Venom IMP-L2 from the Ectoparasitoid Scleroderma guani Regulates the IIS/TOR Pathway in Tenebrio molitor
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. The Expression of IIS/TOR Pathway Related Genes in Different Developmental Stages of T. molitor
2.3. Regulation of Parasitism on Genes Related to IIS/TOR Signaling Pathway in T. molitor
2.4. Cloning and Sequence Analysis of IMP-L2 from S. guani
2.5. Expression Analysis of IMP-L2 in S. guani Venom Organs, Developmental Stages, and Parasitic Process
2.6. Construction of the Expression Vector pFast-bac1-IMP-L2 and Recombinant Baculovirus Expression Vector (Bacmid)
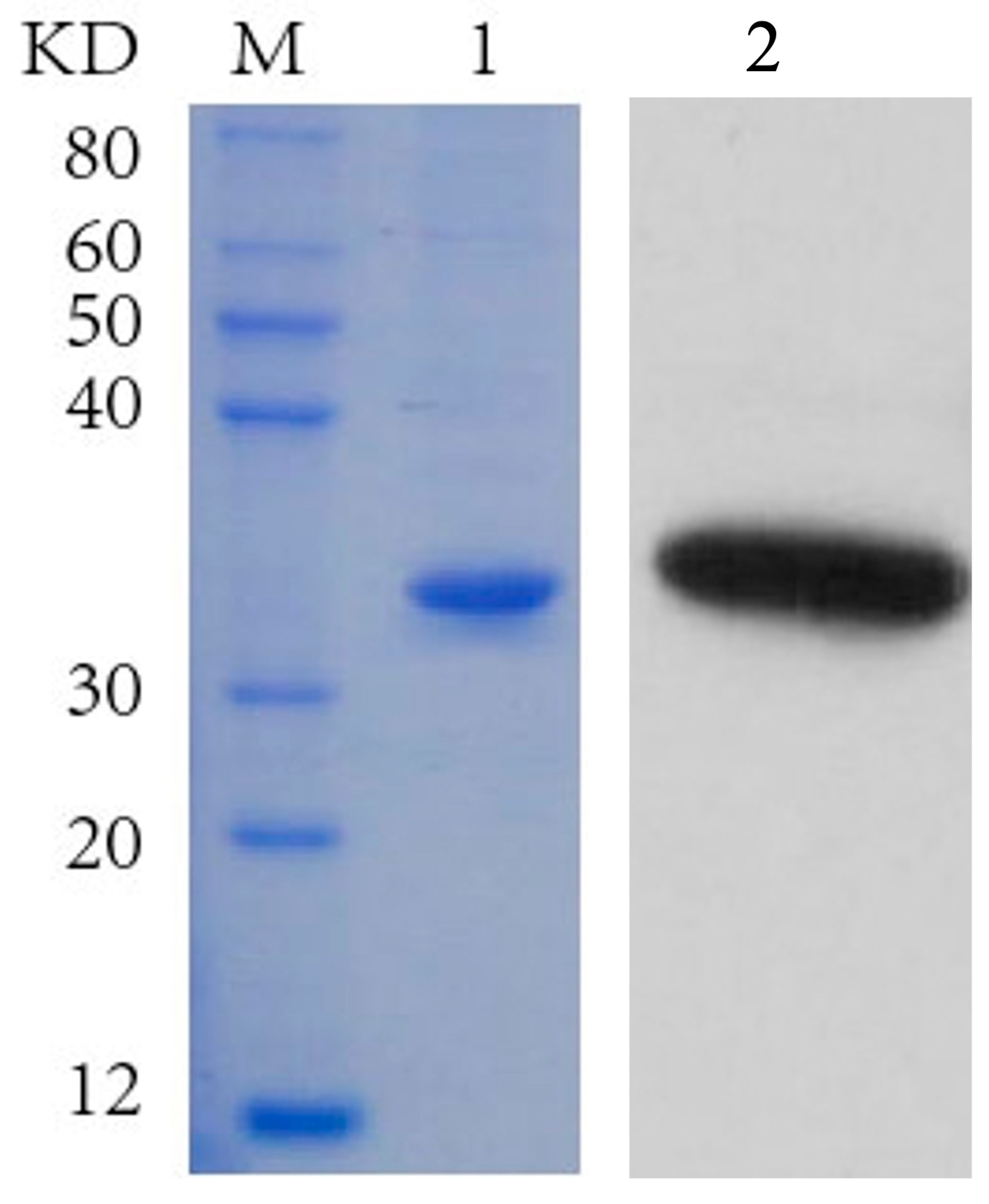
2.7. Expression and Purification of the IMP-L2 Protein
2.8. SDS-PAGE and Western Blot Analysis
2.9. The Regulation of Recombinant IMP-L2 Protein on the Genes Related to the IIS/TOR Signaling Pathway of T. molitor
2.10. Synthesis of dsRNA and RNA Interference
2.11. Records of Survival Pupae of T. molitor
2.12. Statistical Analyses
3. Results
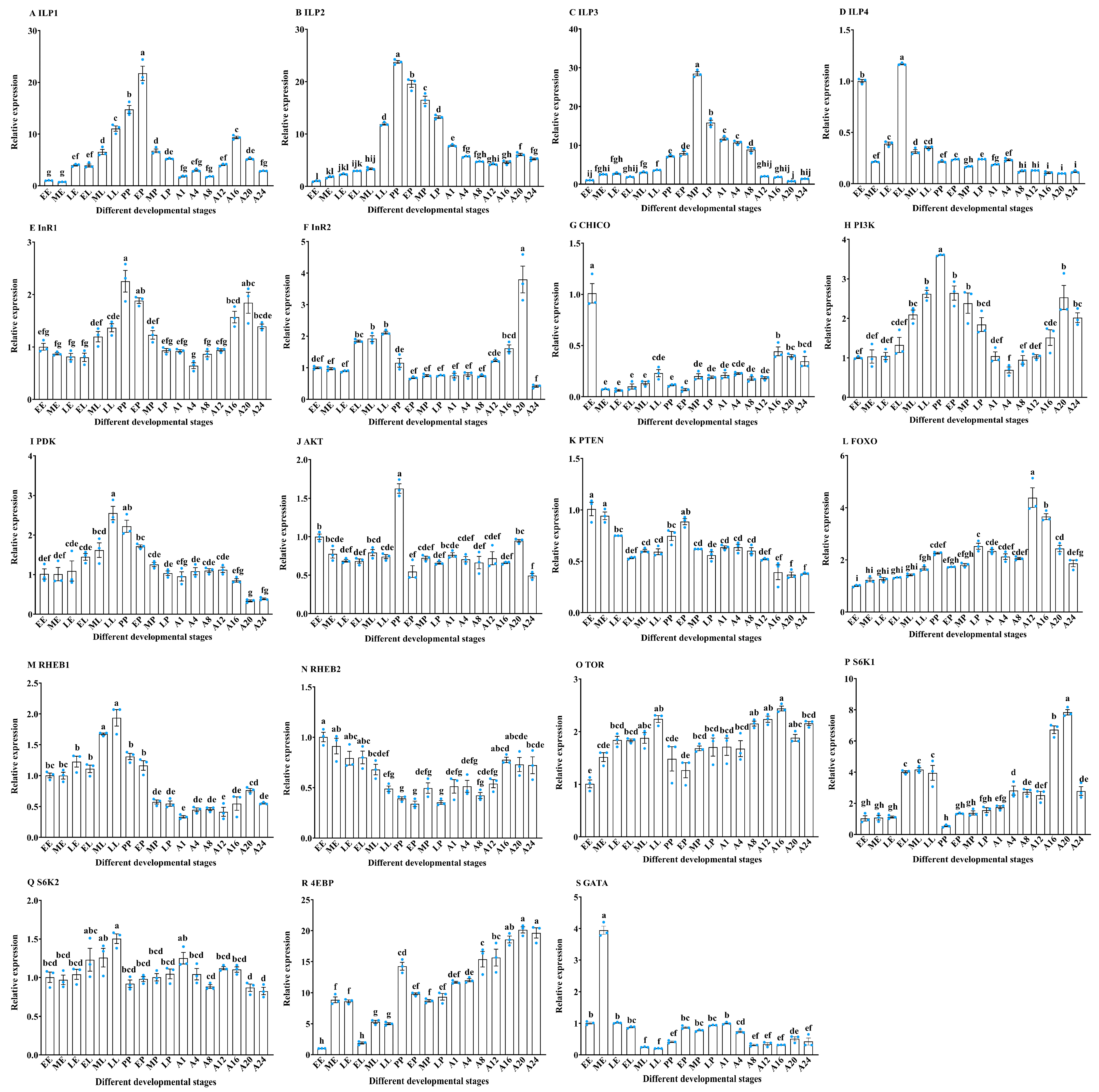
3.1. The Expression Pattern Analysis of IIS/TOR Signaling Pathway Related Genes in Different Developmental Stages of T. molitor
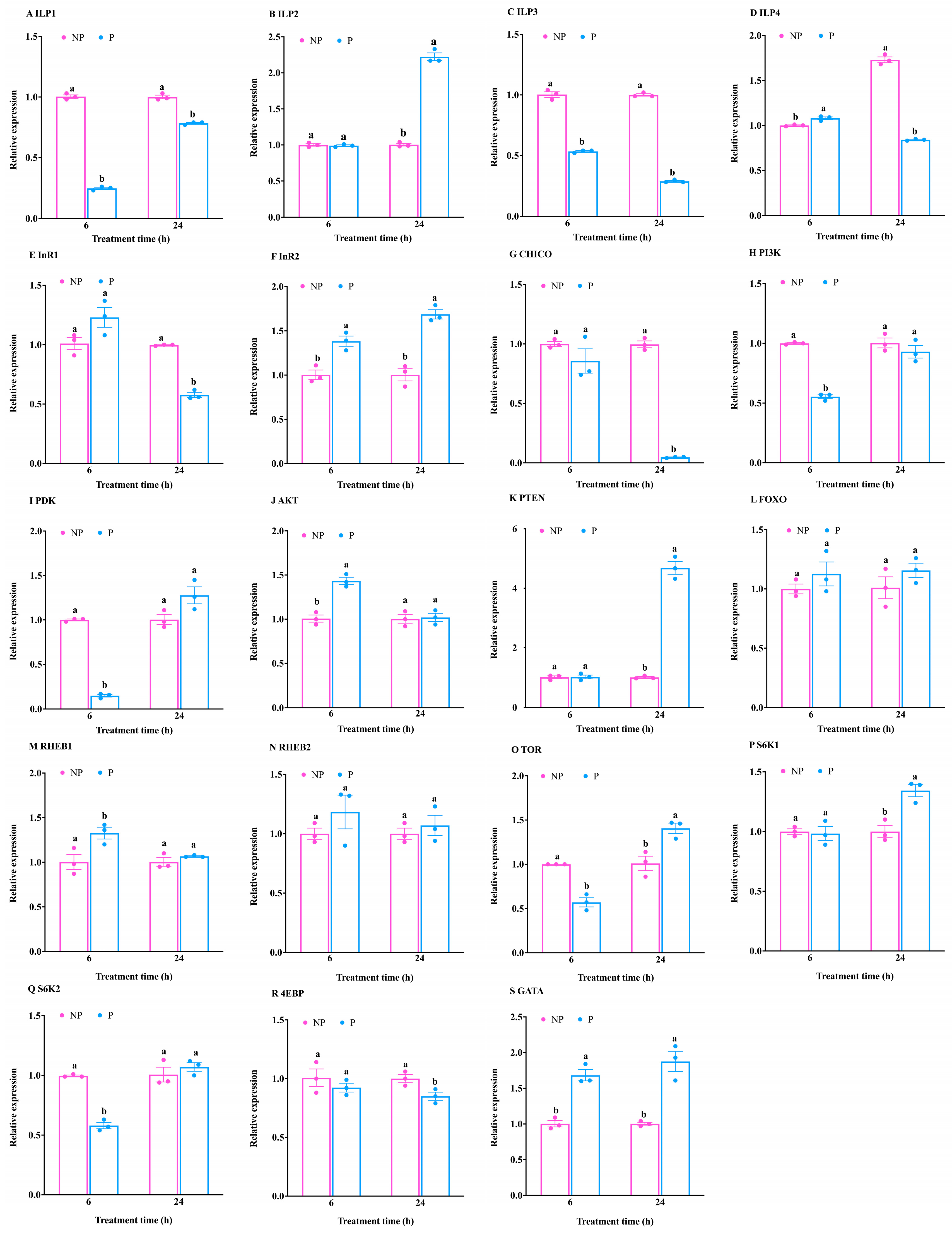
3.2. Expression Pattern Analysis of Genes Related to the IIS/TOR Signaling Pathway of T. molitor in Response to S. guani Parasitism
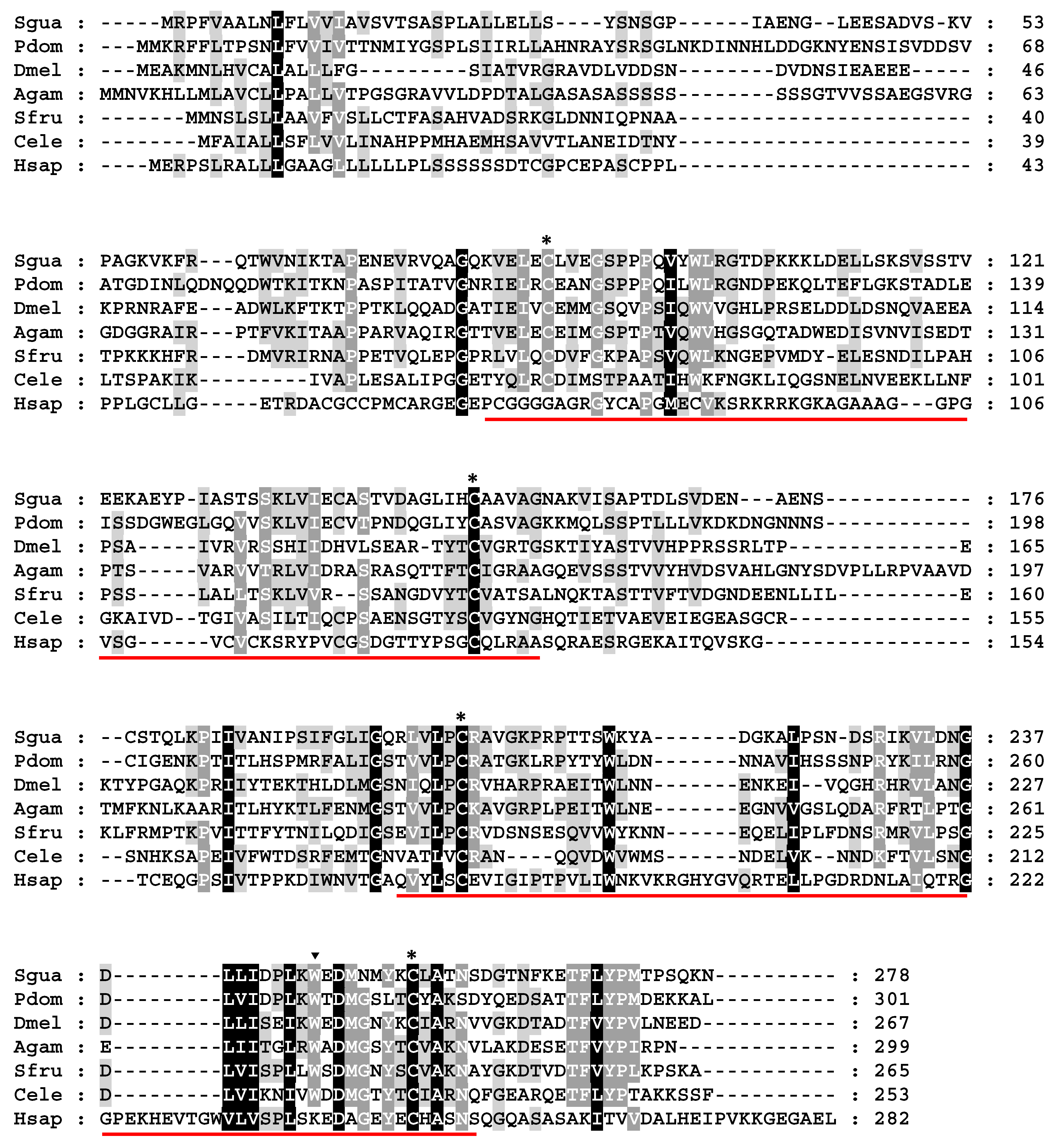
3.3. Cloning and Expression Analysis of IMP-L2 Gene from S. guani Venom
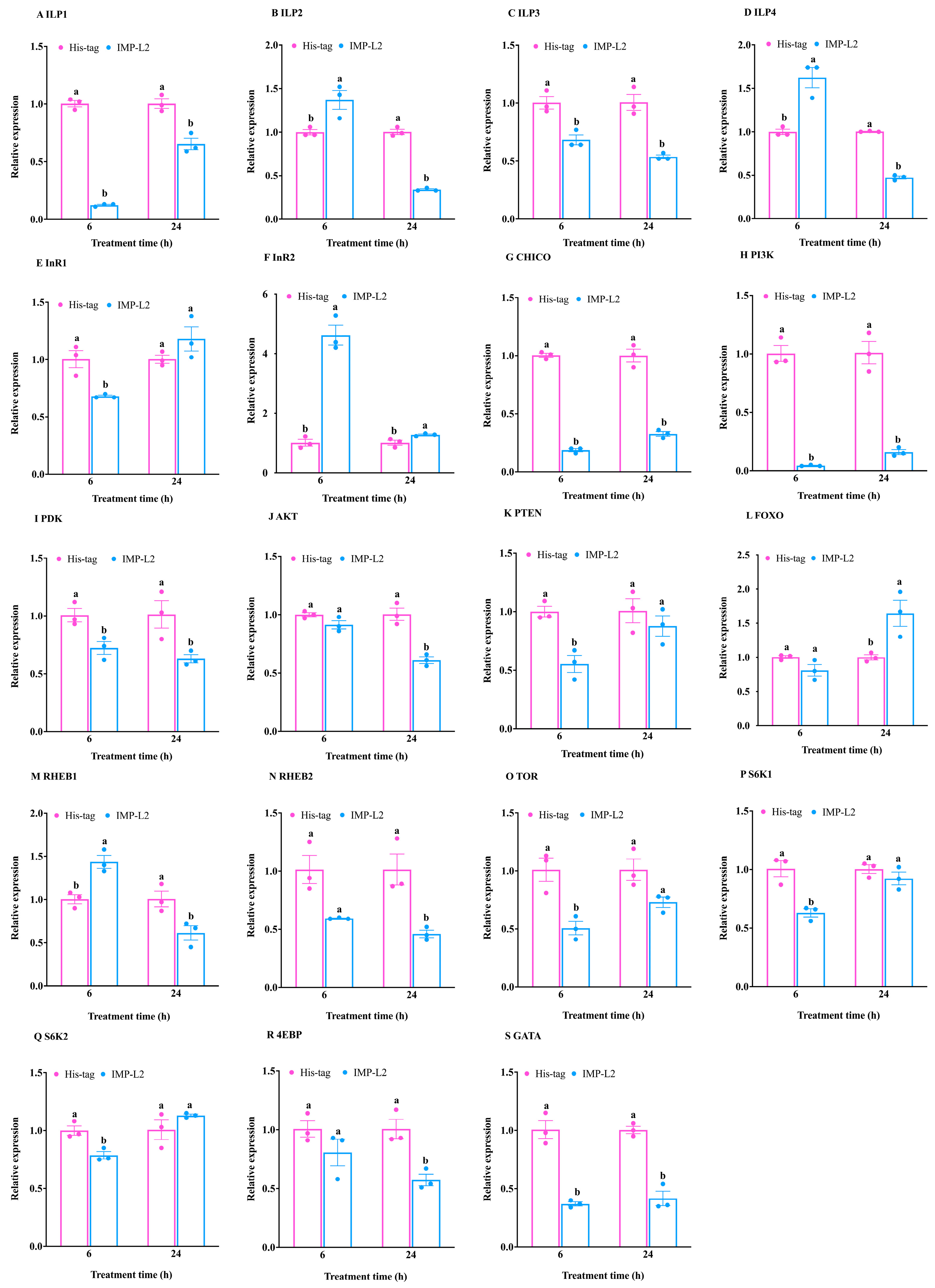
3.4. Analysis of the Expression Pattern of Genes Related to the IIS/TOR Signaling Pathway in T. molitor After Injection of Recombinant IMP-L2 Protein
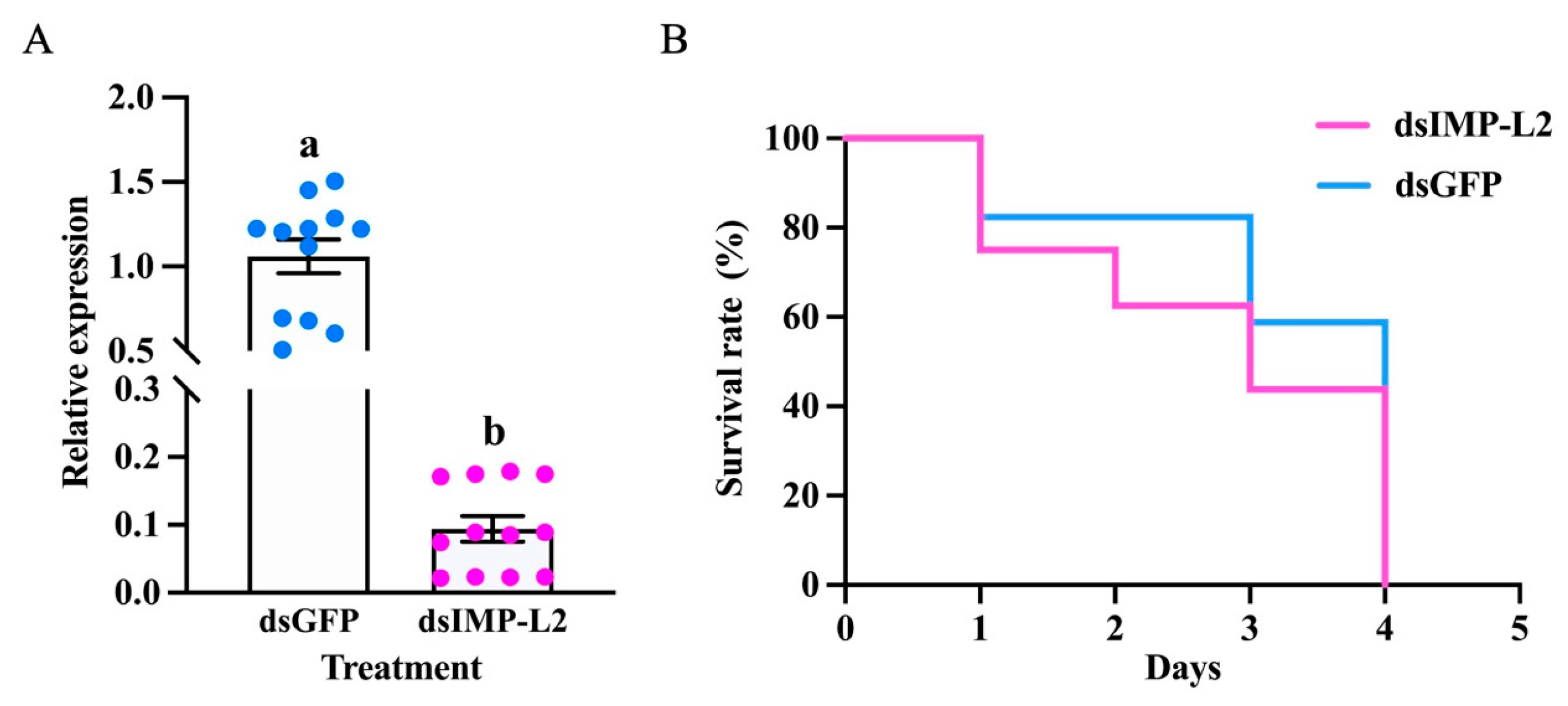
3.5. S. guani IMP-L2 Affects the Longevity of T. molitor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becchimanzi, A.; Avolio, M.; Bostan, H.; Colantuono, C.; Cozzolino, F.; Mancini, D.; Chiusano, M.L.; Pucci, P.; Caccia, S.; Pennacchio, F. Venomics of the ectoparasitoid wasp Bracon nigricans. BMC Genom. 2020, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Samková, A.; Hadrava, J.; Skuhrovec, J.; Janšta, P. Host specificity of the parasitic wasp Anaphes flavipes (Hymenoptera: Mymaridae) and a new defence in its hosts (Coleoptera: Chrysomelidae: Oulema spp.). Insects 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, F.; De Mandal, S.; Zeng, L.; Zhang, Y.; Hua, Y.; Hong, Y.; Zhao, C.; Li, J.; Li, D.; et al. Insights into the venom protein components of the egg parasitoid Anastatus japonicus (Hymenoptera: Eupelmidae). Pest Manag. Sci. 2020, 76, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.R. Common themes in three independently derived endogenous nudivirus elements in parasitoid wasps. Curr. Opin. Insect Sci. 2019, 32, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.J.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Yang, L.; Zhang, J.; Qiu, L.; Fang, Q.; Yao, H.; Poirié, M.; Gatti, J.L.; Ye, G. The venom of the ectoparasitoid wasp Pachycrepoideus vindemiae (Hymenoptera: Pteromalidae) induces apoptosis of Drosophila melanogaster Hemocytes. Insects 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- De Loof, A. Ecdysteroids, juvenile hormone and insect neuropeptides: Recent successes and remaining major challenges. Gen. Comp. Endocrinol. 2008, 155, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin–IGF axis. Trends Endocrinol. Metab. 2006, 17, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, S.V.; Hartfelder, K. The insulin signaling pathway in honey bee (Apis mellifera) caste development—differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol. 2008, 54, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Smagghe, G. Roles of the insulin signaling pathway in insect development and organ growth. Peptides 2019, 122, 169923. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.G.; Pietri, J.E.; Smithers, H.M.; Nuss, A.; Antonova, Y.; Drexler, A.L.; Riehle, M.A.; Brown, M.R.; Luckhart, S. Insulin-like peptides in the mosquito Anopheles stephensi: Identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 2011, 173, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Kubrak, O.I.; Liu, Y.; Luo, J.; Lushchak, O.V. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 2013, 4, 252. [Google Scholar] [CrossRef] [PubMed]
- Riehle, M.A.; Fan, Y.; Cao, C.; Brown, M.R. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: Expression, cellular localization, and phylogeny. Peptides 2006, 27, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Hun, L.V.; Cheung, K.W.; Brooks, E.; Zudekoff, R.; Luckhart, S.; Riehle, M.A. Increased insulin signaling in the Anopheles stephensi fat body regulates metabolism and enhances the host response to both bacterial challenge and Plasmodium falciparum infection. Insect Biochem. Mol. Biol. 2021, 139, 103669. [Google Scholar] [CrossRef] [PubMed]
- Rulifson, E.J.; Kim, S.K.; Nusse, R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science 2002, 296, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Kang, P.; Tatar, M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 2012, 11, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Mendes, C.C.; Mirth, C.K. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 2013, 4, 263. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.P.M.; Korb, J.; Bornberg-Bauer, E. Reconstructed evolution of insulin receptors in insects reveals duplications in early insects and cockroaches. J. Exp. Zool. Part B Mol. Dev. Evol. 2018, 330, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Neufeld, T.P.; Pan, D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 2000, 221, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Goberdhan, D.C.; Paricio, N.; Goodman, E.C.; Mlodzik, M.; Wilson, C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999, 13, 3244–3258. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.M.; Davidge, J.T.; Lockyer, J.M.; Staveley, B.E. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev. Biol. 2003, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Jünger, M.A.; Rintelen, F.; Stocker, H.; Wasserman, J.D.; Végh, M.; Radimerski, T.; Greenberg, M.E.; Hafen, E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.; Giannakou, M.E.; Foley, A.; Goss, M.; Partridge, L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 2011, 10, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Y.; Arrazola, P.; Hino, O.; Kobayashi, T.; Yeung, R.S.; Ru, B.; Pan, D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 2002, 4, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Pan, D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001, 15, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Zwartkruis, F.J.; Nobukuni, T.; Joaquin, M.; Roccio, M.; Stocker, H.; Kozma, S.C.; Hafen, E.; Bos, J.L.; Thomas, G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 2003, 11, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Bennett-Keki, S.; Fowler, E.K.; Folkes, L.; Moxon, S.; Chapman, T. Sex-biased gene expression in nutrient-sensing pathways. Proc. R. Soc. B Biol. Sci. 2023, 290, 20222086. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Raikhel, A.S. Amino acid-dependent regulation of insulin-like peptide signaling is mediated by TOR and GATA factors in the disease vector mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2023, 120, e2303234120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, X.; Dai, X.; Han, S.; Wu, X.; Wang, L.; Wei, W.; Zhang, N.; Xie, W.; Guo, J. S6K1-mediated phosphorylation of PDK1 impairs AKT kinase activity and oncogenic functions. Nat. Commun. 2022, 13, 1548. [Google Scholar] [CrossRef] [PubMed]
- Teleman, A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2009, 425, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Hietakangas, V.; Cohen, S.M. Re-evaluating AKT regulation: Role of TOR complex 2 in tissue growth. Genes Dev. 2007, 21, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Garbe, J.C.; Yang, E.; Fristrom, J.W. IMP-L2: An essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development 1993, 119, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Sloth Andersen, A.; Hertz Hansen, P.; Schaffer, L.; Kristensen, C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J. Biol. Chem. 2000, 275, 16948–16953. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Wilson, E.M.; Rosenfeld, R.G.; Oh, Y. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J. Biol. Chem. 1997, 272, 30729–30734. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Nagalla, S.R.; Yamanaka, Y.; Kim, H.S.; Wilson, E.; Rosenfeld, R.G. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J. Biol. Chem. 1996, 271, 30322–30325. [Google Scholar] [CrossRef] [PubMed]
- Roed, N.K.; Viola, C.M.; Kristensen, O.; Schluckebier, G.; Norrman, M.; Sajid, W.; Wade, J.D.; Andersen, A.S.; Kristensen, C.; Ganderton, T.R.; et al. Structures of insect Imp-L2 suggest an alternative strategy for regulating the bioavailability of insulin-like hormones. Nat. Commun. 2018, 9, 3860. [Google Scholar] [CrossRef] [PubMed]
- Alic, N.; Hoddinott, M.P.; Vinti, G.; Partridge, L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 2011, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, E.; Song, W.; Perrimon, N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 2013, 155, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Honegger, B.; Galic, M.; Köhler, K.; Wittwer, F.; Brogiolo, W.; Hafen, E.; Stocker, H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Han, G.; Yun, H.M.; Lim, J.J.; Noh, S.; Lee, J.; Hyun, S. Steroid signaling mediates nutritional regulation of juvenile body growth via IGF-binding protein in Drosophila. Proc. Natl. Acad. Sci. USA 2018, 115, 5992–5997. [Google Scholar] [CrossRef] [PubMed]
- Taghert, P.H.; Hewes, R.S.; Park, J.H.; O’Brien, M.A.; Han, M.; Peck, M.E. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6673–6686. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.; Sarraf-Zadeh, L.; Peters, M.; Moderau, N.; Stocker, H.; Köhler, K.; Pankratz, M.J.; Hafen, E. The IGFBP7 homolog Imp-L2 promotes insulin signaling in distinct neurons of the Drosophila brain. J. Cell Sci. 2013, 126, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Veenstra, J.A.; Park, J.H.; Taghert, P.H. Mapping peptidergic cells in Drosophila: Where DIMM fits in. PLoS ONE 2008, 3, e1896. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y. Deciphering the main venom components of the ectoparasitic ant-like bethylid wasp, Scleroderma guani. Toxicon 2016, 113, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.H.; Kim, W.Y.; Kim, M.G. An insulin-binding protein from the venom of a solitary wasp Eumenes pomiformis binds to apolipophorin III in lepidopteran hemolymph. Toxicon 2016, 111, 62–64. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, D.C.; Aerts, M.; Brunain, M.; Desjardins, C.A.; Jacobs, F.J.; Werren, J.H.; Devreese, B. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Mol. Biol. 2010, 19 (Suppl. S1), 11–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Yang, P.; Zhang, Z.; Wu, G.X.; Yang, B. Transcriptomic immune response of Tenebrio molitor pupae to parasitization by Scleroderma guani. PLoS ONE 2013, 8, e54411. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, F. Role of the subtilisin-like serine protease CJPRB from Cordyceps javanica in eliciting an immune response in Hyphantria cunea. Int. J. Mol. Sci. 2023, 24, 4170. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schiesari, L.; Andreatta, G.; Kyriacou, C.P.; O’Connor, M.B.; Costa, R. The insulin-like proteins dILPs-2/5 determine diapause inducibility in Drosophila. PLoS ONE 2016, 11, e0163680. [Google Scholar] [CrossRef] [PubMed]
- Riehle, M.A.; Brown, J.M. Characterization of phosphatase and tensin homolog expression in the mosquito Aedes aegypti: Six splice variants with developmental and tissue specificity. Insect Mol. Biol. 2007, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Palli, S.R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011, 41, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dou, W.; He, L.Q.; Yu, S.S.; Chen, J.Q.; Zheng, L.Y.; Wang, L.; Smagghe, G.; Wang, J.J. Pannier is a key regulator of embryogenesis, pupal development and female reproduction in the insect pest Bactrocera dorsalis. Pest Manag. Sci. 2023, 79, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Katsuki, M.; Okamoto, N.; Fujioka, H.; Okada, K. A specific type of insulin-like peptide regulates the conditional growth of a beetle weapon. PLoS Biol. 2019, 17, e3000541. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Ma, L.; Tian, L.; Wang, S.; Sheng, Z.; Jiang, R.J.; Bendena, W.G.; Li, S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 2010, 56, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Honjoh, S.; Yamamoto, T.; Uno, M.; Nishida, E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 2009, 457, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Kaun, K.R.; Edgar, B.A. The small GTPase Rheb affects central brain neuronal morphology and memory formation in Drosophila. PLoS ONE 2012, 7, e44888. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, C.; Monjon, E.; Van Lommel, J.; Verbakel, L.; Vanden Broeck, J. Peptides in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.G.; Humann, F.C.; Hartfelder, K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Al Baki, M.A.; Lee, D.W.; Jung, J.K.; Kim, Y. Insulin-like peptides of the legume pod borer, Maruca vitrata, and their mediation effects on hemolymph trehalose level, larval development, and adult reproduction. Arch. Insect Biochem. Physiol. 2019, 100, e21524. [Google Scholar] [CrossRef] [PubMed]
- Chowański, S.; Walkowiak-Nowicka, K.; Winkiel, M.; Marciniak, P.; Urbański, A.; Pacholska-Bogalska, J. Insulin-like peptides and cross-talk with other factors in the regulation of insect metabolism. Front. Physiol. 2021, 12, 701203. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, W.; Kang, K.; Pang, R.; Dong, Y.; Liu, K.; Zhang, W. FoxO directly regulates the expression of TOR/S6K and vitellogenin to modulate the fecundity of the brown planthopper. Sci. China Life Sci. 2021, 64, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; Sieglaff, D.H.; Munro, J.B.; Shiao, S.H.; Cruz, J.; Lee, I.W.; Heraty, J.M.; Raikhel, A.S. Forkhead transcription factors regulate mosquito reproduction. Insect Biochem. Mol. Biol. 2007, 37, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-K.; Luo, Y.-J.; Deng, Y.-M.; Li, Y.; Pang, X.-Q.; Xu, C.-D.; Wang, S.-G.; Tang, B. Insulin receptors regulate the fecundity of Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). J. Asia-Pac. Entomol. 2020, 23, 1151–1159. [Google Scholar] [CrossRef]
- Grönke, S.; Clarke, D.F.; Broughton, S.; Andrews, T.D.; Partridge, L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010, 6, e1000857. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Geng, S.-L.; Zhang, X.-S.; Xu, W.-H. P–S6K is associated with insect diapause via the ROS/AKT/ S6K/CREB/HIF-1 pathway in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2020, 120, 103262. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, P.; Zid, B.M.; Harper, T.; Koslover, D.; Sapin, V.; Benzer, S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004, 14, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Alic, N.; Bjedov, I.; Piper, M.D.W. Ageing in Drosophila: The role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 2011, 46, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Rahbe, Y.; Digilio, M.C.; Febvay, G.; Guillaud, J.; Fanti, P.; Pennacchio, F. Metabolic and symbiotic interactions in amino acid pools of the pea aphid, Acyrthosiphon pisum, parasitized by the braconid Aphidius ervi. J. Insect Physiol. 2002, 48, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Mrinalini; Siebert, A.L.; Wright, J.; Martinson, E.; Wheeler, D.; Werren, J.H. Parasitoid venom induces metabolic cascades in flyhosts. Metabolomics 2014, 11, 1–17. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, Z.; Ren, X.; Wu, C.; Zhu, J. Venom IMP-L2 from the Ectoparasitoid Scleroderma guani Regulates the IIS/TOR Pathway in Tenebrio molitor. Insects 2025, 16, 763. https://doi.org/10.3390/insects16080763
Wang W, Zhang Z, Ren X, Wu C, Zhu J. Venom IMP-L2 from the Ectoparasitoid Scleroderma guani Regulates the IIS/TOR Pathway in Tenebrio molitor. Insects. 2025; 16(8):763. https://doi.org/10.3390/insects16080763
Chicago/Turabian StyleWang, Wenxiu, Zhiquan Zhang, Xuemin Ren, Chaoyan Wu, and Jiaying Zhu. 2025. "Venom IMP-L2 from the Ectoparasitoid Scleroderma guani Regulates the IIS/TOR Pathway in Tenebrio molitor" Insects 16, no. 8: 763. https://doi.org/10.3390/insects16080763
APA StyleWang, W., Zhang, Z., Ren, X., Wu, C., & Zhu, J. (2025). Venom IMP-L2 from the Ectoparasitoid Scleroderma guani Regulates the IIS/TOR Pathway in Tenebrio molitor. Insects, 16(8), 763. https://doi.org/10.3390/insects16080763




