Edge Effects on the Spatial Distribution and Diversity of Drosophilidae (Diptera) Assemblages in Deciduous Forests of Central European Russia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
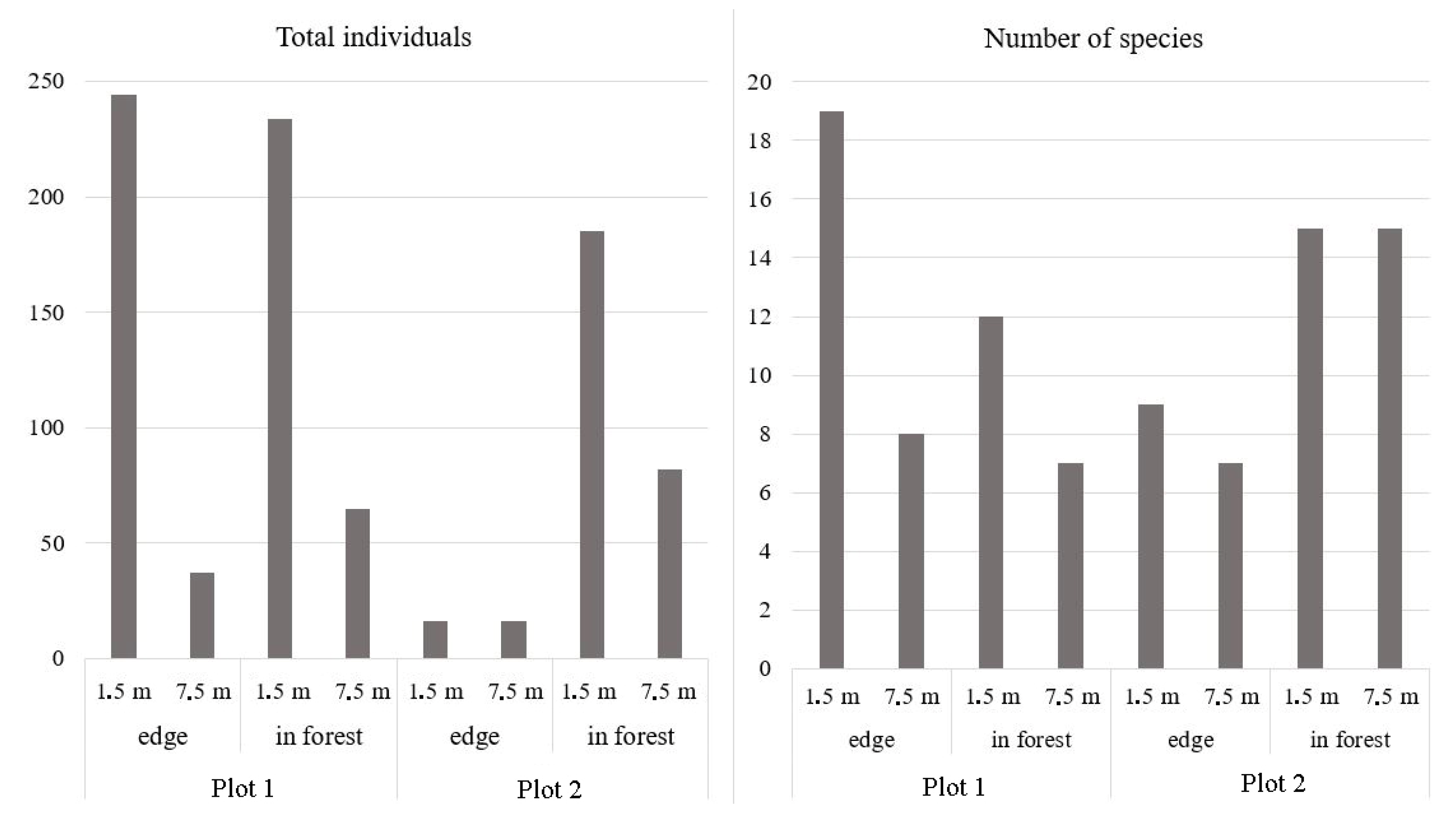
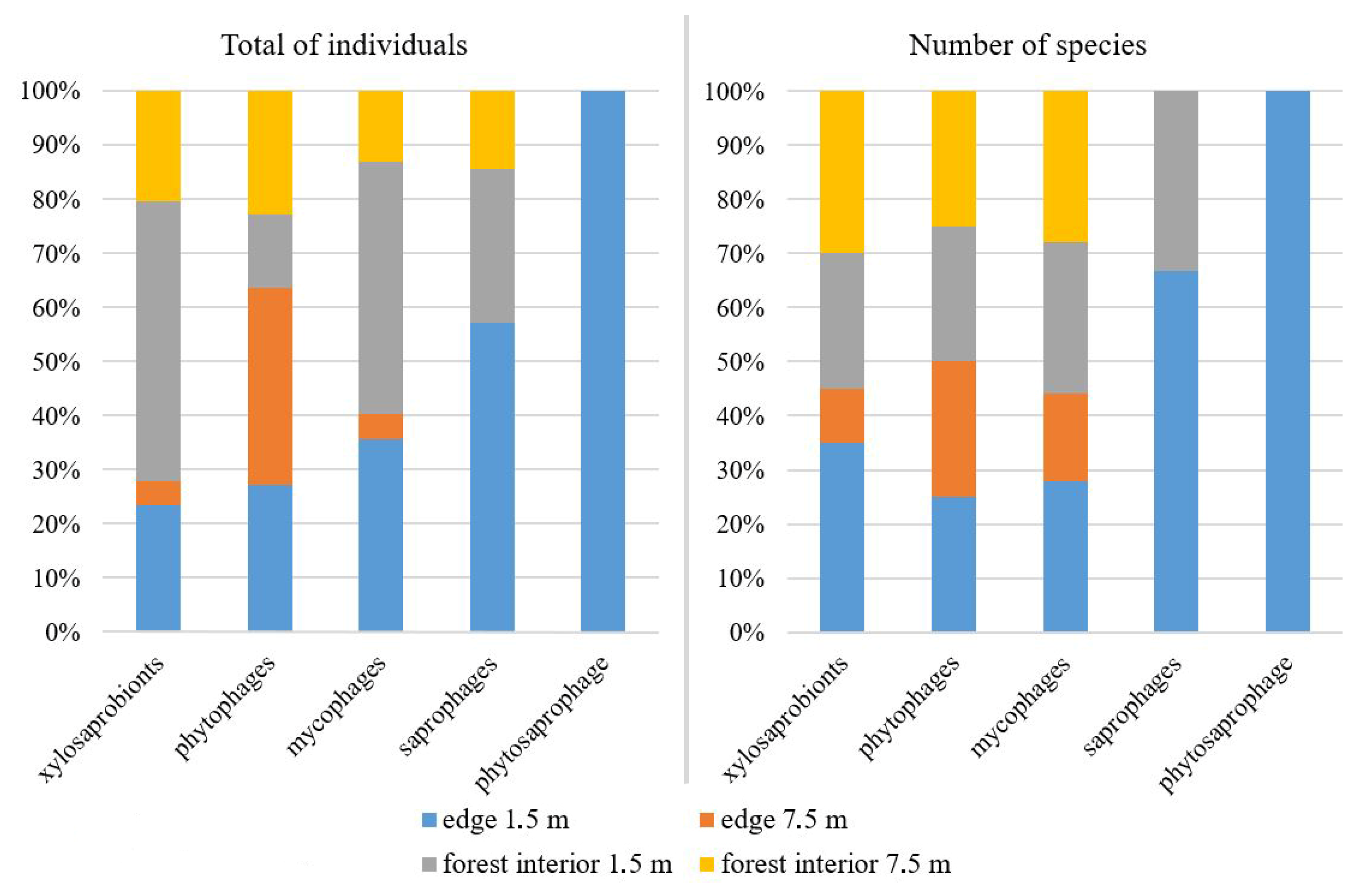
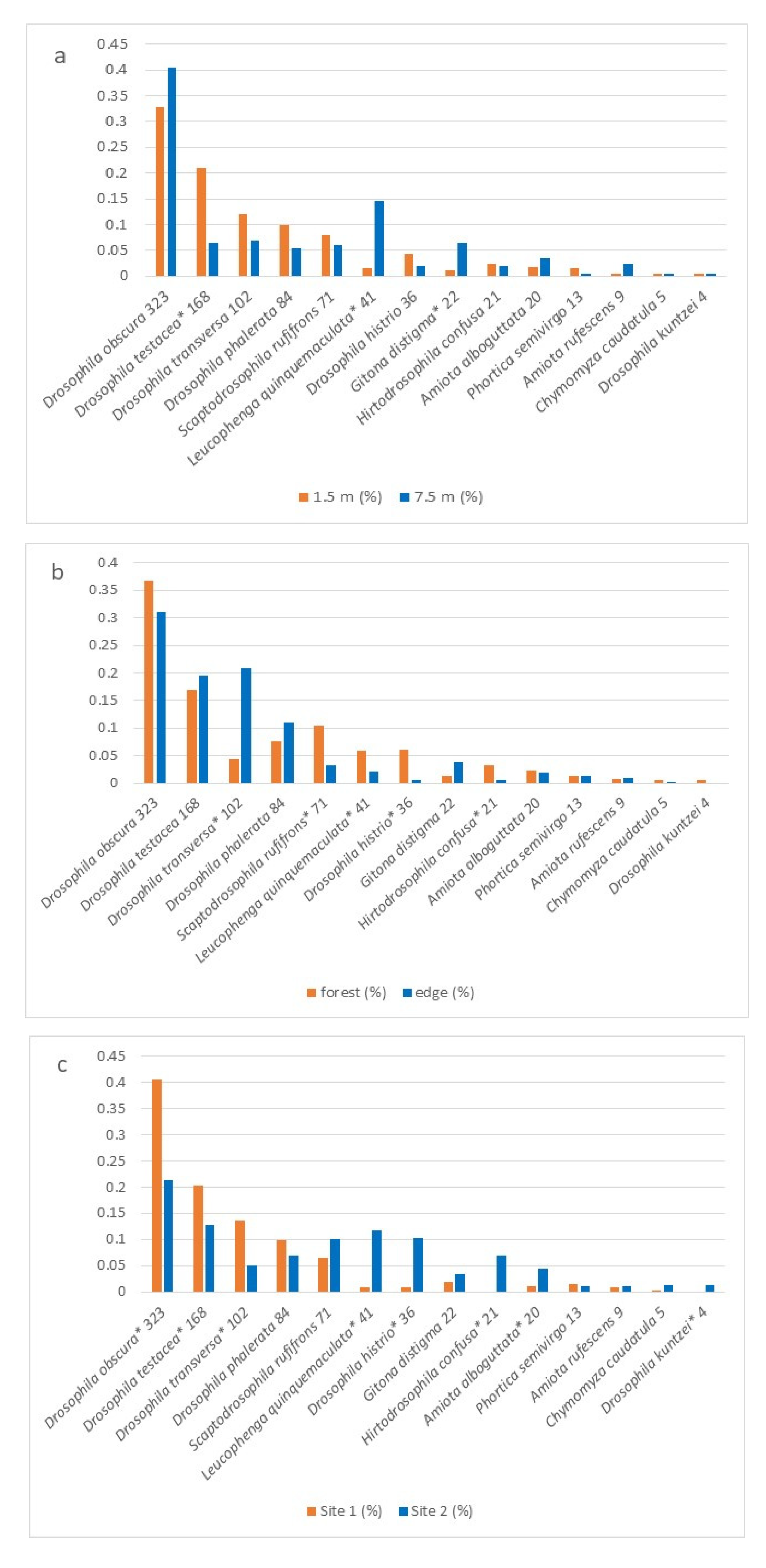
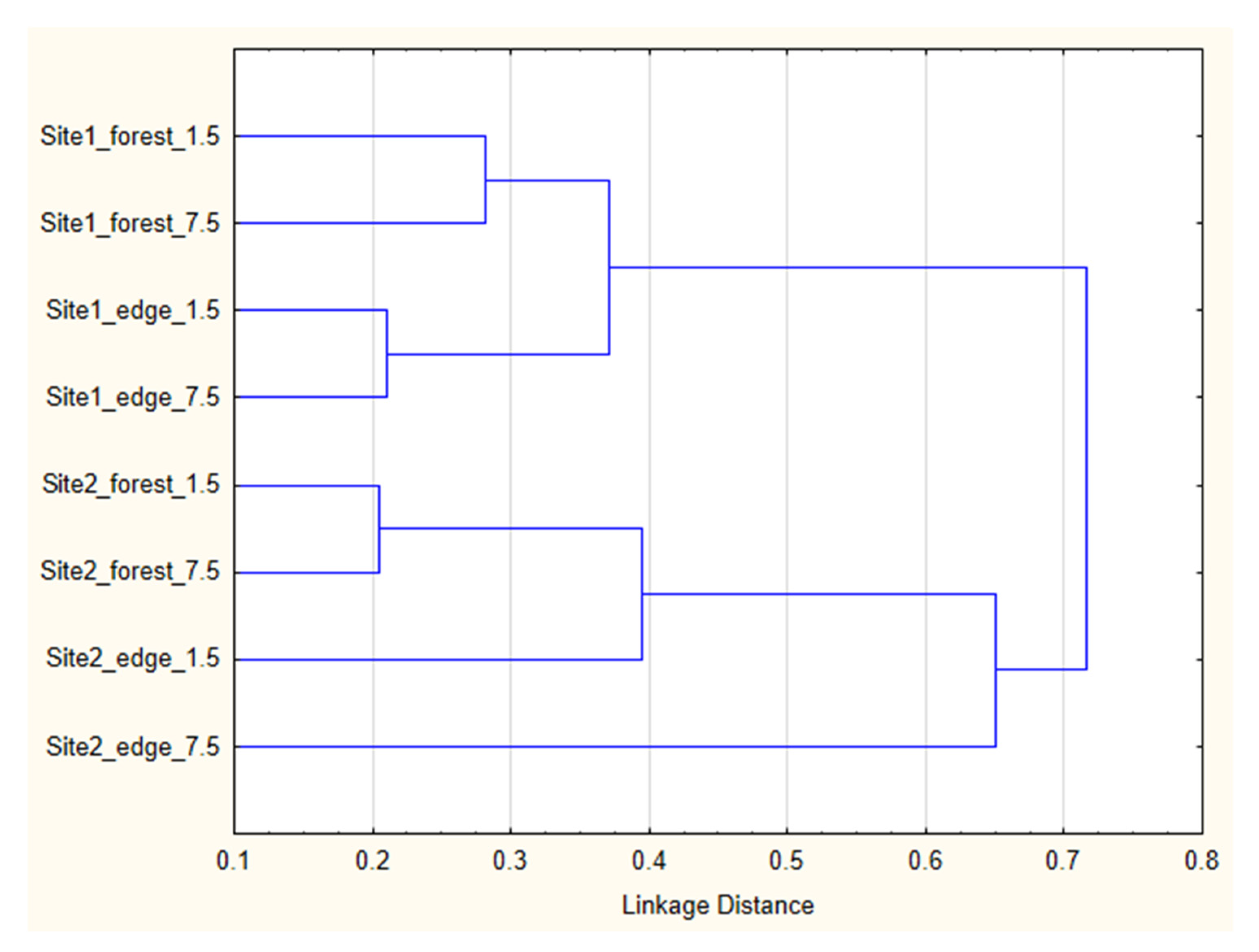
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bächli, G. TaxoDros: The Database on Taxonomy of Drosophilidae. 2024. Available online: https://www.taxodros.uzh.ch/ (accessed on 10 November 2024).
- Gottschalk, M.S.; De Toni, D.C.; Valente, V.L.S.; Hofmann, P.R.P. Changes in Brazilian Drosophilidae (Diptera) assemblages across an urbanisation gradient. Neotrop. Entomol. 2007, 36, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Hochmüller, C.J.C.; Lopes-da-Silva, M.; Valente, V.L.S.; Schmitz, H.J. The drosophilid fauna (Diptera, Drosophilidae) of the transition between the Pampa and Atlantic Forest Biomes in the state of Rio Grande do Sul, southern Brazil: First records. Papéis Avulsos Zool. 2010, 50, 285–295. [Google Scholar] [CrossRef]
- Gornostaev, N.G.; Lyupina, Y.V.; Lazebny, O.E.; Kulikov, A.M. Seasonal Dynamics of Fruit Flies (Diptera: Drosophilidae) in Natural Parks of Moscow City, Russia. Insects 2024, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, H.; Van Achterberg, K.; Nordlander, G.; Kimura, M.T. Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J. Nat. Hist. 2007, 41, 1731–1738. [Google Scholar] [CrossRef]
- Batista, M.R.; Uno, F.; Chaves, R.D.; Tidon, R.; Rosa, C.A.; Klaczko, L.B. Differential attraction of drosophilids to banana baits inoculated with Saccharomyces cerevisiae and Hanseniaspora uvarum within a Neotropical forest remnant. PeerJ 2017, 5, e3063. [Google Scholar] [CrossRef] [PubMed]
- Pipkin, S.B.; Rodriguez, R.L.; Leon, J. Plant host specificity among flower-feeding neotropical Drosophila (Diptera: Drosophilidae). Am. Nat. 1966, 100, 135–156. [Google Scholar] [CrossRef]
- Papp, L.; Rácz, O.; Bachli, G. Revision of the European species of the Scaprodrosophila rufifrons group (Diptera, Drosophilidae). Mitteilungen-Schweiz. Entomol. Ges. 1999, 72, 105–117. [Google Scholar]
- Schmitz, H.J.; da Silva Valente, V.L. The flower flies and the unknown diversity of Drosophilidae (Diptera): A biodiversity inventory in the Brazilian fauna. Papéis Avulsos Zool. 2019, 59, e20195945. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Padró, J.; De Panis, D.; Soto, I.M.; Carreira, V.P. Protein–alkaloid interaction in larval diet affects fitness in cactophilic Drosophila (Diptera: Drosophilidae). Biol. J. Linn. Soc. 2019, 127, 44–55. [Google Scholar] [CrossRef]
- Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Invasive Agricultural Pest Drosophila suzukii (Diptera, Drosophilidae) Appeared in the Russian Caucasus. Insects 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Castellan, I.; Duménil, C.; Rehermann, G.; Eisenstecken, D.; Bianchi, F.; Robatscher, P.; Spitaler, U.; Favaro, R.; Schmidt, S.; Becher, P.G.; et al. Chemical and Electrophysiological Characterisation of Headspace Volatiles from Yeasts Attractive to Drosophila suzukii. J. Chem. Ecol. 2024, 50, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Ostrauskas, H.; Pakalniškis, S.; Taluntytė, L. The species composition of plant mining dipterous (Insecta: Diptera) of greenhouse surroundings in Lithuania. Ekologija 2003, 3, 3–11. [Google Scholar]
- Whiteman, N.K.; Gloss, A.D.; Sackton, T.B.; Groen, S.C.; Humphrey, P.T.; Lapoint, R.T.; Sønderby, I.E.; Halkier, B.A.; Kocks, C.; Ausubel, F.M.; et al. Genes involved in the evolution of herbivory by a leaf-mining, drosophilid fly. Genome Biol. Evol. 2012, 4, 900–916. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, P.; Swathy, K.; Sarayut, P.; Patcharin, K. Classification, biology and entomopathogenic fungi-based management and their mode of action against Drosophila species (Diptera: Drosophilidae): A review. Front. Microbiol. 2024, 15, 1443651. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.F.; Blauth, M.L.; Santos, L.A.D.; Gaiesky, V.L.S.V.; Gottschalk, M.S. Temporal edge effects structure the assemblages of Drosophilidae (Diptera) in a Restinga forest fragment in Southern Brazil. Neotrop. Biol. Conserv. 2021, 16, 299–315. [Google Scholar] [CrossRef]
- Kark, S. Ecotones and Ecological Gradients. In Ecological Systems; Leemans, R., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.; Laurance, S.G.; Sampaio, E. Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L. The permeability of natural versus anthropogenic forest edges modulates the abundance of ground beetles of different dispersal power and habitat affinity. Diversity 2020, 12, 320. [Google Scholar] [CrossRef]
- Peltonen, M.; Heliövaara, K. Attack density and breeding success of bark beetles (Coleoptera, Scolytidae) at different distances from forest-clearcut edge. Agric. For. Entomol. 1999, 1, 237–242. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L. The type of forest edge governs the spatial distribution of different-sized ground beetles. Acta Zool. Acad. Sci. Hung. 2020, 66, 69–96. [Google Scholar] [CrossRef]
- Ruchin, A.B. Seasonal dynamics and spatial distribution of lepidopterans in selected locations in Mordovia, Russia. Biodiversitas 2021, 22, 2569–2575. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Edge Effects in the Distribution of Coleoptera in the Forests of the Center of the European Part of Russia. Insects 2023, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, M.A.; Pereira-Balbino, V.L.; Faccenda, O. Ecotone effect on the fruit fly assemblages (Diptera: Tephritidae) in natural and anthropized environments. Braz. J. Biol. 2023, 83, e273399. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Naz, F.; Nalwaya, S.; Saxena, K. Impact of anthropogenic disturbance on beetle diversity, with special reference to ground beetles (Coleoptera-Carabidae) and dung beetles (Coleoptera-Scarabaeidae): A review. Int. J. Entomol. Res. 2023, 8, 47–51. [Google Scholar]
- Esin, M.N.; Ruchin, A.B. Edge effects on Diptera distribution in deciduous forests of the centre of European Russia. J. Wildl. Biodivers. 2024, 8, 16–37. [Google Scholar] [CrossRef]
- Penariol, L.V.; Madi-Ravazzi, L. Edge-interior differences in the species richness and abundance of drosophilids in a semideciduous forest fragment. SpringerPlus 2013, 2, 114. [Google Scholar] [CrossRef] [PubMed]
- Bächli, G.; Flückiger, P.F.; Obrist, M.K.; Duelli, P. On the microdistribution of species of Drosophilidae and some other Diptera across a forest edge. Mitteilungen Schweiz. Entomol. Ges. 2006, 79, 117–126. [Google Scholar]
- Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Lazebny, O.E.; Kulikov, A.M. Vertical distribution of fruit flies (Diptera: Drosophilidae) in deciduous forests in the center of European Russia. Insects 2023, 14, 822. [Google Scholar] [CrossRef] [PubMed]
- Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Kulikov, A.M. Seasonal dynamics of fruit flies (Diptera: Drosophilidae) in forests of the European Russia. Insects 2022, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423, 623–659. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Chapman & Hall: London, UK, 1996. [Google Scholar]
- McDonald, J.H. G–est of goodness-of-fit. In Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, ML, USA, 2014. [Google Scholar]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Sol, D.; Lapiedra, O.; Legendre, P. A framework for estimating niche metrics using the resemblance between qualitative resources. Oikos 2011, 120, 1341–1350. [Google Scholar] [CrossRef]
- Orlandin, E.; Santos, E.B.; Schneeberger, A.H.; Souza, V.O.; Favretto, M.A. Habitat use by Neotropical mosquitoes (Diptera: Culicidae): Vegetation structure and edge effects. Austral Entomol. 2020, 59, 541–548. [Google Scholar] [CrossRef]
- Dantur Juri, M.J.; Almiron, W.R.; Claps, G.L. Population fluctuation of Anopheles (Diptera: Culicidae) in forest and forest edge habitats in Tucumán province, Argentina. J. Vector Ecol. 2010, 35, 28–34. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Salvo, A.; Valladares, G. Natural vegetation cover in the landscape and edge effects: Differential responses of insect orders in a fragmented forest. Insect Sci. 2017, 24, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Piekarska-Boniecka, H.; Siatkowski, M.; Trzciński, P.; Siatkowski, I. The impact of the vegetation of apple orchard edges on quantity and quality structure of predatory hoverflies (Diptera: Syrphidae) communities. Turk. Entomoloji Derg. 2015, 39, 333–343. [Google Scholar] [CrossRef]
- Okada, T. The oriental drosophilids breeding in flowers. Kontyu 1975, 43, 356–363. [Google Scholar][Green Version]
- Gornostaev, N.G. Ecological classification of drosophilid flies (Diptera, Drosophilidae). Entomol. Obozr. 1996, 75, 698–705. [Google Scholar][Green Version]
- Krivosheina, N.P. Macromycete fruit bodies as a habitat for dipterans (Insecta, Diptera). Entomol. Rev. 2008, 88, 778–792. [Google Scholar] [CrossRef]
- Shorrocks, B. An ecological classification of European Drosophila species. Oecologia 1977, 26, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Gornostaev, N.G. Addition to the fauna of drosophilid flies (Diptera, Drosophilidae) of Russia. Russ. Entomol. J. 1997, 6, 113–118. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvorak, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Begon, M.; Shorrocks, B. The feeding-and breeding-sites of Drosophila obscura Fallen and D. subobscura Collin. J. Nat. Hist. 1978, 12, 137–151. [Google Scholar] [CrossRef]
- Máca, J. Revision of Palaearctic species of Amiota subg. Phortica (Diptera, Drosophilidae). Acta Ent. Bohemoslov. 1977, 74, 115–130. [Google Scholar]
- Bächli, G.; Schatzmann, E.; Haring, E. On some population parameters of drosophilids in Switzerland (Diptera, Drosophilidae). Mitteilungen-Schweiz. Entomol. Ges. 2008, 81, 243–260. [Google Scholar]
- Jakovlev, J. Fungal hosts of mycetophilids (Diptera: Sciaroidea excluding Sciaridae): A review. Mycology 2012, 3, 11–23. [Google Scholar] [CrossRef]
- Ševčík, J. Czech and Slovak Diptera Associated with Fungi; Slezské Zemské Museum: Opava, Czech Republic, 2010. [Google Scholar]
- Sörensson, M. Pilotinventering av den Saproxyliska Insektsfaunan i Dalby Söderskog 2008; Länsstyrelsen i Skåne: Malmö, Sweden, 2012. [Google Scholar]
- Bächli, G.; Thunes, K. Leucophenga quinquemaculata Strobl (Diptera, Drosophilidae) from Norway. Fauna Nor. 1992, 39, 81–84. [Google Scholar]
- Jonsell, M.; Nordlander, G.; Jonsson, M. Colonization patterns of insects breeding in wood-decaying fungi. J. Insect Conserv. 1999, 3, 145–161. [Google Scholar] [CrossRef]
- Edwards, F.W. Amiota alboguttata Wahlb. in Dorset (Diptera, Drosophilidae). Entomologist 1936, 69, 218. [Google Scholar]
- Buxton, P.A. British Diptera associated with fungi. III. Flies of all families reared from about 150 species of fungi. Ent. Month. Mag. 1960, 96, 61–94. [Google Scholar]
- Wakely, S. Amiota alboguttata (Wahlb.) (Dipt., Drosophilidae) in Surrey. Entomologist 1953, 86, 199. [Google Scholar]
- De Bree, E. Acalyptratae flies (Diptera: Schizophora) from vinegar traps from the Valbona valley national park (Albania). Acta Entomol. Serbica 2022, 27, 91–97. [Google Scholar]
- Rotheray, G.E. Ecomorphology of Cyclorrhaphan Larvae (Diptera); Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Nartshuk, E.P. Fruit flies (Diptera: Drosophilidae) of the Russian Arctic. Zoosyst. Ross. 2014, 23, 256–263. [Google Scholar] [CrossRef]
- Hodge, S.; Ward, D.F.; Merfield, C.N.; Liu, W.Y.Y.; Gunawardana, D. Seasonal patterns of drosophilid flies and parasitoid wasps attracted to rotting fruit and vegetable baits in Canterbury, New Zealand. N. Z. Entomol. 2017, 40, 7–15. [Google Scholar] [CrossRef]
- Michalska, K.; Mrowińska, A.; Studnicki, M. Ectoparasitism of the Flightless Drosophila melanogaster and D. hydei by the Mite Blattisocius mali (Acari: Blattisociidae). Insects 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Maside, X.; Charlesworth, B. Patterns of molecular variation and evolution in Drosophila americana and its relatives. Genetics 2007, 176, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, B.V.; Sorokina, S.Y.; Mugue, N.S.; Reznik, N.L.; Mitrofanov, V.G. Dynamics of mitochondrial polymorphism in a natural population of Drosophila littoralis. Russ. J. Genet. 2008, 44, 159–164. [Google Scholar] [CrossRef]
- Sidorenko, V.C. Drosophilids (Diptera, Drosophilidae) related to fungi of the genus Pleurotus (Fr.) Kumm. (Agaricales, Pleurotaceae). Bulletin of the Moscow Society of Nature Testers. Dep. Biol. 2009, 114, 33–37. [Google Scholar]
- Máca, J. Czechoslovak species of the genus Scaptomyza Hardy (Diptera, Drosophilidae) and their bionomics. Acta Entomol. Bohemoslov. 1972, 69, 119–132. [Google Scholar]
- Toda, M.J. Three-dimensional dispersion of drosophilid flies in a cool temperate forest of northern Japan. Ecol. Res. 1992, 7, 283–295. [Google Scholar] [CrossRef]
- Beuk, P.L. The species of the Drosophila repleta group in northwestern Europe with special reference to The Netherlands (Diptera: Drosophilidae). Entomol. Ber. 1993, 53, 96–98. [Google Scholar]
- Lewis, T. The effects of an artificial windbreak on the aerial distribution of flying insects. Ann. Appl. Biol. 1965, 55, 503–512. [Google Scholar] [CrossRef]
- Bernaschini, M.L.; Valladares, G.; Salvo, A. Edge effects on insect–plant food webs: Assessing the influence of geographical orientation and microclimatic conditions. Ecol. Entomol. 2020, 45, 806–820. [Google Scholar] [CrossRef]

| Species | Edge | Forest Interior | ||
|---|---|---|---|---|
| 1.5 m | 7.5 m | 1.5 m | 7.5 m | |
| Amiota albilabris (Roth, 1860) | 0 | 0 | 0 | 2 |
| Amiota alboguttata (Wahlberg, 1839) | 3 | 4 | 10 | 3 |
| Amiota rufescens (Oldenberg, 1914) | 2 | 2 | 2 | 3 |
| Amiota subtusradiata Duda, 1934 | 0 | 1 | 0 | 0 |
| Gitona distigma Meigen, 1830 | 6 | 8 | 3 | 5 |
| Leucophenga quinquemaculata Strobl, 1893 | 3 | 5 | 9 | 24 |
| Phortica semivirgo (Máca, 1977) | 5 | 0 | 7 | 1 |
| Stegana coleoptrata (Scopoli 1763) | 1 | 0 | 0 | 0 |
| Stegana furta (Linnaeus, 1767) | 0 | 0 | 0 | 1 |
| Chymomyza amoena (Loew, 1862) | 1 | 0 | 1 | 0 |
| Chymomyza caudatula Oldenberg, 1914 | 1 | 0 | 3 | 1 |
| Drosophila funebris (Fabricius, 1787) | 1 | 0 | 0 | 0 |
| Drosophila histrio Meigen, 1830 | 2 | 0 | 30 | 4 |
| Drosophila hydei Sturtevant, 1921 | 1 | 0 | 0 | 0 |
| Drosophila kuntzei Duda, 1924 | 0 | 0 | 3 | 1 |
| Drosophila littoralis Meigen, 1830 | 1 | 0 | 0 | 0 |
| Drosophila melanogaster Meigen, 1830 | 1 | 0 | 0 | 0 |
| Drosophila obscura Fallén, 1823 | 100 | 15 | 142 | 66 |
| Drosophila phalerata Meigen, 1830 | 37 | 4 | 36 | 7 |
| Drosophila repleta Wollaston, 1858 | 0 | 0 | 1 | 0 |
| Drosophila subobscura Collin, 1936 | 1 | 0 | 2 | 0 |
| Drosophila testacea von Roser, 1840 | 70 | 2 | 85 | 11 |
| Drosophila transversa Fallén, 1823 | 68 | 9 | 20 | 5 |
| Hirtodrosophila confusa (Staeger, 1844) | 2 | 0 | 15 | 4 |
| Hirtodrosophila trivittata (Strobl, 1893) | 1 | 0 | 0 | 0 |
| Scaptodrosophila rufifrons (Loew, 1873) | 9 | 3 | 50 | 9 |
| Scaptomyza pallida (Zetterstedt, 1847) | 1 | 0 | 0 | 0 |
| Total of individuals | 317 | 53 | 419 | 147 |
| Total of individuals (forest edge + interior) | 370 | 566 | ||
| Number of species | 22 | 10 | 17 | 16 |
| Number of species (forest edge + interior) | 23 | 19 | ||
| Margalef Index | 3.65 | 2.27 | 2.65 | 3.01 |
| Margalef Index (forest edge + interior) | 3.72 | 2.84 | ||
| Shannon Index | 1.89 | 2.04 | 2.03 | 1.94 |
| Shannon Index (forest edge + interior) | 1.98 | 2.08 | ||
| Simpson Index | 0.79 | 0.84 | 0.81 | 0.76 |
| Simpson Index (forest edge + interior) | 0.80 | 0.81 | ||
| Samples Grouped by Feature | G | d.f. | p-Value |
|---|---|---|---|
| Forest layer—1.5:7.5 | 124.81 | 26 | 7.10 × 10−15 |
| Edge–forest interior | 146.48 | 26 | 9.23 × 10−19 |
| Plot 1–Plot 2 | 241.68 | 26 | 7.43 × 10−37 |
| Species | Layer 1.5 m:7.5 m | Edge–Forest Interior | Plot 1–Plot 2 | ||||
|---|---|---|---|---|---|---|---|
| n | χ2 | p | χ2 | p | χ2 | p | |
| A. albilabris | 2 | 7.38 | 0.0066 | 1.31 | 0.2524 | 4.23 | 0.0388 |
| A. alboguttata | 20 | 2.26 | 0.1327 | 0.18 | 0.6753 | 10.27 | 0.0014 |
| A. rufescens | 9 | 6.32 | 0.0119 | 0.09 | 0.7619 | 0.01 | 0.9284 |
| A. subtusradiata | 1 | 3.68 | 0.0549 | 1.53 | 0.2159 | 2.13 | 0.1442 |
| G. distigma | 22 | 19.08 | 0.0000 | 5.48 | 0.0193 | 1.89 | 0.1690 |
| L. quinquemaculata | 41 | 62.19 | 0.0000 | 7.19 | 0.0073 | 56.29 | 0.0000 |
| P. semivirgo | 11 | 1.47 | 0.2258 | 0.01 | 0.9368 | 0.48 | 0.4899 |
| S. coleoptrata | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 0.47 | 0.4930 |
| S. furta | 1 | 3.68 | 0.0549 | 0.65 | 0.4185 | 2.13 | 0.1442 |
| C. amoena | 2 | 0.54 | 0.4605 | 0.09 | 0.7618 | 0.94 | 0.3321 |
| C. caudatula | 5 | 0.01 | 0.9404 | 0.8 | 0.3705 | 5.34 | 0.0209 |
| D. funebris | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 0.47 | 0.4930 |
| D. histrio | 35 | 2.34 | 0.1258 | 18.08 | 0.0000 | 50.53 | 0.0000 |
| D. hydei | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 2.13 | 0.1442 |
| D. kuntzei | 4 | 0.03 | 0.8590 | 2.63 | 0.1051 | 8.56 | 0.0034 |
| D. littoralis | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 0.47 | 0.4930 |
| D. melanogaster | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 0.47 | 0.4930 |
| D. obscura | 303 | 4.04 | 0.0444 | 3.18 | 0.0745 | 33.38 | 0.0000 |
| D. phalerata | 80 | 3.76 | 0.0525 | 3.32 | 0.0683 | 2.05 | 0.1525 |
| D. repleta | 1 | 0.27 | 0.6020 | 0.65 | 0.4185 | 0.47 | 0.4930 |
| D. subobscura | 3 | 0.82 | 0.3658 | 0.05 | 0.8260 | 1.67 | 0.1964 |
| D. testacea | 162 | 22.64 | 0.0000 | 0.95 | 0.3302 | 8.19 | 0.0042 |
| D. transversa | 82 | 3.98 | 0.0461 | 70.66 | 0.0000 | 15.65 | 0.0001 |
| H. confusa | 21 | 0.07 | 0.7931 | 8.09 | 0.0045 | 45.77 | 0.0000 |
| H. trivittata | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 0.47 | 0.4930 |
| S. rufifrons | 67 | 0.91 | 0.3396 | 16.46 | 0.0000 | 3.76 | 0.0526 |
| S. pallida | 1 | 0.27 | 0.6020 | 1.53 | 0.2159 | 0.47 | 0.4930 |
| B-H corr. | 0.05 | 0.0074 | 0.0093 | 0.0148 | |||
| Species | Layer 1.5:7.5 | Forest Depth | Plots 1:2 | p-Value |
|---|---|---|---|---|
| D. testacea | 1.5 | - | - | 0.007 |
| D. phalerata | 1.5 | - | - | 0.0264 |
| P. semivirgo | 1.5 | - | - | 0.0284 |
| D. kuntzei | - | forest | Plot 2 | 0.0360 |
| H. confusa | - | forest | Plot 2 | 0.0360 |
| C. caudatula | - | forest | Plot 2 | 0.0450 |
| L. quinquemaculata | - | - | Plot 2 | 0.0080 |
| H. confusa | - | - | Plot 2 | 0.0250 |
| L. quinquemaculata | 7.5 | - | Plot 2 | 0.0360 |
| G. distigma | - | edge | Plot 2 | 0.0370 |
| S. rufifrons | 1.5 | forest | - | 0.0430 |
| Pairs and Triplets of Species | Layer 1.5:7.5 | Sites1:2 | p-Value |
|---|---|---|---|
| D. obscura + D. phalerata + D. testacea | - | site 1 | 0.0278 |
| D. phalerata + D. testacea | - | site 1 | 0.0278 |
| D. testacea + D. transversa | 1.5 | - | 0.0149 |
| D. obscura + D. transversa | 1.5 | - | 0.0353 |
| D. testacea + D. obscura | 1.5 | - | 0.0097 |
| D. testacea + P. semivirgo | 1.5 | - | 0.0174 |
| D. phalerata + D. testacea | 1.5 | - | 0.0017 |
| D. phalerata + D. obscura | 1.5 | - | 0.0193 |
| D. phalerata + D. transversa | 1.5 | - | 0.0353 |
| D.phalerata + L. quinquemaculata | 1.5 | - | 0.0433 |
| P. semivirgo + D. obscura | 1.5 | - | 0.0307 |
| P. semivirgo + D. phalerata | 1.5 | - | 0.0307 |
| P. semivirgo + D. transversa | 1.5 | - | 0.0307 |
| P. semivirgo + S. rufifrons | 1.5 | - | 0.0307 |
| D. testacea + S. rufifrons | 1.5 | - | 0.0449 |
| D. phalerata + S. rufifrons | 1.5 | - | 0.0430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornostaev, N.G.; Ruchin, A.B.; Lazebny, O.E.; Kulikov, A.M.; Esin, M.N. Edge Effects on the Spatial Distribution and Diversity of Drosophilidae (Diptera) Assemblages in Deciduous Forests of Central European Russia. Insects 2025, 16, 762. https://doi.org/10.3390/insects16080762
Gornostaev NG, Ruchin AB, Lazebny OE, Kulikov AM, Esin MN. Edge Effects on the Spatial Distribution and Diversity of Drosophilidae (Diptera) Assemblages in Deciduous Forests of Central European Russia. Insects. 2025; 16(8):762. https://doi.org/10.3390/insects16080762
Chicago/Turabian StyleGornostaev, Nikolai G., Alexander B. Ruchin, Oleg E. Lazebny, Alex M. Kulikov, and Mikhail N. Esin. 2025. "Edge Effects on the Spatial Distribution and Diversity of Drosophilidae (Diptera) Assemblages in Deciduous Forests of Central European Russia" Insects 16, no. 8: 762. https://doi.org/10.3390/insects16080762
APA StyleGornostaev, N. G., Ruchin, A. B., Lazebny, O. E., Kulikov, A. M., & Esin, M. N. (2025). Edge Effects on the Spatial Distribution and Diversity of Drosophilidae (Diptera) Assemblages in Deciduous Forests of Central European Russia. Insects, 16(8), 762. https://doi.org/10.3390/insects16080762








