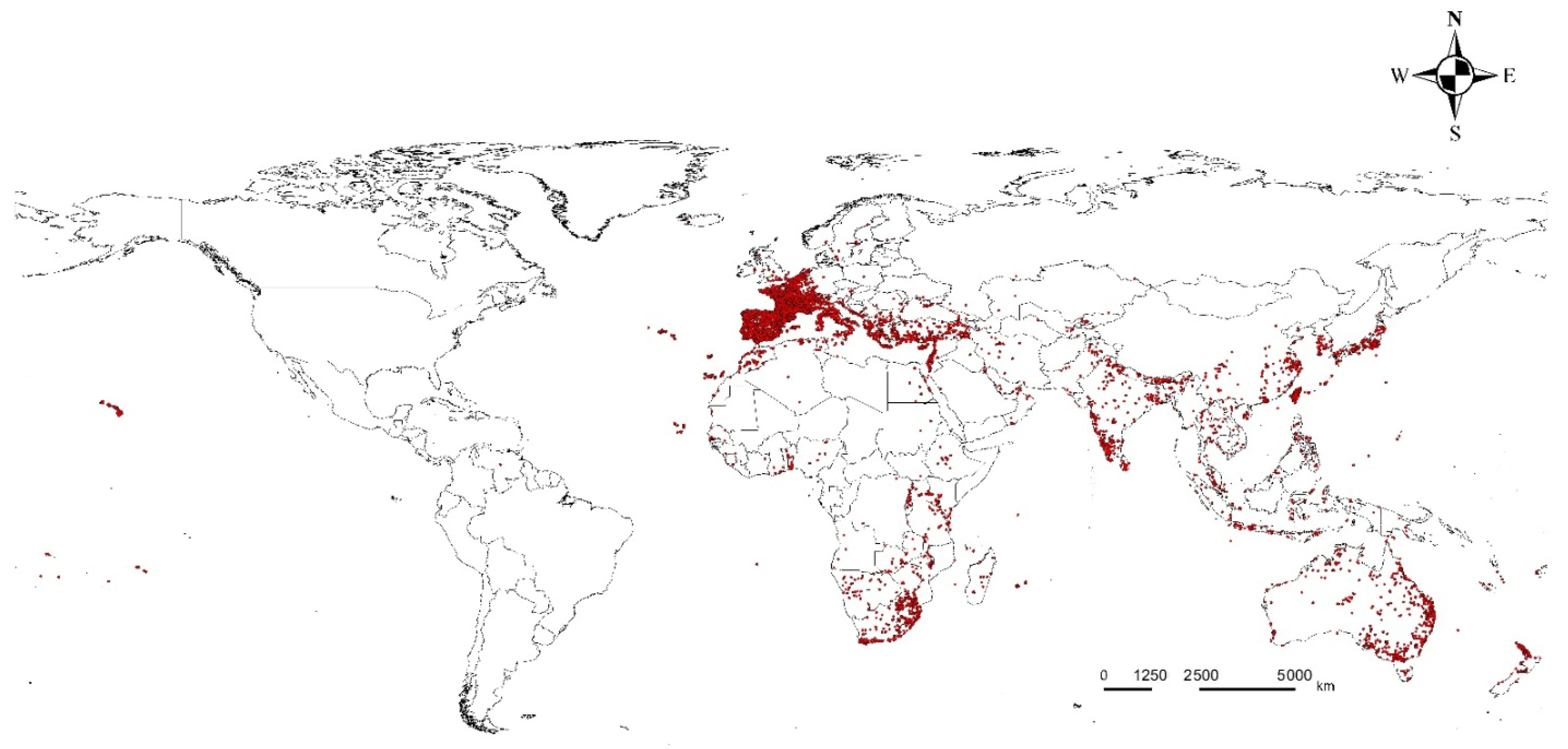
2.1. Occurrence and Background Data Processing
2.2. Model Variables and Their Selection
2.3. Optimal Model Operating Conditions
2.4. Model Performance Test
3. Results
3.1. Results of Model Performance Test for L. boeticus
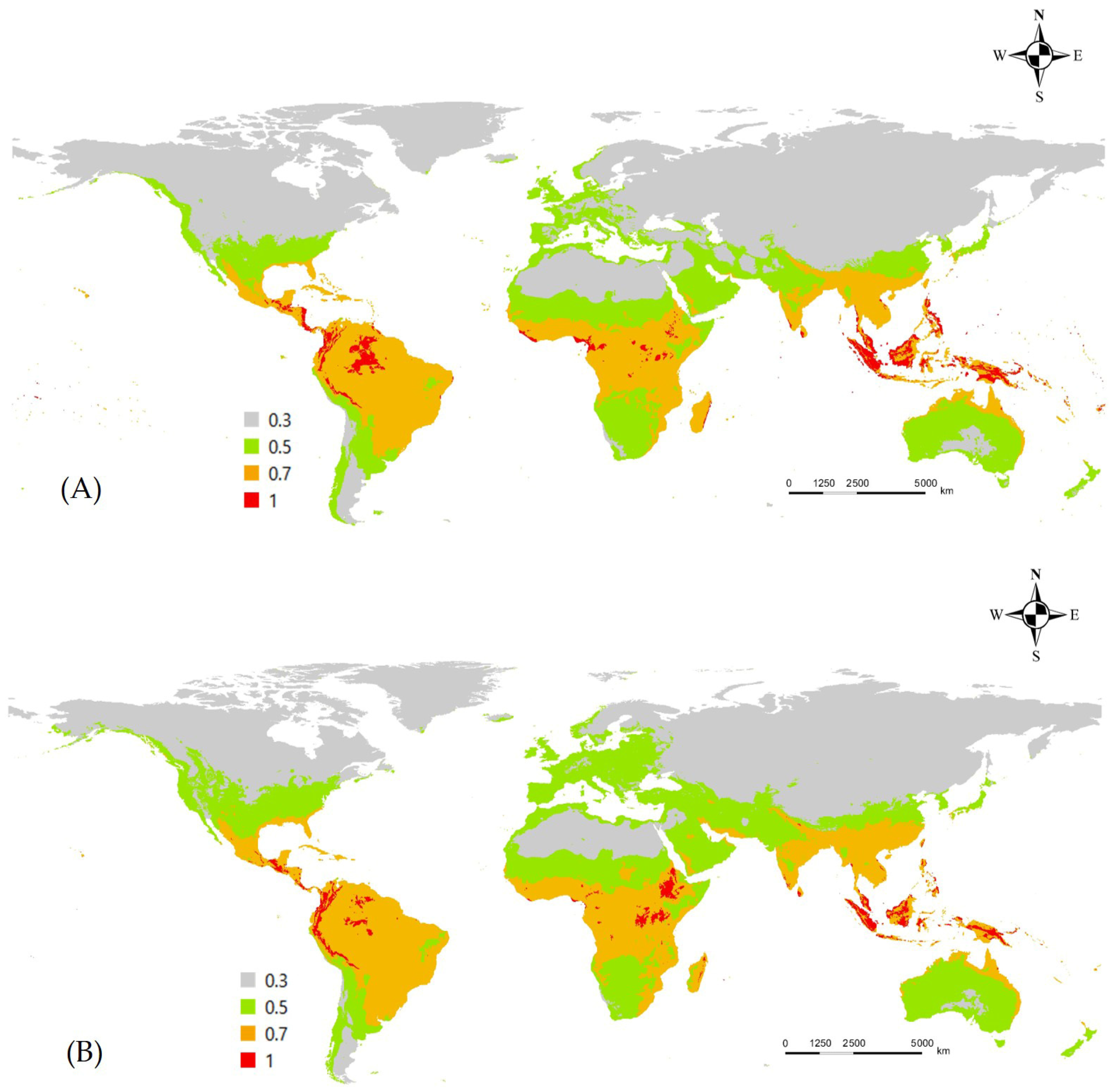
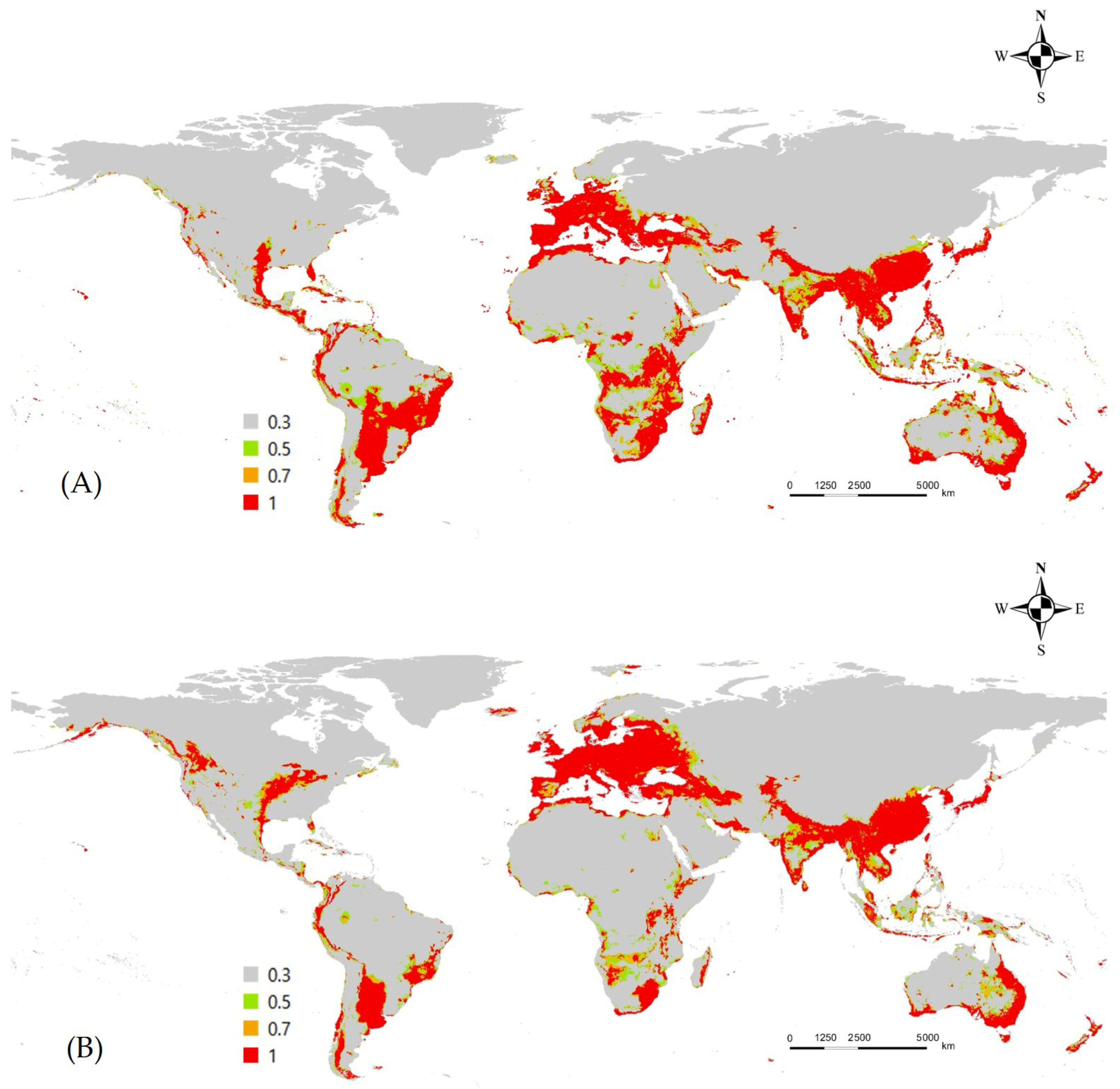
3.2. Occurrence, Variables, and Potential Distribution of L. boeticus by Single Model Algorithm
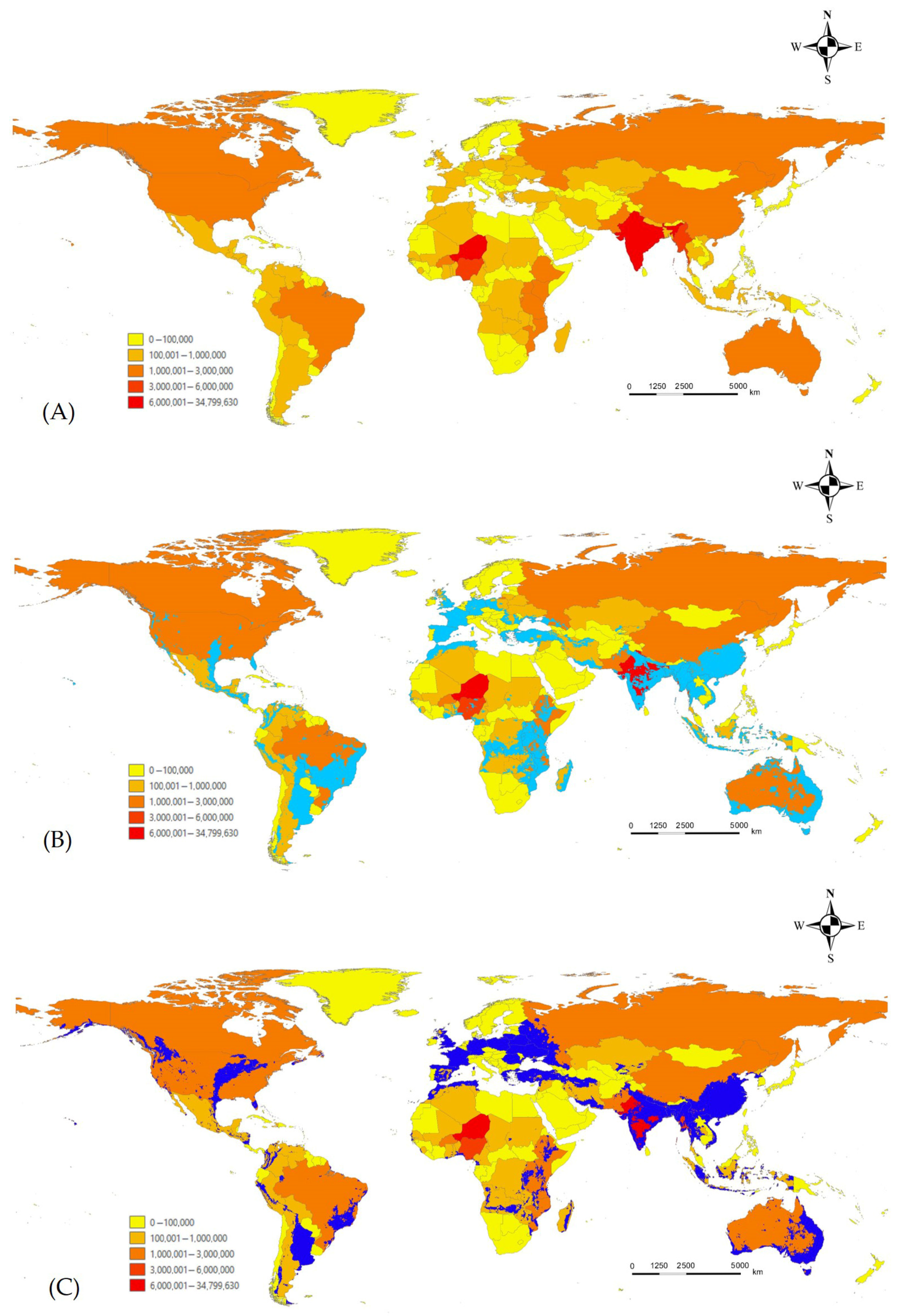
3.3. Ensemble Prediction of L. boeticus Potential Distribution with Consideration of Host Production and Global Pulse-Crop Production Area with Potential Risk Area Assessment
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lohman, D.J.; Peggie, D.; Pierce, N.E.; Meier, R. Phylogeography and genetic diversity of a widespread Old World butterfly, Lampides boeticus (Lepidoptera: Lycaenidae). BMC Evol. Biol. 2008, 8, 301. [Google Scholar] [CrossRef]
- Barbudo, J.; González, J.R.; Haeger, J.F. Capparis spinosa (Capparidaceae): An oviposition substrate for Lampides boeticus Linnaeus, in southern Spain (Lepidoptera: Lycaenidae). Nota Lepidopterol. 1987, 10, 218–223. [Google Scholar]
- Jordano, D.; Rodríguez, J. Nuevas citas de plantas nutricias para tres especies de ropalóceros. Shilap Revta. Lepid. 1987, 10, 218–223. [Google Scholar]
- Natural History Museum (NHM). HOSTS—A Database of the World’s Lepidopteran Hostplants. Available online: https://data.nhm.ac.uk/dataset/hosts/resource/877f387a-36a3-486c-a0c1-b8d5fb69f85a (accessed on 25 May 2024).
- Abdallah, Y.E. Effect of plant traps and sowing dates on population density of major soybean pests. J. Basic Appl. Zool. 2012, 65, 37–46. [Google Scholar] [CrossRef]
- Askari, A.; Zare, N. Effect of foliar application of four insecticides on the yield of cowpea affected by the bean butterfly, Lycaena boetica L. (Lepidoptera: Lycaenidae). Indian J. Agric. Sci. 1975, 45, 470–472. [Google Scholar]
- Dialoke, S.A.; Akparobi, S.O.; Abraham, N.; Kennedy, O.; Frank, O.O.; Christopher, E. Effect of Color Sticky Traps on the Population of Major Pests of Improved Pigeon Pea (Cajanus cajan (L.) Millsp) Cultivar and Its Yield in Owerri, Imo State, Nigeria. Int. J. Sci. Technoledge 2019, 7, 12–20. [Google Scholar] [CrossRef]
- Hiroshi, H.; Tetsuro, S.; Yoshiharu, M. Oviposition and Larval Feeding Habits of the Pea Blue, Lampides boeticus L. (Lepidoptera: Lycaenidae) on the Lablab Bean, Dolichos lablab L. Jpn. J. Appl. Entomol. Zool. 1985, 29, 26–30. [Google Scholar]
- Khidher, K.Q. Susceptibility of Cowpea varieties to infestation by Pea blue Butterfly, Lampides boeticus (Linnaeus) (Lepidoptera: Lycaenidae). Tikrit J. Agric. Sci. 2024, 24, 1–8. [Google Scholar] [CrossRef]
- Marchal, P. Report on injurious insects in France during the year 1913. Rev. De. Phytopathol. Appl. 1914, 19, 9–13. [Google Scholar]
- Minja, E.M.; Shanower, T.G.; Silim, S.N.; Karuru, O. Efficacy of different insecticides for pigeonpea pest management in Kenya. Int. Chickpea Pigeonpea Newsl. 2000, 7, 53–55. [Google Scholar]
- Muthukrishnan, N. In-Vivo and field evaluation of spinetoram 12 SC against Lampides boeticus on pigeonpea. Legume Res. Int. J. 2017, 40, 1126–1132. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Srinivasan, R.; Wu, M.H.; Bhattarai, M.; Patricio, R.; Yule, S.; Quang, V.H.; Hop, B.T.H. Safe and sustainable management of legume pests and diseases in Thailand and Vietnam: A situational analysis. Int. J. Trop. Insect Sci. 2014, 34, 88–97. [Google Scholar] [CrossRef]
- Durairaj, C. Evaluation of certain Neem formulations and insecticides against pigon pea pod fly. Indian J. Pulses Res. 2006, 19, 269–270. [Google Scholar]
- AL-Janabi, N.H.; Al-Karboli, H.H. Damage assessment of the pea blue butterfly Lampides boeticus L. (Lepidoptera: Lycaenidae) on cowpea (Vignaung uiculata). J. Biodivers. Environ. Sci. 2017, 11, 268–273. [Google Scholar]
- National Institute of Biological Resources (NIBR). Available online: https://species.nibr.go.kr/home/mainHome.do?cont_link=009&subMenu=009002&contCd=009002&ktsn=120000034429 (accessed on 25 May 2024).
- Colvin, M. Rearing the Long-Tailed Blue—Personal Observations. Available online: http://www.dispar.org/reference.php?id=5 (accessed on 25 January 2024).
- Ma, G.; Peng, Q.; Pan, X.; Xie, M.; Liao, J.; Wu, C.; Yang, M. Maximum Entropy Model Prediction of the Distributions of Two Sympatric Bean Weevil Species, Megabruchidius dorsalis (Fahraeus, 1839) and Bruchidius coreanus (Chûjô, 1937), under Various Climate Scenarios in Guizhou Province, China. Forests 2024, 15, 300. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Ding, W.; Li, H.; Zhang, A. Predicting habitat suitability for the soybean pod borer Leguminivora glycinivorella (Matsumura) using optimized MaxEnt models with multiple variables. J. Econ. Entomol. 2024, 117, 1796–1808. [Google Scholar] [CrossRef]
- Byeon, D.H.; Jung, S.; Lee, W.H. Review of CLIMEX and MaxEnt for studying species distribution in South Korea. J. Asia Pac. Biodivers. 2018, 11, 325–333. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, H.; Zhao, W.; Zhao, Q. Niche shifts and the potential distribution of Phenacoccus solenopsis (Hemiptera: Pseudococcidae) under climate change. PLoS ONE 2017, 12, e0180913. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. NCEAS predicting species distributions working group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Evans, J.S.; Murphy, M.A.; Holden, Z.A.; Cushman, S.A. Modeling Species Distribution and Change Using Random Forest; Springer: New York, NY, USA, 2011; pp. 139–159. [Google Scholar]
- Lee, W.H.; Song, J.W.; Yoon, S.H.; Jung, J.M. Spatial evaluation of machine learning-based species distribution models for prediction of invasive ant species distribution. Appl. Sci. 2022, 12, 10260. [Google Scholar] [CrossRef]
- Centre for Agriculture and Bioscience International (CABI). PlantwisePlus Knowledge Bank. Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.29761 (accessed on 25 May 2024).
- Global Biodiversity Information Facility (GBIF). Occurrence Download. Available online: https://doi.org/10.15468/39omei (accessed on 25 May 2024).
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- McQuillan, M.A.; Rice, A.M. Differential effects of climate and species interactions on range limits at a hybrid zone: Potential direct and indirect impacts of climate change. Ecol. Evol. 2015, 5, 5120–5137. [Google Scholar] [CrossRef]
- Mudereri, B.T.; Mukanga, C.; Mupfiga, E.T.; Gwatirisa, C.; Kimathi, E.; Chitata, T. Analysis of potentially suitable habitat within migration connections of an intra-African migrant-the Blue Swallow (Hirundo atrocaerulea). Ecol. Inf. 2020, 57, 101082. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-038-721-706-2. [Google Scholar]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, S.H.; Yoon, S.; Jung, S.; Kim, D.H.; Lee, W.H. Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model. Insects 2022, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef]
- Kim, G.Y.; Lee, W.H. Prediction of the spatial distribution of vine weevil under climate change using multiple variable selection methods. Sci. Rep. 2025, 15, 7845. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘dismo’. Circles 2017, 9, 1–68. [Google Scholar]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Huercha; Song, R.; Ma, Y.; Hu, Z.; Li, Y.; Li, M.; Wu, L.; Li, C.; Dao, E.; Fan, X.; et al. MaxEnt Modeling of Dermacentor marginatus (Acari: Ixodidae) Distribution in Xinjiang, China. J. Med. Entomol. 2020, 57, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Ujagir, R. Effect of temperature and relative humidity on pod borer in pigeonpea. J. Food Legumes 2007, 20, 121. [Google Scholar]
- Subharani, S.; Singh, T.K. Population dynamics of pod borer complex in pigeonpea in relation to abiotic factors. Indian J. Entomol. 2009, 71, 215–218. [Google Scholar]
- Amdouni, J.; Conte, A.; Ippoliti, C.; Candeloro, L.; Tora, S.; Sghaier, S.; Hassine, T.B.; Fakhfekh, E.A.; Hammami, S. Culex pipiens distribution in Tunisia: Identification of suitable areas through Random Forest and MaxEnt approaches. Vet. Med. Sci. 2022, 8, 2703–2715. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F.; et al. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Guo, J.; Liang, X.; Wang, X.; Fan, Y.; Liu, L. Potential benefits of limiting global warming for the mitigation of temperature extremes in China. Npj Clim. Atmos. Sci. 2023, 6, 106. [Google Scholar] [CrossRef]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Iturbide, M.; Bedia, J.; Herrera, S.; del Hierro, O.; Pinto, M.; Gutiérrez, J.M. A framework for species distribution modelling with improved pseudo-absence generation. Ecol. Model. 2015, 312, 166–174. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards Jr, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Guilhaumon, F.; Albouy, C.; Somot, S.; Thuiller, W.; Mouillot, D. The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob. Change Biol. 2010, 16, 3233–3245. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 3 February 2025).
- Buden, D.W.; Tennent, W.J. Butterflies (Lepidoptera) of the Republic of the Marshall Islands. Pac. Sci. 2017, 71, 511–522. [Google Scholar] [CrossRef]
- Duffey, E. The terrestrial ecology of Ascension Island. J. Appl. Ecol. 1964, 1, 219–251. [Google Scholar] [CrossRef]
- Schreiner, I.H.; Nafus, D.M. Butterflies of Micronesia; Agricultural Experiment Station, College of Agriculture and Life Sciences, University of Guam: Guam, GU, USA, 1997; pp. 1–40. [Google Scholar]
- Smithers, C.N. A species list and bibliography of the insects recorded from Norfolk Island. Tech. Rep. Aust. Mus. 1998, 13, 1–55. [Google Scholar] [CrossRef]
- Pungavi, R.; Kandibane, M. Seasonal Abundance of Spotted pod borer Maruca vitrata and Pea blue butterfly Lampides boeticus on Urd bean. Int. J. Res. Anal. Rev. 2021, 8, 463–471. [Google Scholar][Green Version]
- Shantibala, T.; Singh, T.K. Population dynamics of Lampides boeticus (Linnaeus) on pea crop in Manipur. Shashpa 2003, 10, 133–137. [Google Scholar][Green Version]
- Shantibala, T.; Subharani, S.; Singh, T.K. Seasonal incidence of Lampides boeticus (Linnaeus) and influence of weather factors on its abundance on pigeonpea, Cajanus cajan (L.) Millsp. in Manipur. Indian J. Entomol. 2004, 66, 198–201. [Google Scholar][Green Version]
- Mota, J.D.S.; Barbosa, L.R.; Marchioro, C.A. Suitable areas for invasive insect pests in Brazil and the potential impacts for eucalyptus forestry. Pest Manag. Sci. 2022, 78, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Q.; Yang, J.; Liu, B.; Deng, X.; Gan, T.; Liao, X.; Li, X.; Xu, D.; Zhuo, Z. Evaluating the Impact of Climate Change on the Asia Habitat Suitability of Troides helena Using the MaxEnt Model. Insects 2025, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xian, X.; Zhao, Z.; Zhang, G.; Liu, W.; Wan, F. Climate change increases the expansion risk of Helicoverpa zea in China according to potential geographical distribution estimation. Insects 2022, 13, 79. [Google Scholar] [CrossRef]
- Ashe-Jepson, E.; Cobo, S.A.; Basset, Y.; Bladon, A.J.; Kleckova, I.; Laird-Hopkins, B.C.; Mcfarlane, A.; Sam, K.; Savage, A.F.; Zamora, A.C.; et al. Tropical Butterflies Use Thermal Buffering and Thermal Tolerance as Alternative Strategies to Cope with Temperature Increase. J. Anim. Ecol. 2023, 92, 1759–1770. [Google Scholar] [CrossRef]
- Bonebrake, T.C.; Deutsch, C.A. Climate Heterogeneity Modulates Impact of Warming on Tropical Insects. Ecology 2012, 93, 449–455. [Google Scholar] [CrossRef]
- Morris, H.; Waterhouse, D.F. The Distribution and Importance of Arthropod Pests and Weeds of Agriculture in Myanmar; Australian Centre for International Agricultural Research: Canberra, Australia, 2001; p. 73.
- Wang, W.L.; Suman, D.O.; Zhang, H.H.; Xu, Z.B.; Ma, F.Z.; Hu, S.J. Butterfly conservation in China: From science to action. Insects 2020, 11, 661. [Google Scholar] [CrossRef]
- Quishpe, A.L.C.; Gallegos, P.G. Análisis de riesgo de plagas para la importación de fréjol seco (Phaseolus vulgaris L.) proveniente de China. quito–ecuador. 2007. RUMIPAMBA 2008, 24, 1–14. [Google Scholar]

| Measure | MaxEnt | Random Forest |
|---|---|---|
| Test AUC | 0.6694 | 0.9015 |
| Accuracy | 0.6545 | 0.9012 |
| TSS | 0.5155 | 0.8027 |
| Variable Code a | Description | Percent Contribution (MaxEnt) | Mean Decrease Gini (Random Forest) |
|---|---|---|---|
| Bio2 | Mean diurnal range a,b | 0.3 | 264.94 |
| Bio6 | Minimum temperature of the coldest month | 59 | 853.61 |
| Bio8 | Mean temperature of the wettest quarter | 2 | 210.48 |
| Bio13 | Precipitation of wettest month | 16 | 251.73 |
| Bio14 | Precipitation of driest month | 0.2 | 149.53 |
| Bio15 | Precipitation seasonality (Coefficient of variation) | 2.8 | 132.13 |
| Bio18 | Precipitation of warmest quarter | 15.1 | 182.77 |
| Bio19 | Precipitation of the coldest quarter | 1.6 | 375.96 |
| Elevation | Altitude data | 2.9 | 129.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.H.; Yoon, S.; Lee, W.-H. Evaluation of Spatial Distribution of Pulse Blue Butterfly (Lampides boeticus), Pest of Legume Crops, in Response to Climate Change. Insects 2025, 16, 826. https://doi.org/10.3390/insects16080826
Hwang JH, Yoon S, Lee W-H. Evaluation of Spatial Distribution of Pulse Blue Butterfly (Lampides boeticus), Pest of Legume Crops, in Response to Climate Change. Insects. 2025; 16(8):826. https://doi.org/10.3390/insects16080826
Chicago/Turabian StyleHwang, Jeong Ho, Sunhee Yoon, and Wang-Hee Lee. 2025. "Evaluation of Spatial Distribution of Pulse Blue Butterfly (Lampides boeticus), Pest of Legume Crops, in Response to Climate Change" Insects 16, no. 8: 826. https://doi.org/10.3390/insects16080826
APA StyleHwang, J. H., Yoon, S., & Lee, W.-H. (2025). Evaluation of Spatial Distribution of Pulse Blue Butterfly (Lampides boeticus), Pest of Legume Crops, in Response to Climate Change. Insects, 16(8), 826. https://doi.org/10.3390/insects16080826






