Maturation of Eupyrene Sperm upon Ejaculation Is Influenced by a Male Accessory Gland-Derived Serine Protease in Grapholita molesta
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Cloning of GmAGSP1 Based on Quantitative Proteomic Data
2.3. qRT-PCR
2.4. RNA Interference
2.5. Behavioral and Fertility Assay
2.6. Determination of Eupyrene Sperm Morphology and Viability
2.7. Metabolome Analysis
2.8. Data Analysis
3. Results
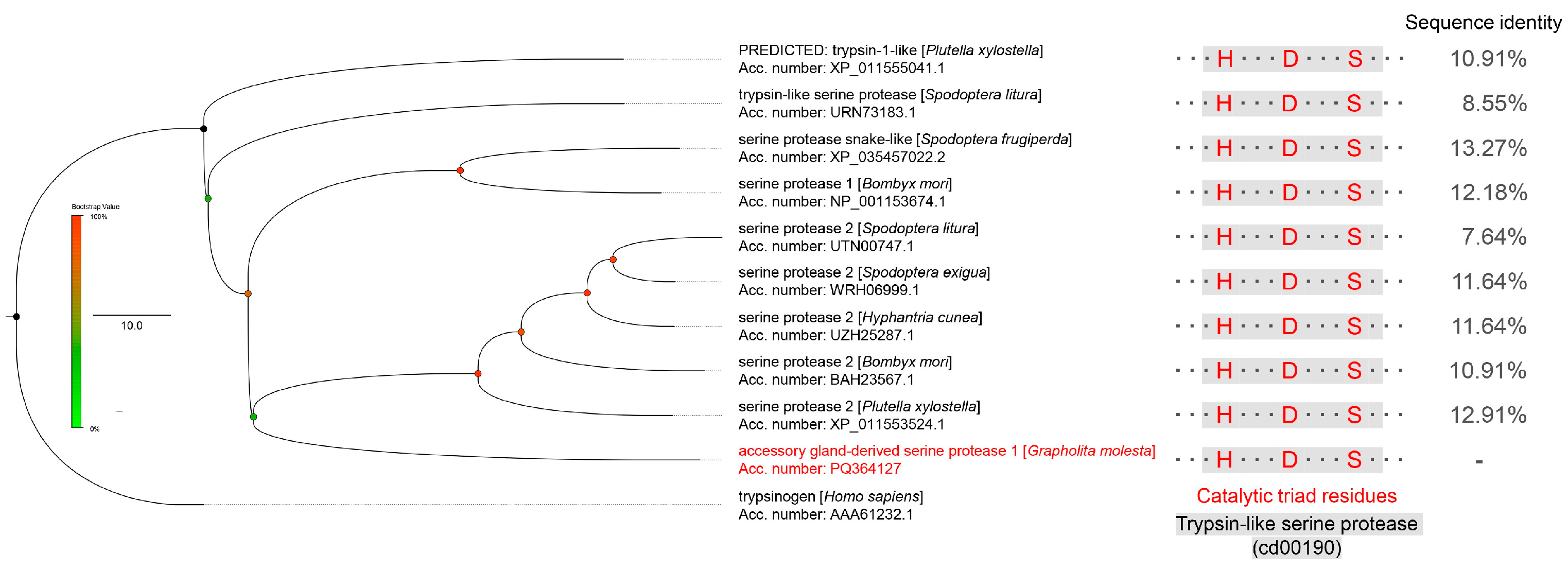
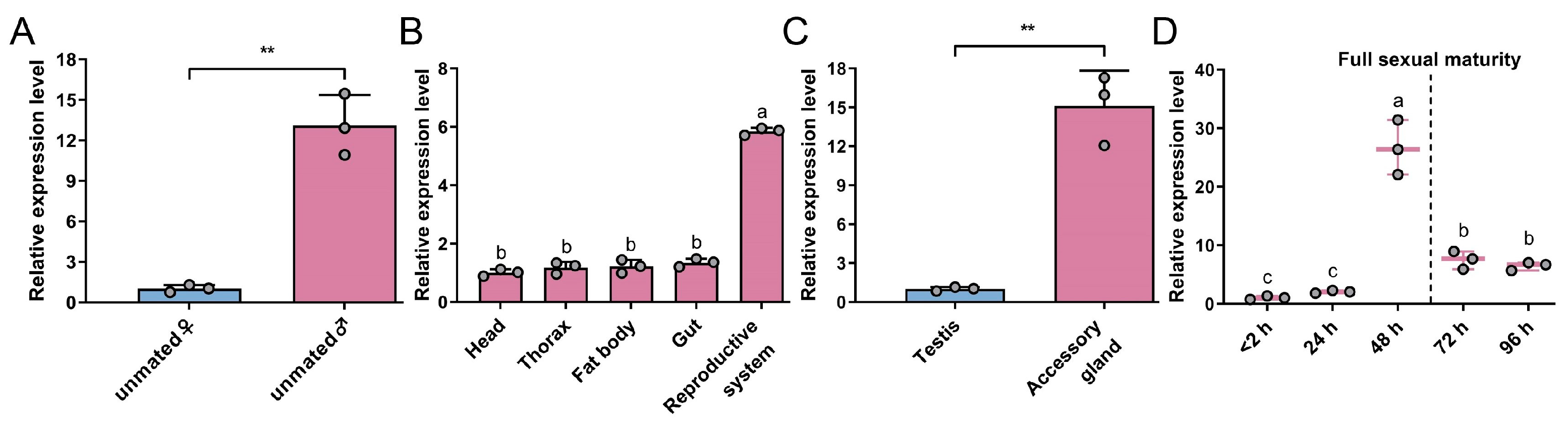
3.1. Characterization of GmAGSP1
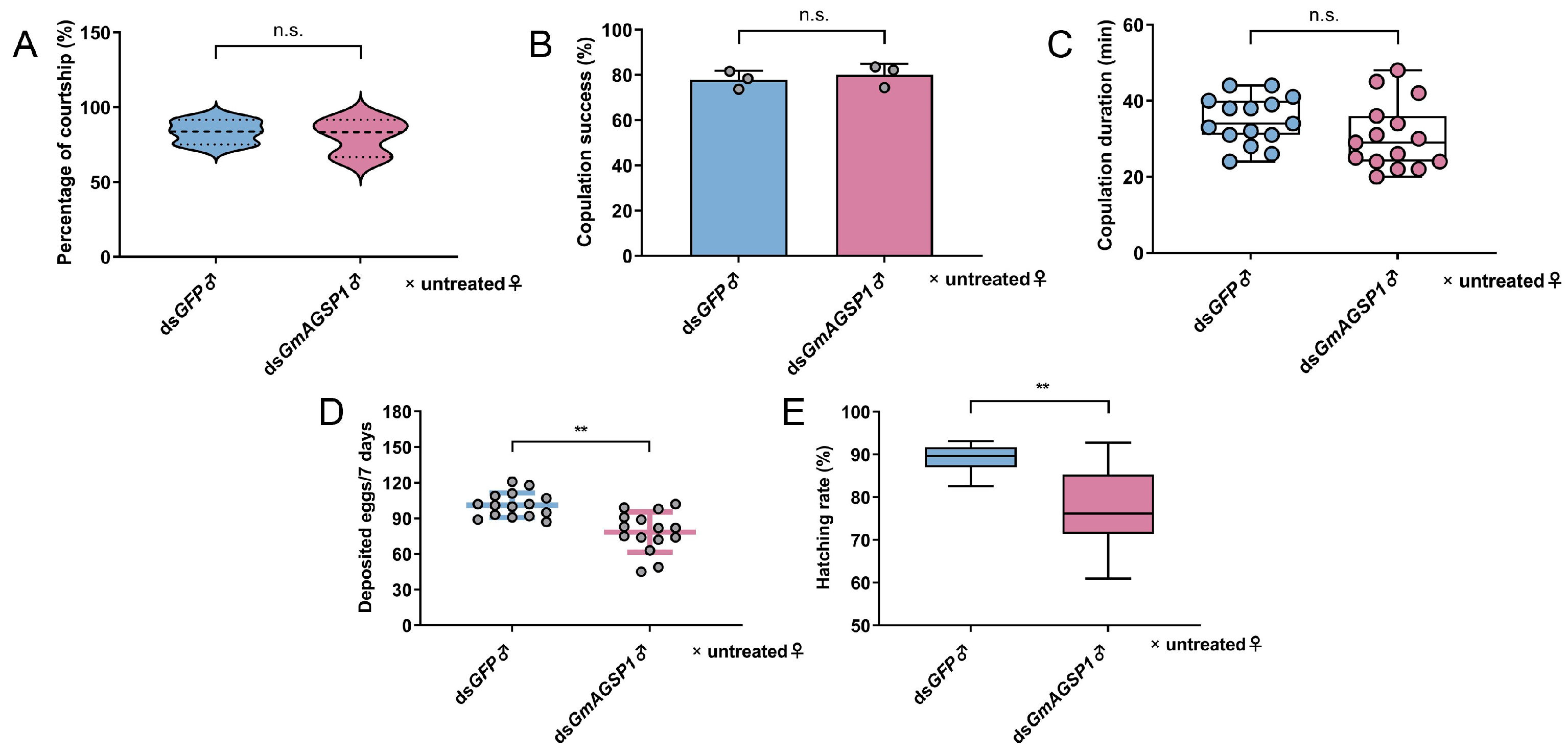
3.2. GmAGSP1 Affects Male Fertility Without Influencing Sexual Behavior
3.3. GmAGSP1 Promotes Eupyrene Sperm Maturation in the Spermatophore
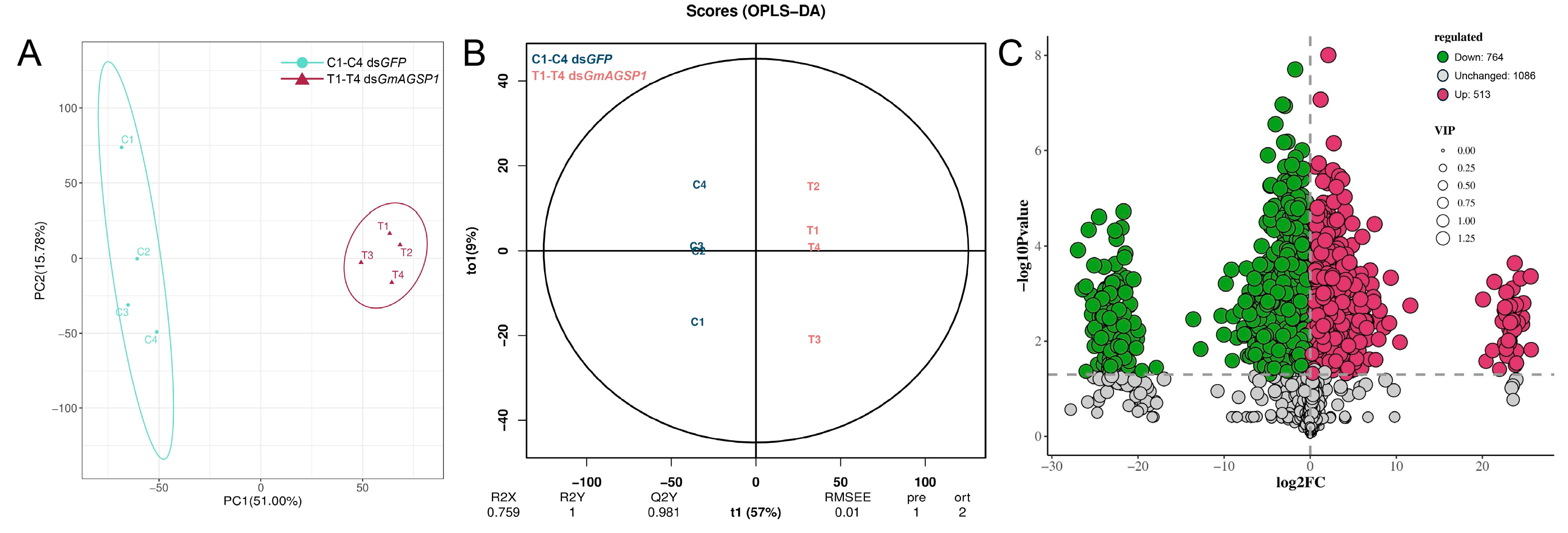
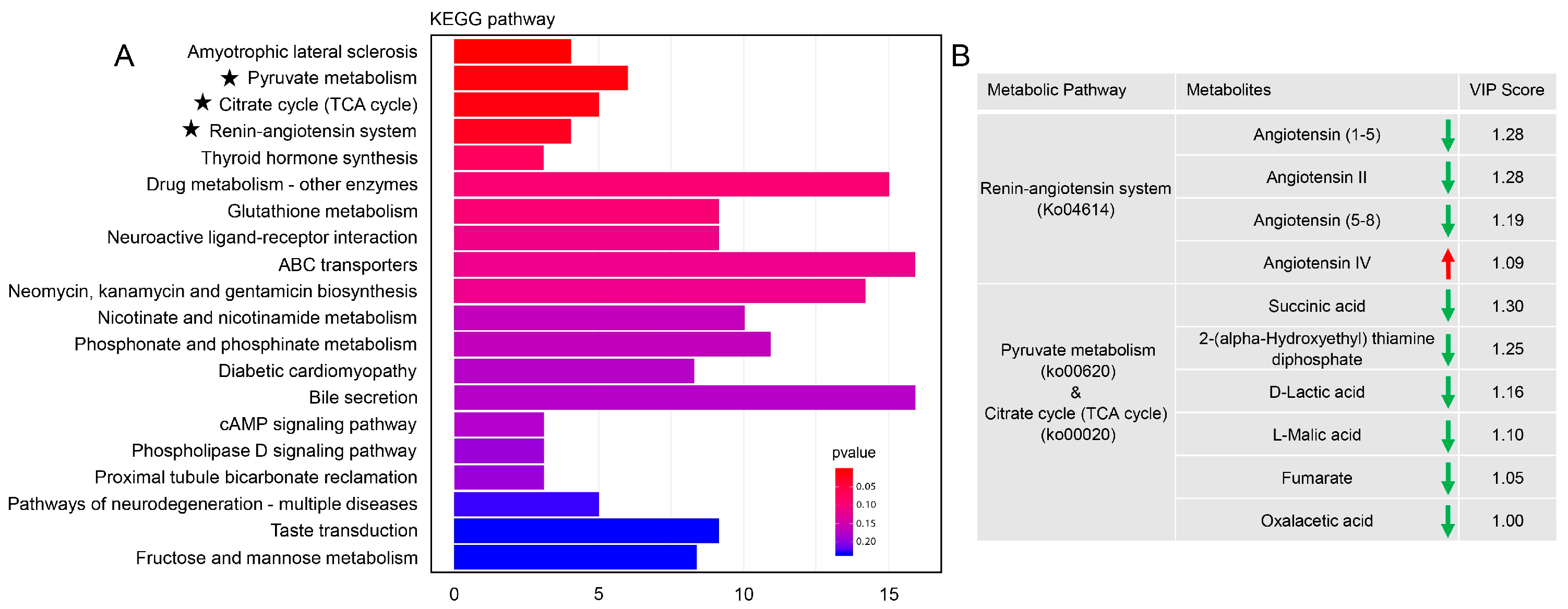
3.4. Metabolomic Analysis in the Spermatophore of Females upon GmAGSP1 Knockdown
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, X.L.; He, Y.; Hu, Y.X.; Zhang, X.F.; Wang, Y.; Zou, Z.; Chen, Y.R.; Blissard, G.W.; Kanost, M.R.; Jiang, H.B. Sequence conservation, phylogenetic relationships, and expression profiles of nondigestive serine proteases and serine protease homologs in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 51–63. [Google Scholar] [CrossRef]
- Lin, H.L.; Xia, X.F.; Yu, L.Y.; Vasseur, L.; Gurr, G.M.; Yao, F.L.; Yang, G.; You, M.S. Genome-wide identification and expression profiling of serine proteases and homologs in the diamondback moth, Plutella xylostella (L.). BMC Genom. 2015, 16, 1054. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- Yu, B.; Li, D.T.; Lu, J.B.; Zhang, W.X.; Zhang, C.X. Seminal fluid protein genes of the brown planthopper, Nilaparvata lugens. BMC Genom. 2016, 17, 654. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Li, F.; Song, Y.; Zhang, Z.F.; Fan, Y.L.; Liu, T.X. Proteome analysis of male accessory gland secretions in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Insects 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.H.; Bi, H.L.; Xu, J.; Liu, Z.L.; Niu, C.Y.; He, L.; James, A.A.; Li, K.; Huang, Y.P. Mutation of the seminal protease gene, serine protease 2, results in male sterility in diverse lepidopterans. Insect Biochem. Mol. Biol. 2020, 116, 103243. [Google Scholar] [CrossRef]
- Bi, H.L.; Xu, X.; Li, X.W.; Wang, Y.H.; Zhou, S.T.; Huang, Y.P. CRISPR/Cas9-mediated Serine protease 2 disruption induces male sterility in Spodoptera litura. Front. Physiol. 2022, 13, 931824. [Google Scholar] [CrossRef]
- Zou, P.; Wu, L.Y.; Wen, S.; Pei, Y.K.; Hu, Z.N.; Zuo, Y.Y. Disruption of Spodoptera exigua serine protease 2 (Ser2) results in male sterility by CRISPR/Cas9 technology. Pest Manag. Sci. 2025, 81, 498–506. [Google Scholar] [CrossRef]
- Li, X.W.; Liu, Q.; Bi, H.L.; Wang, Y.H.; Xu, X.; Sun, W.; Zhang, Z.; Huang, Y.P. piggyBac-based transgenic RNAi of serine protease 2 results in male sterility in Hyphantria cunea. Insect Biochem. Mol. Biol. 2022, 143, 103726. [Google Scholar] [CrossRef]
- Omura, S. Artificial insemination of Bombyx mori. J. Frac. Agric. Hokkaido Imp. Univ. 1936, 38, 135–150. [Google Scholar]
- Nagaoka, S.; Kato, K.; Takata, Y.; Kamei, K. Identification of the sperm-activating factor initiatorin, a prostatic endopeptidase of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2012, 42, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.; Jeshtadi, A.; Reynolds, S.E. The structural mechanism of trypsin-induced intrinsic motility in Manduca sexta spermatozoa in vitro. J. Insect Physiol. 2001, 47, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.S.; Yang, X.; Xu, X.M.; Yang, D.H.; Zhu, C.X.; Yi, M.Y.; Bi, H.L.; Wang, Y.H.; Huang, Y.P. SPSL1 is essential for spermatophore formation and sperm activation in Spodoptera frugiperda. PLoS Genet. 2023, 19, e1011073. [Google Scholar] [CrossRef]
- Yadav, P.; Seth, R.K.; Reynolds, S.E. A sperm-activating trypsin-like protease from the male reproductive tract of Spodoptera litura: Proteomic identification, sequence characterization, gene expression profile, RNAi and the effects of ionizing radiation. J. Insect Physiol. 2024, 156, 104664. [Google Scholar] [CrossRef]
- Osanai, M.; Isono, M. Dissociation of eusperm bundles by acids, especially by succinate accumulated in the spermatophore of the silkmoth, Bombyx mori. Invertebr. Reprod. Dev. 1997, 31, 99–108. [Google Scholar] [CrossRef]
- Al-Wathiqui, N.; Fallon, T.R.; South, A.; Weng, J.K.; Lewis, S.M. Molecular characterization of firefly nuptial gifts: A multi-omics approach sheds light on postcopulatory sexual selection. Sci. Rep. 2016, 6, 38556. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, P.; Zhu, L.; Zhu, F.; Tian, Z.Q.; Shen, Z.J.; Liu, X.M.; Liu, X.X. Corazonin signaling modulates the synthetic activity of male accessory gland in Grapholita molesta. Int. J. Biol. Macromol. 2022, 216, 446–455. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, L.; Zhu, F.; Zhao, P.; Li, Q.X.; Lu, Z.H.; Zhang, S.D.; Li, Z.; Liu, X.X. Peroxiredoxin 1 transfer during mating protects eupyrene sperm against oxdative stress in Grapholita molesta. Pest Manag. Sci. 2023, 79, 2823–2830. [Google Scholar] [CrossRef]
- Seth, R.K.; Yadav, P.; Reynolds, S.E. Dichotomous sperm in Lepidopteran insects: A biorational target for pest management. Front. Insect Sci. 2023, 3, 1198252. [Google Scholar] [CrossRef]
- Lyu, Z.H.; Chen, J.X.; Lyu, J.; Guo, P.P.; Liu, J.H.; Liu, J.H.; Zhang, W.Q. Spraying double-stranded RNA targets UDP-N-acetylglucosamine pyrophosphorylase in the control of Nilaparvata lugens. Int. J. Biol. Macromol. 2024, 271, 132455. [Google Scholar] [CrossRef] [PubMed]
- Karr, T.L.; Walters, J.R. Panning for sperm gold: Isolation and purification of apyrene and eupyrene sperm from lepidopterans. Insect Biochem. Mol. Biol. 2015, 63, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Shi, J.L.; Hu, Y.Y.; Su, Y.C.; Zhang, Y.H.; Wu, X.B. Flumethrin exposure perturbs gut microbiota structure and intestinal metabolism in honeybees (Apis mellifera). J. Hazard. Mater. 2024, 480, 135886. [Google Scholar] [CrossRef]
- Laflamme, B.A.; Ram, K.R.; Wolfner, M.F. The Drosophila melanogaster seminal fluid protease “Seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet. 2012, 8, e1002435. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.; Cardullo, R.A.; Thaler, C.D. Culex pipiens sperm motility is initiated by a trypsin-like protease from male accessory glands. Mol. Reprod. Devel. 2018, 85, 440–448. [Google Scholar] [CrossRef]
- Marshall, J.L.; Huestis, D.L.; Hiromasa, Y.; Wheeler, S.; Oppert, C.; Marshall, S.A.; Tomich, J.M.; Oppert, B. Identification, RNAi knockdown, and functional analysis of an ejaculate protein that mediates a postmating, prezygotic phenotype in a cricket. PLoS ONE 2009, 4, e7537. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Travers, R.J.; Morrisey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef]
- Osanai, M.; Aigaki, T.; Kasuga, H. Energy metabolism in the spermatophore of the silkmoth, Bombyx mori, associated with accumulation of alanine derived from arginine. Insect Biochem. 1987, 17, 71–75. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Gianzo, M.; Subirán, N. Regulation of male fertility by the Renin-Angiotensin System. Int. J. Mol. Sci. 2020, 21, 7943. [Google Scholar] [CrossRef]
- Kohn, F.M.; Muller, C.; Drescher, D.; Neukamm, C.; el Mulla, K.F.; Henkel, R.; Hagele, W.; Hinsch, E.; Habenicht, U.F.; Schill, W.B. Effect of angiotensin converting enzyme (ACE) and angiotensins on human sperm functions. Andrologia 1998, 30, 207–215. [Google Scholar] [CrossRef]
- Vinson, G.P.; Mehta, J.; Evans, S.; Matthews, S.; Puddefoot, J.R.; Saridogan, E.; Holt, W.V.; Djahanbakhch, O. Angiotensin II stimulates sperm motility. Regul. Pept. 1996, 67, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Kawasaki, S.; Kawasaki, H.; Kamei, K. The angiotensin converting enzyme (ACE) inhibitor, captopril disrupts the motility activation of sperm from the silkworm, Bombyx mori. J. Insect Physiol. 2017, 103, 18–28. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Guo, T.; Zhou, Z.; Wei, W.; Liang, Y.; Xiang, H.; Ma, R.; Shen, Z.; Zhao, Z.-G. Maturation of Eupyrene Sperm upon Ejaculation Is Influenced by a Male Accessory Gland-Derived Serine Protease in Grapholita molesta. Insects 2025, 16, 782. https://doi.org/10.3390/insects16080782
Cheng J, Guo T, Zhou Z, Wei W, Liang Y, Xiang H, Ma R, Shen Z, Zhao Z-G. Maturation of Eupyrene Sperm upon Ejaculation Is Influenced by a Male Accessory Gland-Derived Serine Protease in Grapholita molesta. Insects. 2025; 16(8):782. https://doi.org/10.3390/insects16080782
Chicago/Turabian StyleCheng, Jie, Tai Guo, Zhongyan Zhou, Wei Wei, Yu Liang, Huiming Xiang, Ruiyan Ma, Zhongjian Shen, and Zhi-Guo Zhao. 2025. "Maturation of Eupyrene Sperm upon Ejaculation Is Influenced by a Male Accessory Gland-Derived Serine Protease in Grapholita molesta" Insects 16, no. 8: 782. https://doi.org/10.3390/insects16080782
APA StyleCheng, J., Guo, T., Zhou, Z., Wei, W., Liang, Y., Xiang, H., Ma, R., Shen, Z., & Zhao, Z.-G. (2025). Maturation of Eupyrene Sperm upon Ejaculation Is Influenced by a Male Accessory Gland-Derived Serine Protease in Grapholita molesta. Insects, 16(8), 782. https://doi.org/10.3390/insects16080782






