Living Root-Mediated Soil Temperature Amplifies the Effects of Experimental Warming on Soil Microarthropod Communities in a Quercus mongolica Forest in Northeast China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
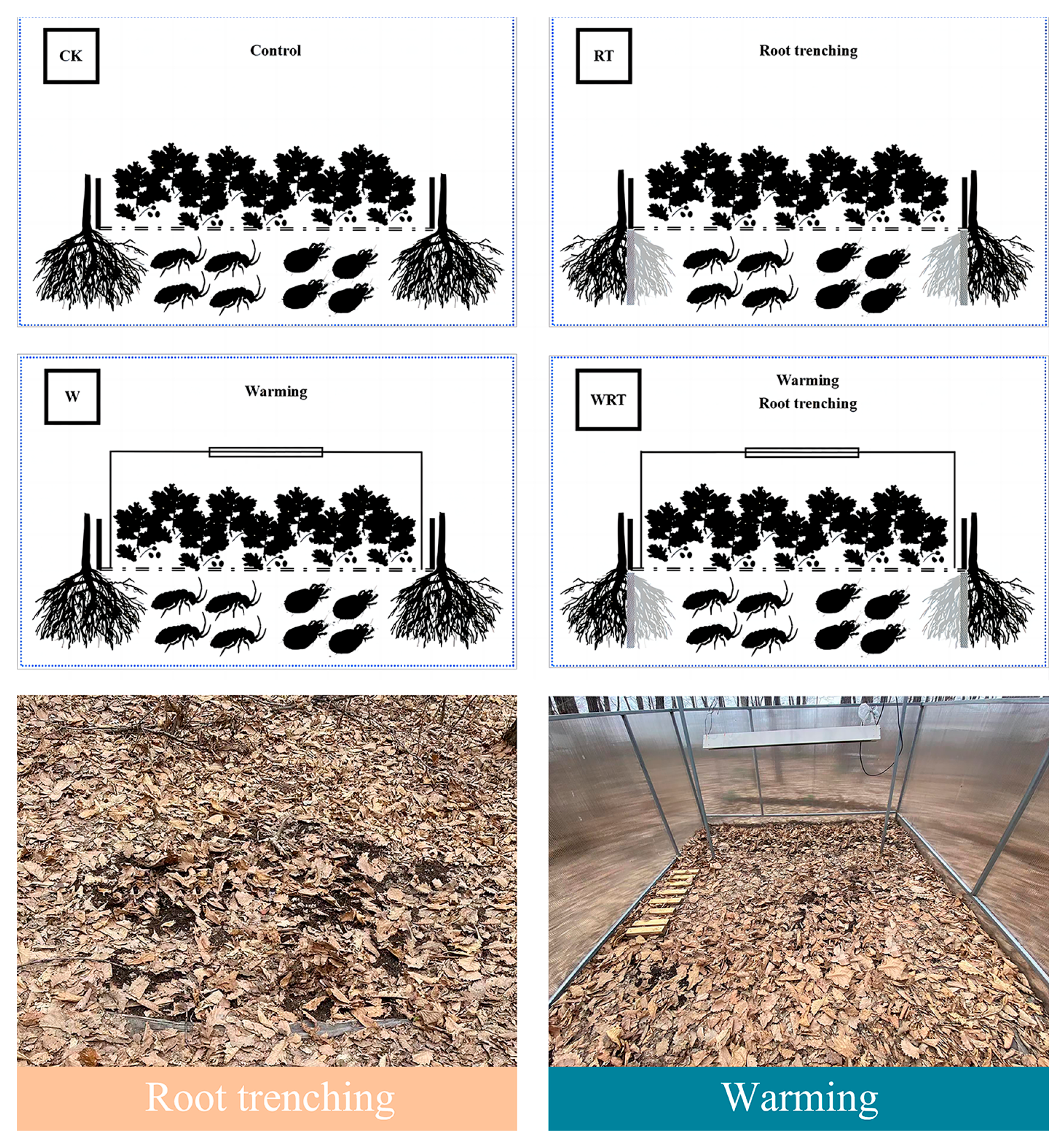
2.2. Experimental Design and Set-Up
2.3. Soil Microclimate Measurements
2.4. Sampling, Extraction and Identification of Soil Microarthropods
2.5. Statistical Analysis
3. Results
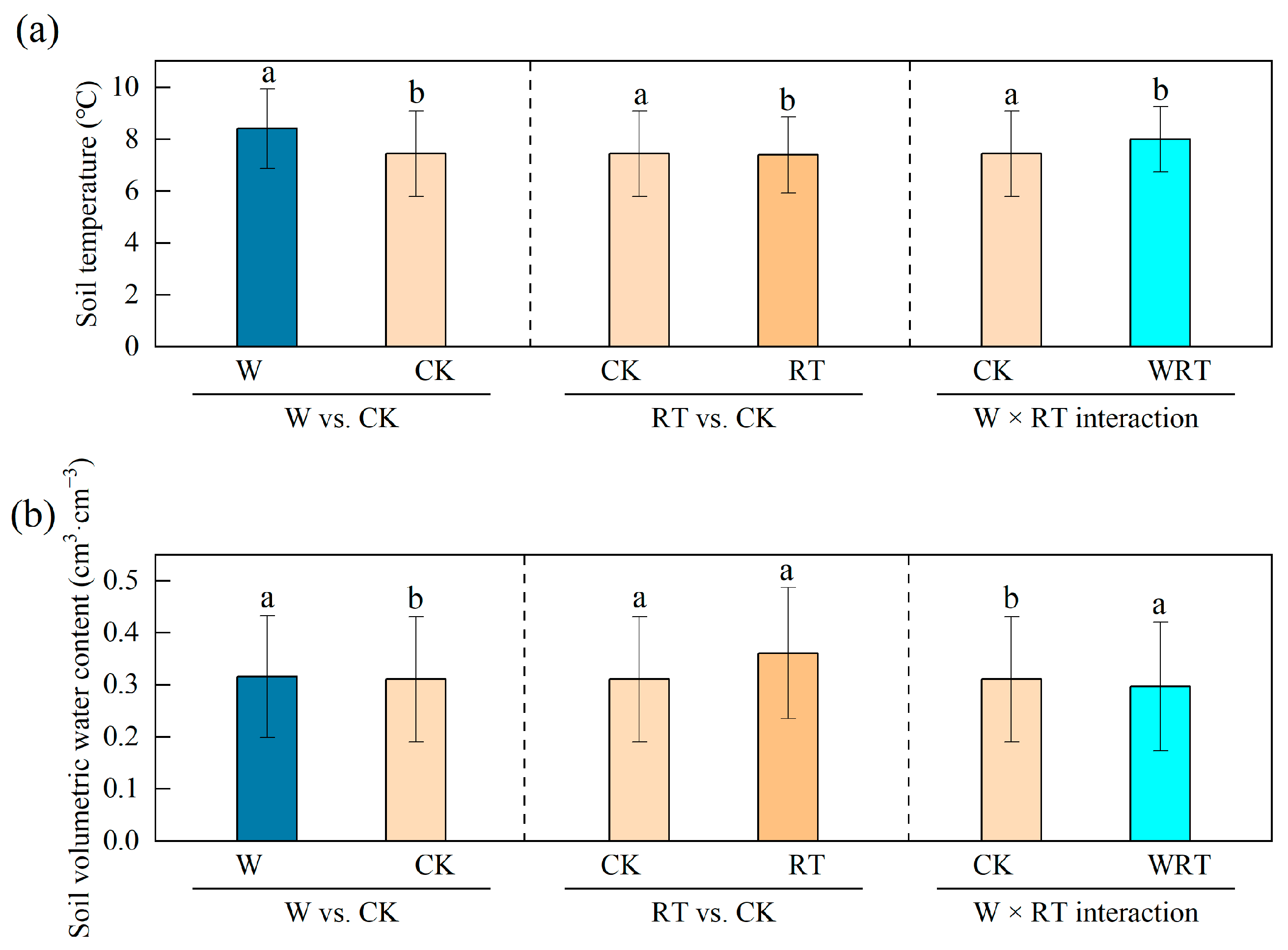
3.1. Variation in Soil Microclimate
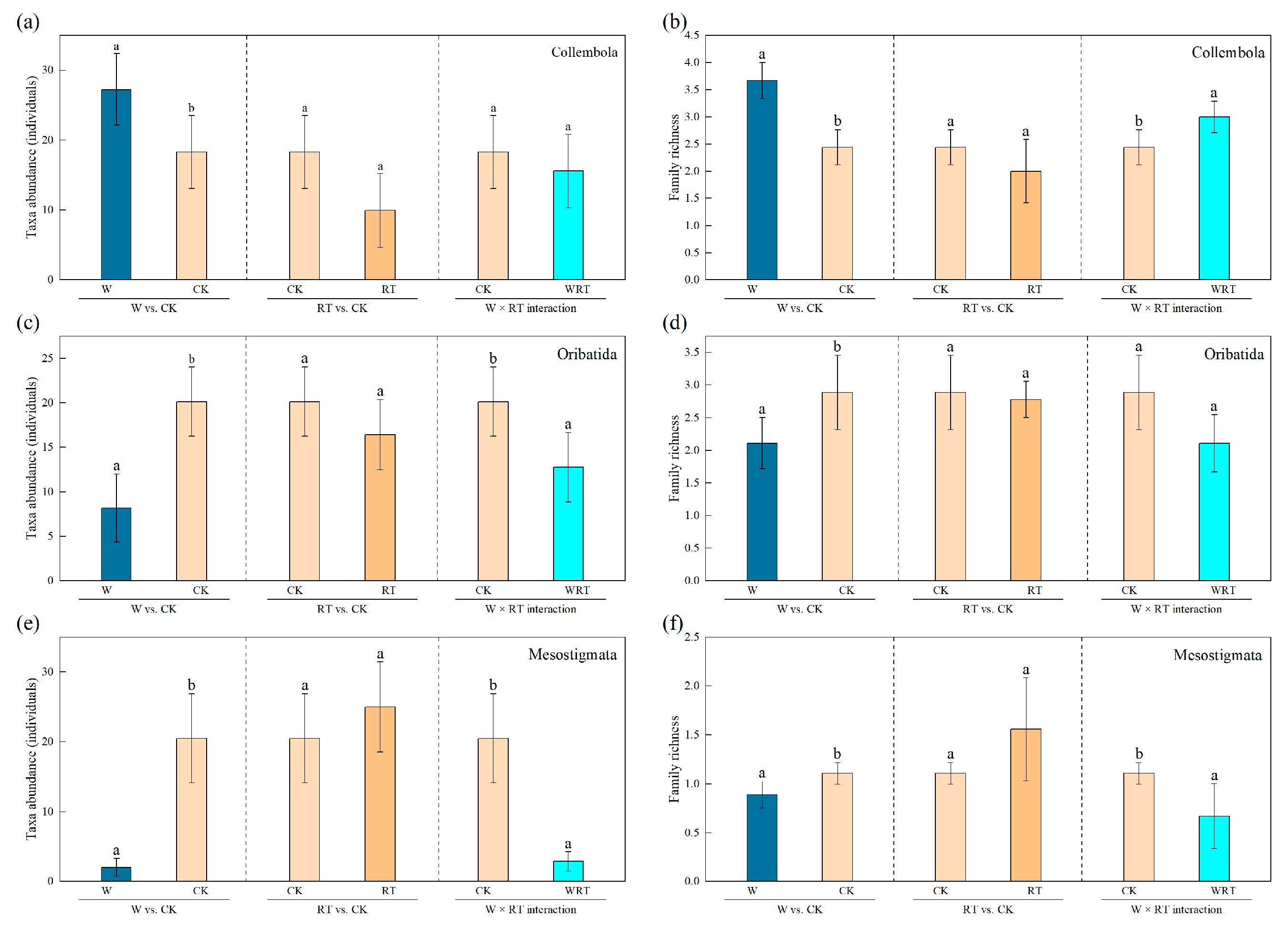
3.2. Taxa Abundance and Family Richness of Soil Microarthropods Under Warming and Root Trenching Treatments
3.3. Predominant Expression of Family of Soil Microarthropods Under Different Treatments
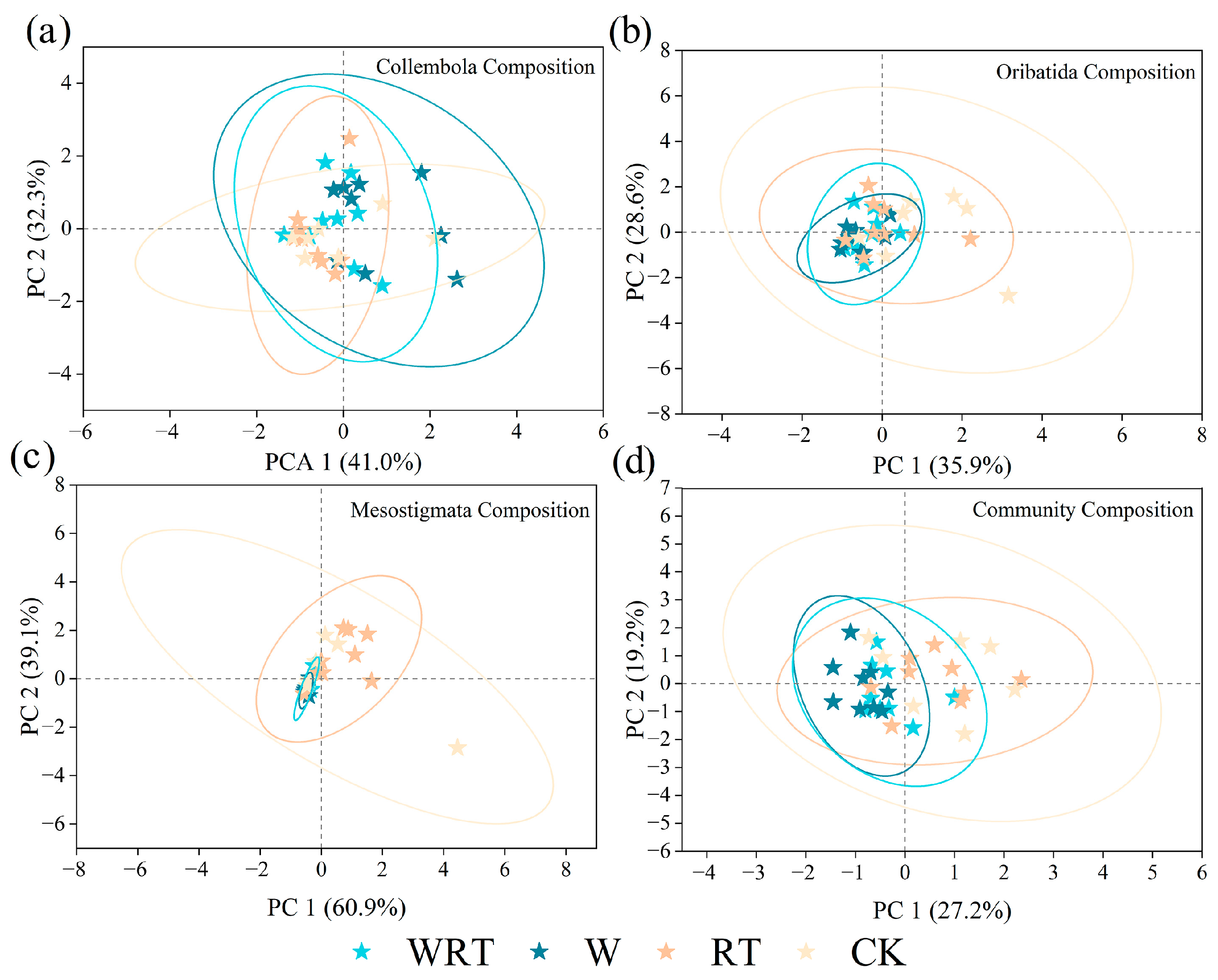
3.4. The Relationship Between Soil Microclimate and Soil Microarthropod Community
3.5. Contributions of Warming, Root Trenching and Soil Temperature to Soil Microarthropod Community
4. Discussion
4.1. Effects of Experimental Warming
4.2. Effects of Root Trenching
4.3. Effects of Experimental Warming and Root Trenching
4.4. Study Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | CK | W | RT | WRT |
|---|---|---|---|---|
| Soil temperature (°C) | 7.4 ± 1.6 | 8.4 ± 1.5 | 7.3 ± 1.5 | 8.0 ± 1.3 |
| Soil volumetric water content (cm3∙cm−3) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Groups | CK | W | RT | WRT | |
|---|---|---|---|---|---|
| Collembola | Onychiuridae | 7.4 ± 8.2 | 5.9 ± 3.0 | 1.4 ± 2.1 | 3.3 ± 2.2 |
| Neanuridae | 3.2 ± 5.1 | 6.7 ± 3.9 | 2.4 ± 4.9 | 4.6 ± 4.8 | |
| Isotomidae | 5.7 ± 7.4 | 12.9 ± 11.3 | 5.1 ± 5.5 | 6.9 ± 7.5 | |
| Tomoceridae | 1.7 ± 3.0 | 1.8 ± 1.8 | 1.0 ± 1.3 | 0.8 ± 0.7 | |
| Oribatida | Phthiracaridae | 4.2 ± 5.3 | 1.3 ± 1.9 | 2.1 ± 3.1 | 1.9 ± 1.8 |
| Oppiidae | 5.4 ± 4.6 | 1.7 ± 1.7 | 6.1 ± 3.2 | 5.6 ± 7.2 | |
| Nothridae | 7.2 ± 6.3 | 4.9 ± 3.1 | 6.9 ± 5.8 | 5.3 ± 6.0 | |
| Protoribatidae | 3.2 ± 2.4 | 0.3 ± 1.0 | 1.3 ± 1.5 | 0.0 ± 0.0 | |
| Mesostigmata | Digamasellidae | 16.4 ± 17.1 | 1.9 ± 2.5 | 20.8 ± 12.7 | 2.8 ± 4.8 |
| Ascidae | 4.0 ± 10.9 | 0.1 ± 0.3 | 4.2 ± 4.0 | 0.1 ± 0.3 | |
| Astigmata | Acaridae | 3.6 ± 5.4 | 1.9 ± 2.9 | 2.1 ± 4.9 | 0.7 ± 1.0 |
References
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Setala, H.; Huhta, V. Soil fauna increase Betula pendula growth: Laboratory experiments with coniferous forest floor. Ecology 1991, 72, 665–671. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.H.; Bradford, M.A.; John, M.G.S.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voigt, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Change Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef]
- George, P.B.L.; Keith, A.M.; Creer, S.; Barrett, G.L.; Lebron, I.; Emmett, B.A.; Robinson, D.A.; Jones, D.L. Evaluation of mesofauna communities as soil quality indicators in a national-level monitoring programme. Soil Biol. Biochem. 2017, 115, 537–546. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil health and arthropods: From complex system to worthwhile investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- David, J.F. The role of litter-feeding macroarthropods in decomposition processes: A reappraisal of common views. Soil Biol. Biochem. 2014, 76, 109–118. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Wang, B.; Yin, J.; Wu, F.; Wang, D.; Jiang, Z.; Song, X. Climate change did not alter the effects of Bt maize on soil Collembola in northeast China. Sci. Rep. 2022, 12, 13435. [Google Scholar] [CrossRef] [PubMed]
- Kærsgaard, C.W.; Holmstrup, M.; Malte, H.; Bayley, M. The importance of cuticular permeability, osmolyte production, and body size for the desiccation resistance of nine species of Collembola. J. Insect Physiol. 2004, 50, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Raschmanová, N.; Šustr, V.; Kováč, Ľ.; Parimuchová, A.; Devetter, M. Testing the climatic variability hypothesis in edaphic and subterranean Collembola (Hexapoda). J. Therm. Biol. 2018, 78, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.; Jones, T.H.; Bardgett, R.D.; Black, H.I.J.; Boag, B.; Bonkowski, M.; Cook, R.; Eggers, T.; Gange, A.; Grayston, S.J. Impacts of soil faunal community composition on model grassland ecosystems. Science 2002, 298, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, Y.; Ball, B.A.; Bradford, M.A.; Jordan, C.F.; Molina, M. Soil fauna alter the effects of litter composition on nitrogen cycling in a mineral soil. Soil Biol. Biochem. 2011, 43, 1440–1449. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Potential impacts of climate change on interactions among saprotrophic cord-forming fungal mycelia and grazing soil invertebrates. Fung. Ecol. 2014, 10, 34–43. [Google Scholar] [CrossRef]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Potapov, A.; Bellini, B.C.; Chown, S.L.; Deharveng, L.; Janssens, F.; Kováč, Ľ.; Kuznetsova, N.; Ponge, J.F.; Potapov, M.; Querner, P.; et al. Towards a global synthesis of Collembola knowledge: Challenges and potential solutions. Soil Org. 2020, 92, 161–188. [Google Scholar] [CrossRef]
- Rousseau, L.; Venier, L.; Hazlett, P.; Fleming, R.; Morris, D.; Handa, I.T. Forest floor mesofauna communities respond to a gradient of biomass removal and soil disturbance in a boreal jack pine (Pinus banksiana) stand of northeastern Ontario (Canada). For. Ecol. Manag. 2018, 407, 155–165. [Google Scholar] [CrossRef]
- Kardol, P.; Reynolds, W.N.; Norby, R.J.; Classen, A.T. Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 2011, 47, 37–44. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2023: Synthesis Report; Lee, H., Romero, J., Eds.; IPCC: Interlaken, Switzerland, 2023; p. 36. [Google Scholar]
- Campbell, J.L.; Rustad, L.E.; Lindsey, E.; Boyer, E.W.; Christopher, S.F.; Driscoll, C.T.; Fernandez, I.J.; Groffman, P.M.; Houle, D.; Kiekbusch, J.; et al. Consequences of climate change for biogeochemical cycling in forests of northeastern North America. Can. J. For. Res. 2009, 39, 264–284. [Google Scholar] [CrossRef]
- Stuble, K.L.; Ma, S.; Liang, J.; Luo, Y.; Classen, A.T.; Souza, L. Long-term impacts of warming drive decomposition and accelerate the turnover of labile, not recalcitrant, carbon. Ecosphere 2019, 10, e02715. [Google Scholar] [CrossRef]
- Pritchard, S.G. Soil organisms and global climate change. Plant Pathol. 2011, 60, 82–99. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Hartmann, M.; Simard, S.W.; Mohn, W.W. Long-term warming alters the composition of Arctic soil microbial communities. FEMS Microbiol. Ecol. 2012, 82, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Change 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Makoto, K.; Arai, M.; Kaneko, N. Change the menu? Species-dependent feeding responses of millipedes to climate warming and the consequences for plant–soil nitrogen dynamics. Soil Biol. Biochem. 2014, 72, 19–25. [Google Scholar] [CrossRef]
- David, J.F.; Gillon, D. Combined effects of elevated temperatures and reduced leaf litter quality on the life—history parameters of a saprophagous macroarthropod. Glob. Change Biol. 2009, 15, 156–165. [Google Scholar] [CrossRef]
- Wolters, V. Long-term dynamics of a collembolan community. Appl. Soil Ecol. 1998, 9, 221–227. [Google Scholar] [CrossRef]
- Jayawickreme, D.H.; Van Dam, R.L.; Hyndman, D.W. Subsurface imaging of vegetation, climate, and root-zone moisture interactions. Geophys. Res. Lett. 2008, 35, 10–26. [Google Scholar] [CrossRef]
- Sha, S.L.; Cai, G.C.; Liu, S.R.; Ahmed, M.A. Roots to the rescue: How plants harness hydraulic redistribution to survive drought across contrasting soil textures. Adv. Biotechnol. 2024, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.O.; Dirmeyer, P.A. Characterizing the relationship between temperature and soil moisture extremes and their role in the exacerbation of heat waves over the contiguous United States. J. Clim. 2021, 34, 2175–2187. [Google Scholar] [CrossRef]
- Denissen, J.M.C.; Teuling, A.J.; Koirala, S.; Reichstein, M.; Balsamo, B.; Vogel, M.M.; Yu, X.; Orth, R. Intensified future heat extremes linked with increasing ecosystem water limitation. Earth Syst. Dynam. 2024, 15, 717–734. [Google Scholar] [CrossRef]
- Haldar, S.; Sengupta, S. Plant-microbe cross-talk in the rhizosphere: Insight and biotechnological potential. Open Microbiol. J. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, W.; Whalley, W.R.; Ren, T. Physical properties of a sandy soil as affected by incubation with a synthetic root exudate: Strength, thermal and hydraulic conductivity, and evaporation. Eur. J. Soil Sci. 2021, 72, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G. Water dynamics of the root zone: Rhizosphere biophysics and its control on soil hydrology. Vadose Zone J. 2012, 11, vzj2011.0111. [Google Scholar] [CrossRef]
- Galindo-Castañeda, T.; Lynch, J.P.; Six, J.; Hartmann, M. Improving soil resource uptake by plants through capitalizing on synergies between root architecture and anatomy and root-associated microorganisms. Front. Plant Sci. 2022, 13, 827369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, J.; He, X.; Liu, T.; Shao, Y.; Zhang, C.; Zhou, Q.; Li, F.; Mao, P.; Tao, L.; et al. Plant leaf litter plays a more important role than roots in maintaining earthworm communities in subtropical plantations. Soil Biol. Biochem. 2020, 144, 107777. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.Z.; Preiser, J.; Widyastuti, R.; Scheu, S.; Potapov, A. Plant roots fuel tropical soil animal communities. Ecol. Lett. 2023, 26, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Zuev, A.G.; Potapov, M.B.; Tiunov, A.V.; Saraeva, A.K. Root trenching and stable isotope analysis uncover trophic links of edaphic collembola species to mycorrhizal mycelium in pine forests. Eur. J. Soil Biol. 2023, 118, 103519. [Google Scholar] [CrossRef]
- Hart, S.C.; Sollins, P. Soil carbon and nitrogen pools and processes in an old-growth conifer forest 13 years after trenching. Can. J. For. Res. 1998, 28, 1261–1265. [Google Scholar] [CrossRef][Green Version]
- Noh, N.J.; Chung, H.; Ryu, S.R.; Son, Y.; Lee, S.K.; Yoon, T.K.; Yang, A.; Kim, J.R. Changes in soil properties of Abies holophylla and Quercus-dominated stands 4 years after trenching. Scand. J. For. Res. 2012, 27, 597–604. [Google Scholar] [CrossRef]
- Siira-Pietikäinen, A.; Haimi, J.; Fritze, H. Organisms, decomposition, and growth of pine seedlings in boreal forest soil affected by sod cutting and trenching. Biol. Fertil. Soils 2002, 37, 163–174. [Google Scholar] [CrossRef]
- Mei-Yee, C.H.I.N.; Lau, S.Y.L.; Midot, F.; Jee, M.S.; Lo, M.L.; Sangok, F.E.; Melling, L. Root exclusion methods for partitioning of soil respiration: Review and methodological considerations. Pedosphere 2023, 33, 683–699. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Impacts of root competition in forests and woodlands: A theoretical framework and review of experiments. Ecol. Mono. 2000, 70, 171–207. [Google Scholar] [CrossRef]
- Petriţan, I.C.; von Lüpke, B.; Petriţan, A.M. Effects of root trenching of overstorey Norway spruce (Picea abies) on growth and biomass of underplanted beech (Fagus sylvatica) and Douglas fir (Pseudotsuga menziesii) saplings. Eur. J. For. Res. 2011, 130, 813–828. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Edwards, D.P.; Diesmos, A.; Williams, S.E.; Evans, T.A. Microhabitats reduce animals’ exposure to climate extremes. Glob. Change Biol. 2014, 20, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Noordwijk, M.V.; Martikainen, P.; Bottner, P.; Cuevas, E.; Rouland, C.; Dhillion, S.S. Global change and root function. Glob. Change Biol. 1998, 4, 759–772. [Google Scholar] [CrossRef]
- Sun, H.; Dai, E.; Li, Y.; Xi, W. Climate change and sustainable forestry: A regional perspective from northeast China. For. Chron. 2018, 94, 201–207. [Google Scholar]
- Peng, J.; Dong, W.; Yuan, W.; Zhang, Y. Responses of grassland and forest to temperature and precipitation changes in Northeast China. Adv. Atmos. Sci. 2012, 29, 1063–1077. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhao, C.Y.; Jia, Q.Y. Impacts of climate change on forest ecosystems in Northeast China. Adv. Clim. Change Res. 2013, 4, 230–241. [Google Scholar] [CrossRef]
- Huang, Y.; Cong, R.Z.; Zhang, J.L.; Wang, X.H. Soil microbial community structure and influencing factors of typical forest types in Sanjiang plain. J. Cent. South Univ. Technol. 2023, 43, 129–140. [Google Scholar]
- Barberis, I.M.; Tanner, E.V.J. Gaps and root trenching increase tree seedling growth in Panamanian semi-evergreen forest. Ecology 2005, 86, 667–674. [Google Scholar] [CrossRef]
- Hagerman, S.M.; Jones, M.D.; Bradfield, G.E.; Gillespie, M.; Durall, D.M. Effects of clear-cut logging on the diversity and persistence of ectomycorrhizae at a subalpine forest. Can. J. For. Res. 1999, 29, 124–134. [Google Scholar] [CrossRef]
- Bluhm, S.L.; Eitzinger, B.; Bluhm, C. The impact of root-derived resources on forest soil invertebrates depends on body size and trophic position. Front. For. Glob. Change 2021, 4, 622370. [Google Scholar] [CrossRef]
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998; pp. 163–592. [Google Scholar]
- Hopkin, S.P. A Key to the Collembola (Springtails) of Britain and Ireland; Field Studies Council Press: Shrewsbury, UK, 2007. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World; United States Department of Agriculture Research Service Press: Beltsville, MD, USA, 2012.
- Ryabinin, N.A.; Liu, D.; Gao, M.X.; Wu, D.H. Checklist of oribatid mites (Acari, Oribatida) of the Russian Far East and North-East of China. Zootaxa 2018, 4472, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wang, B.; Yan, X.; Ma, L.; Reddy, G.V.; Wu, D. Warming limits daytime but not nighttime activity of epigeic microarthropods in Songnen grasslands. Appl. Soil Ecol. 2019, 141, 79–83. [Google Scholar] [CrossRef]
- Chown, S.L.; Janion-Scheepers, C.; Marshall, A.; Aitkenhead, I.J.; Hallas, R.; Liu, W.A.; Phillips, L.M. Indigenous and introduced Collembola differ in desiccation resistance but not its plasticity in response to temperature. Curr. Res. Insect Sci. 2023, 3, 100051. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Holmstrup, M.; Ehlers, B.K.; Slotsbo, S.; Ilieva-Makulec, K.; Sigurdsson, B.D.; Leblans, N.I.; Ellers, J.; Berg, M.P. Functional diversity of Collembola is reduced in soils subjected to short-term, but not long-term, geothermal warming. Funct. Ecol. 2018, 32, 1304–1316. [Google Scholar] [CrossRef]
- Coulson, S.J.; Hodkinson, I.D.; Wooley, C.; Webb, N.R.; Block, W.; Worland, M.R.; Bale, J.S.; Strathdee, A.T. Effects of experimental temperature elevation on high-arctic soil microarthropod populations. Pola. Biol. 1996, 16, 147–153. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.; Convey, P.; Van Bodegom, P.M.; Aerts, R. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem. 2008, 40, 1547–1556. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Ostle, N.J.; McNamara, N.P.; Poskitt, J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 2009, 41, 315–322. [Google Scholar] [CrossRef]
- Kamath, D.; Barreto, C.; Lindo, Z. Nematode contributions to the soil food web trophic structure of two contrasting boreal peatlands in Canada. Pedobiologia 2022, 93, 150809. [Google Scholar] [CrossRef]
- Sylvain, Z.A.; Wall, D.H.; Cherwin, K.L.; Debra, P.C.; Peters, D.P.C.; Reichmann, L.G.; Sala, O.E. Soil animal responses to moisture availability are large-scale, not ecosystem dependent: Insight from a cross-site study. Glob. Change Biol. 2014, 20, 2631–2643. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, J. Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Glob. Change Biol. 2007, 13, 123–131. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Hendry, D.T.; Murdiyarso, D.; Verchot, L.V. Total and heterotrophic soil respiration in a swamp forest and oil palm plantations on peat in Central Kalimantan, Indonesia. Biogeochemistry 2017, 135, 203–220. [Google Scholar] [CrossRef]
- Greiser, C.; Hederová, L.; Vico, G.; Wild, J.; Macek, M.; Kopecký, M. Higher soil moisture increases microclimate temperature buffering in temperate broadleaf forests. Agric. For. Meteorol. 2024, 345, 109828. [Google Scholar] [CrossRef]
- Feng, W.; Zou, X.; Schaefer, D. Above-and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biol. Biochem. 2009, 41, 978–983. [Google Scholar] [CrossRef]
- Khvorostyanov, D.V.; Krinner, G.; Ciais, P.; Heimann, M.; Zimov, S.A. Vulnerability of Permafrost Carbon to Global Warming. Part I: Model Description and Role of Heat Generated by Organic Matter Decomposition. In Tellus B: Chemical and Physical Meteorology; Taylor & Francis Group Press: London, UK, 2008; Volume 60, pp. 250–264. [Google Scholar]
- Xiao, T.; Li, P.; Fei, W.B.; Wang, J.D. Effects of vegetation roots on the structure and hydraulic properties of soils: A perspective review. Sci. Total Environ. 2024, 906, 167524. [Google Scholar] [CrossRef] [PubMed]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
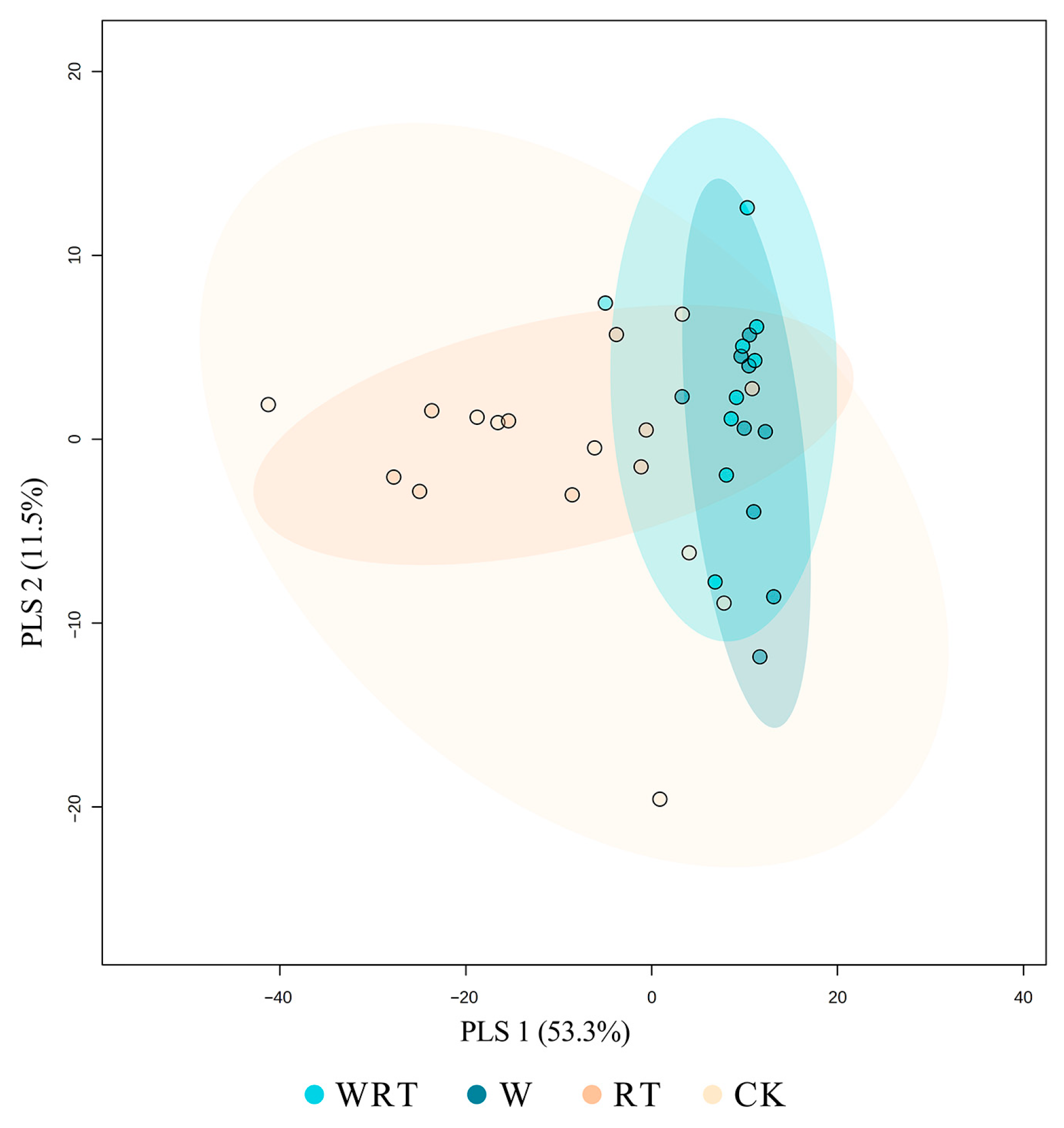
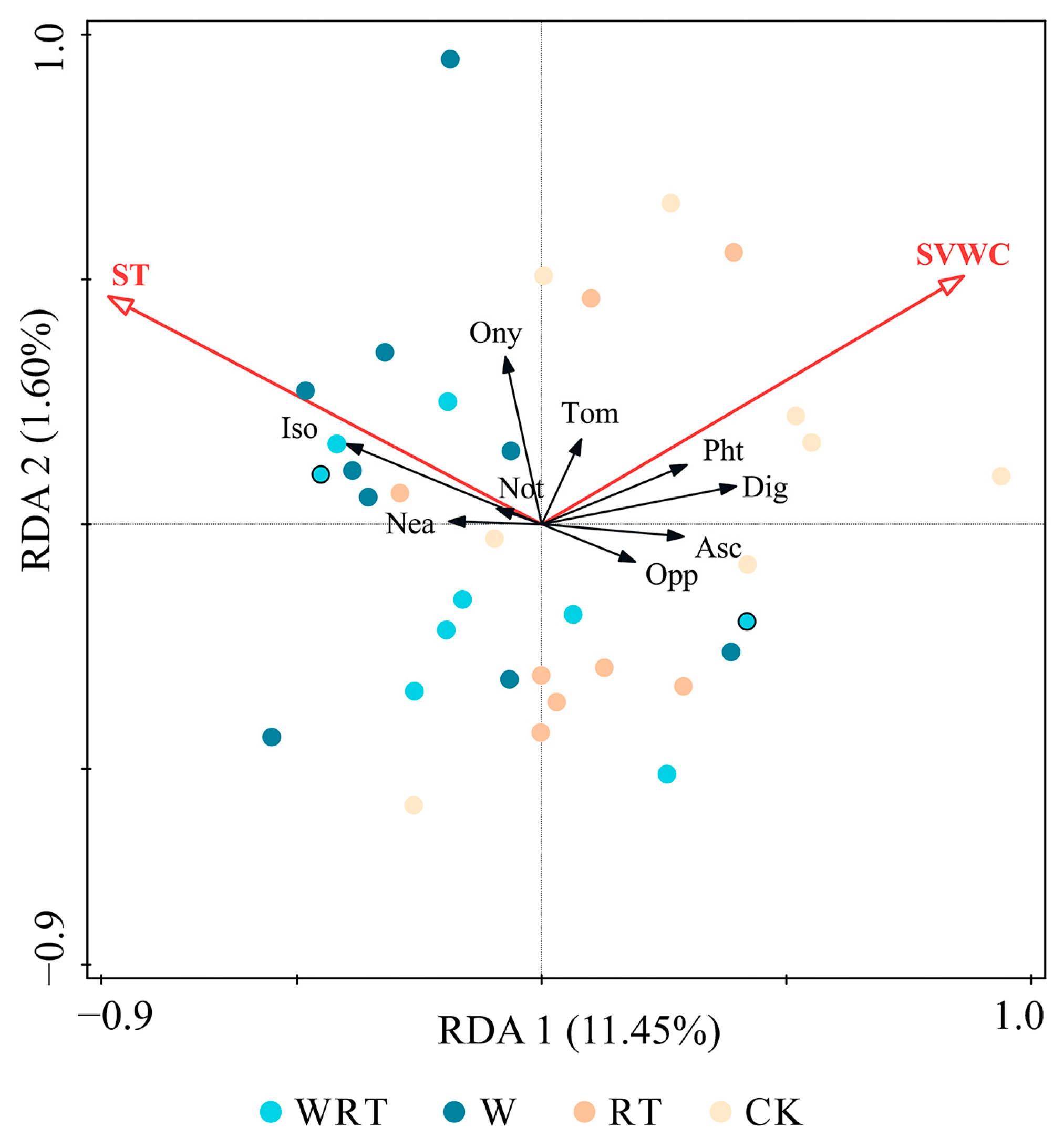
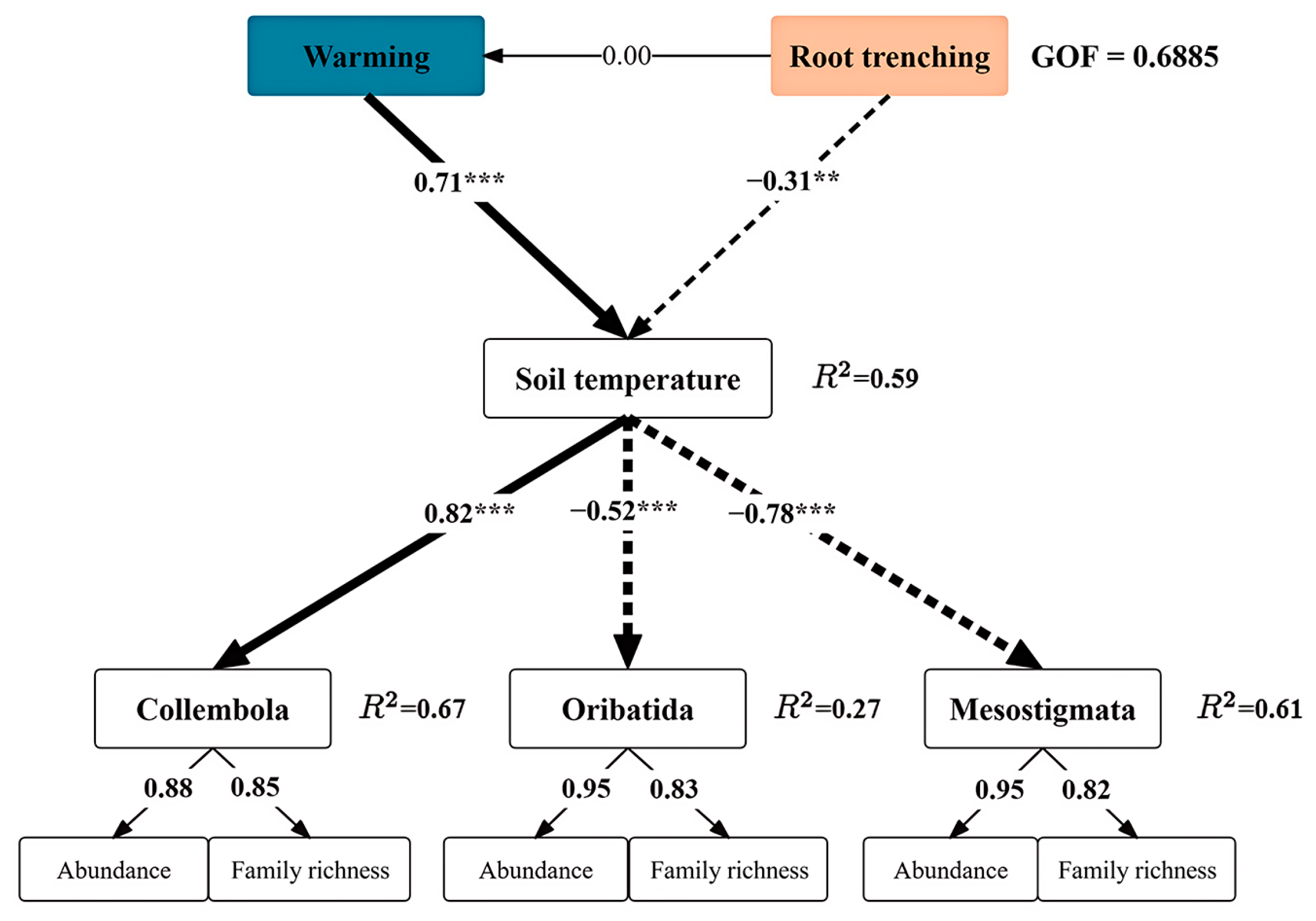
| Variable | Soil Temperature | Soil Volumetric Water Content | |||
|---|---|---|---|---|---|
| df | F Value | p | F Value | p | |
| W | 1, 33 | 48.679 | 0.000 | 11.616 | 0.002 |
| RT | 1, 33 | 10.882 | 0.002 | 0.008 | 0.930 |
| W × RT | 3, 32 | 19.502 | 0.000 | 4.855 | 0.007 |
| Variable | Collembola | Oribatida | Mesostigmata | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa Abundance | Family Richness | Taxa Abundance | Family Richness | Taxa Abundance | Family Richness | ||||||||
| df | F Value | p | F Value | p | F Value | p | F Value | p | F Value | p | F Value | p | |
| W | 1, 33 | 5.606 | 0.024 | 14.233 | 0.001 | 7.559 | 0.010 | 4.638 | 0.039 | 51.950 | 0.000 | 11.861 | 0.002 |
| RT | 1, 33 | 2.899 | 0.098 | 2.844 | 0.101 | 0.233 | 0.632 | 0.024 | 0.878 | 1.945 | 0.172 | 2.274 | 0.141 |
| W × RT | 3, 32 | 2.833 | 0.054 | 5.521 | 0.004 | 3.194 | 0.037 | 1.554 | 0.220 | 17.334 | 0.000 | 6.639 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.; Wang, J.; Cui, R.; Wang, Q.; Zhang, J. Living Root-Mediated Soil Temperature Amplifies the Effects of Experimental Warming on Soil Microarthropod Communities in a Quercus mongolica Forest in Northeast China. Insects 2025, 16, 809. https://doi.org/10.3390/insects16080809
Chi C, Wang J, Cui R, Wang Q, Zhang J. Living Root-Mediated Soil Temperature Amplifies the Effects of Experimental Warming on Soil Microarthropod Communities in a Quercus mongolica Forest in Northeast China. Insects. 2025; 16(8):809. https://doi.org/10.3390/insects16080809
Chicago/Turabian StyleChi, Chenglin, Jiannan Wang, Rong Cui, Qianxue Wang, and Jili Zhang. 2025. "Living Root-Mediated Soil Temperature Amplifies the Effects of Experimental Warming on Soil Microarthropod Communities in a Quercus mongolica Forest in Northeast China" Insects 16, no. 8: 809. https://doi.org/10.3390/insects16080809
APA StyleChi, C., Wang, J., Cui, R., Wang, Q., & Zhang, J. (2025). Living Root-Mediated Soil Temperature Amplifies the Effects of Experimental Warming on Soil Microarthropod Communities in a Quercus mongolica Forest in Northeast China. Insects, 16(8), 809. https://doi.org/10.3390/insects16080809





