Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae) in Sandy Loam Under Constant Moisture and Different Temperature Regimes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Soil
2.3. Observation on Development of Necrophagous Phorid Flies
- (1)
- Larval feeding period: from egg hatching to first larval departure from pork tissue.
- (2)
- Larval period: from egg hatching to first larva pupariation.
- (3)
- Intra-puparial period: from prepupa formation to first adult emergence.
2.4. Measurement of Larval Body Length
2.5. Morphological Changes During the Intra-Puparial Period of Both Species
2.6. Statistical Analysis
3. Results
3.1. Development Time of Larvae and Pupae of Two Necrophagous Phorid Flies
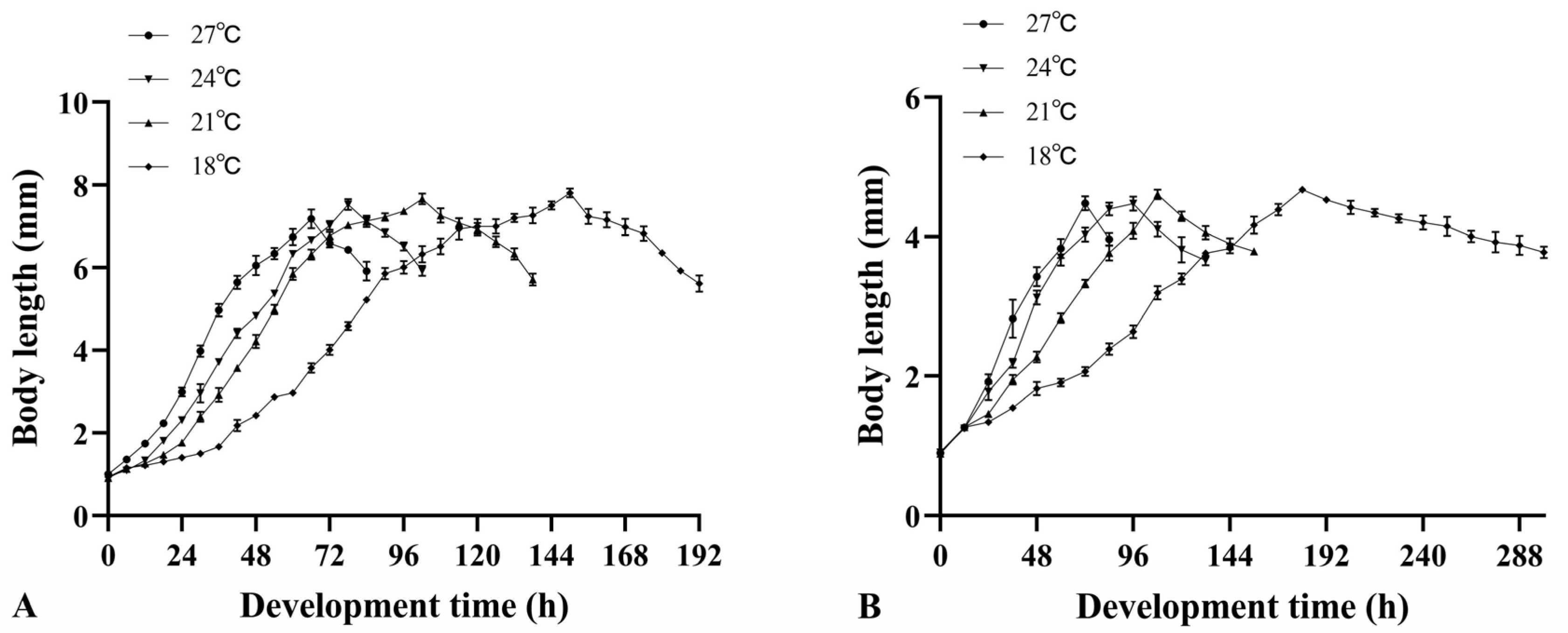
3.2. Changes in Larval Body Length of Two Necrophagous Phorid Flies
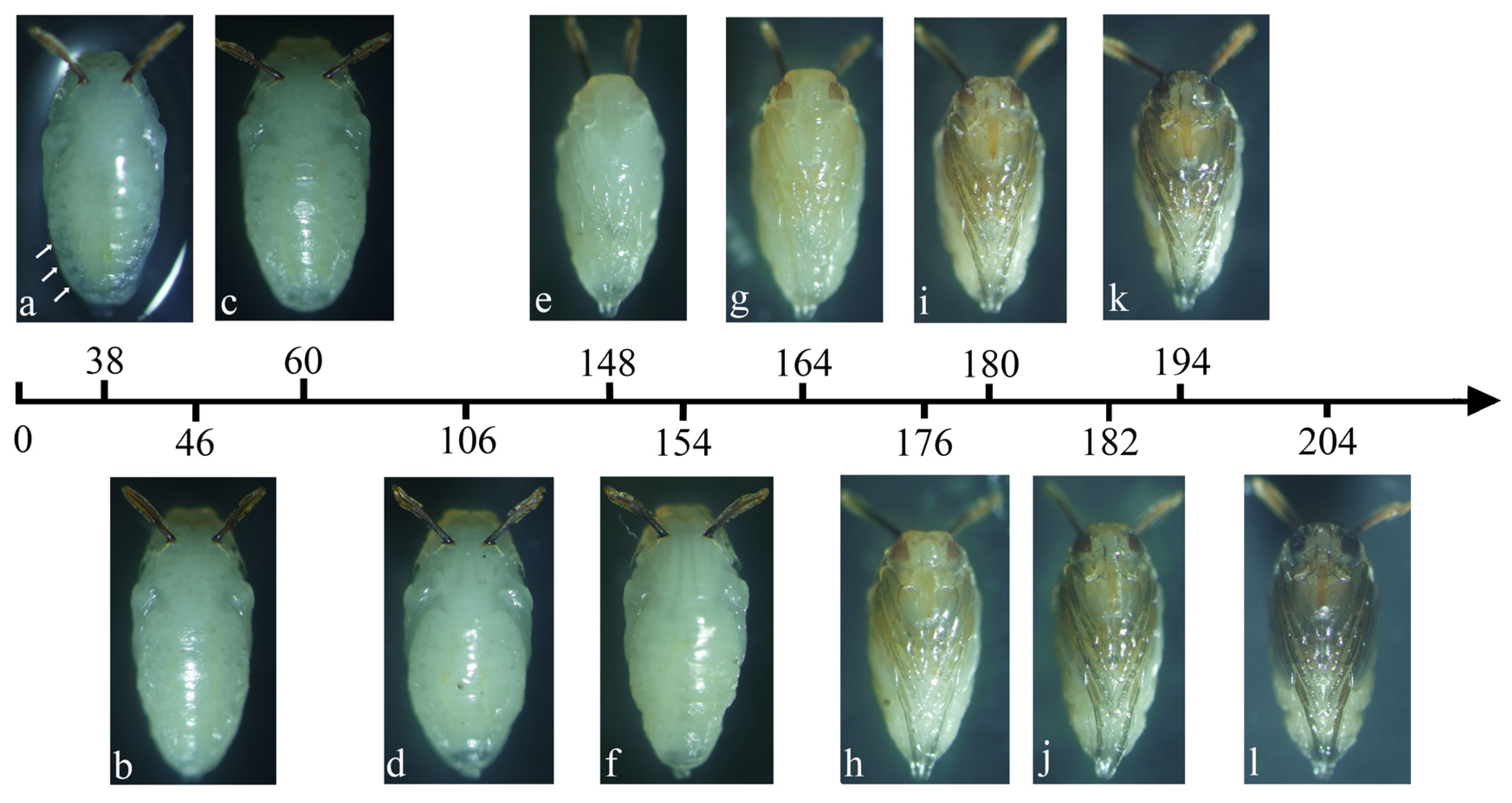
3.3. Intra-Puparial Development of Two Necrophagous Phorid Flies
- Stage 1:
- Protrusion of respiratory horns
- Stage 2:
- Segmentation of thorax and abdomen
- Stage 3:
- Differentiation of dorsal muscle of thorax and segment of abdomen
- Stage 4:
- Pupal cuticle detached from abdominal end
- Stage 5:
- Light yellow eye
- Stage 6:
- Scutellum
- Stage 7:
- Light yellow leg
- Stage 8:
- Light brown leg
- Stage 9:
- Brown leg
- Stage 10:
- Red-brown eye
- Stage 11:
- Black eye
- Stage 12:
- Black wing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srivathsan, A.; Hartop, E.; Puniamoorthy, J.; Lee, W.T.; Kutty, S.N.; Kurina, O.; Meier, R. Rapid, large-scale species discovery in hyperdiverse taxa using 1D MinION sequencing. BMC Biol. 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.C. A Taxonomic Study of Chinese Phorid Flies; Northeastern University Press: Shenyang, China, 2001; pp. 1–6. [Google Scholar]
- Disney, R.H. Natural history of the scuttle fly, Megaselia scalaris. Annu. Rev. Entomol. 2008, 53, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.X.; Wu, J.; Sun, D.P. Intrapuparial age estimation of forensically important Dohrniphora cornuta (Diptera: Phoridae). J. Med. Entomol. 2021, 58, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Disney, R.H.L. Scuttle Flies: The Phoridae; Chapman & Hall: London, UK, 1994; pp. 35–37, 137. [Google Scholar]
- Boehme, P.; Amendt, J.; Disney, R.H.; Zehner, R. Molecular identification of carrion-breeding scuttle flies (Diptera: Phoridae) using COI barcodes. Int. J. Legal. Med. 2010, 124, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Gómez-Gómez, A.; Baz, A. The “coffin fly” Conicera tibialis (Diptera: Phoridae) breeding on buried human remains after a postmortem interval of 18 years. J. Forensic Sci. 2011, 56, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- García-Rojo, A.M.; Martínez-Sánchez, A.; López, R.; García de la Vega, J.M.; Rica, M.; González, M.; Disney, R.H. A mathematical model applied for assisting the estimation of PMI in a case of forensic importance. First record of Conicera similis (Diptera: Phoridae) in a corpse. Forensic Sci. Int. 2013, 231, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Disney, R.H.; Garcia-Rojo, A.; Lindström, A.; Manlove, J.D. Further occurrences of Dohrniphora cornuta (Bigot) (Diptera, Phoridae) in forensic cases indicate likely importance of this species in future cases. Forensic Sci. Int. 2014, 1, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Wells, J.D. Forensic use of Megaselia abdita and M. scalaris (Phoridae: Diptera): Case studies, development rates, and egg structure. J. Med. Entomol. 1998, 35, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Reibe, S.; Madea, B. Use of Megaselia scalaris (Diptera: Phoridae) for post-mortem interval estimation indoors. Parasitol. Res. 2010, 106, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.D.; Soares, T.F.; Costa, D.L. Multiple colonization of a cadaver by insects in an indoor environment: First record of Fannia trimaculata (Diptera: Fanniidae) and Peckia chrysostoma (Sarcophagidae) as colonizers of a human corpse. Int. J. Legal. Med. 2014, 128, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Thevan, K.; Disney, R.H.; Ahmad, A.H. First records of two species of Oriental scuttle flies (Diptera: Phoridae) from forensic cases. Forensic Sci. Int. 2010, 195, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Inda, A.M. Entomofauna of a buried body: Study of the exhumation of a human cadaver in Buenos Aires, Argentina. Forensic Sci. Int. 2014, 237, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Kierbel, I. New records of forensic entomofauna in legally buried and exhumed human infants remains in Buenos Aires, Argentina. J. Forensic. Leg. Med. 2017, 52, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Pittner, S.; Bugelli, V.; Benbow, M.E.; Ehrenfellner, B.; Zissler, A.; Campobasso, C.P.; Oostra, R.J.; Aalders, M.C.G.; Zehner, R.; Lutz, L.; et al. The applicability of forensic time since death estimation methods for buried bodies in advanced decomposition stages. PLoS ONE 2020, 15, 243395. [Google Scholar] [CrossRef] [PubMed]
- Manlove, J.D.; Disney, R.H. The use of Megaselia abdita (Diptera: Phoridae) in forensic entomology. Forensic Sci. Int. 2008, 175, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Disney, R.H.; Manlove, J.D. First occurrences of the Phorid, Megaselia abdita, in forensic cases in Britain. Med. Vet. Entomol. 2005, 19, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Disney, R.H.; Manlove, J.D. First report of Triphleba nudipalpis (Becker) (Diptera: Phoridae) in a forensic case. Forensic Sci. Int. 2009, 191, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Zuha, R.M.; Huong-Wen, S.; Disney, R.H.; Omar, B. Scuttle flies (Diptera: Phoridae) inhabiting rabbit carcasses confined to plastic waste bins in Malaysia include new records and an undescribed species. Trop Life Sci. Res. 2017, 28, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Rabieh, M.M.; Prescher, S.; Alikhani, M.; Arkani, T. Checklist of the scuttle flies (Diptera, Phoridae) of Iran with new records for Iran and Asia. Stud. Dipterol. 2013, 20, 23–30. [Google Scholar]
- Ma, Y.K.; Hu, C.; Min, J.X. A preliminary study on the constitution and succession of insect community on pig carcass in Hangzhou District. Acta Entomol. Sin. 2000, 43, 388–393. [Google Scholar]
- Shalaby, O.A.; deCarvalho, L.M.; Goff, M.L. Comparison of patterns of decomposition in a hanging carcass and a carcass in contact with soil in a xerophytic habitat on the Island of Oahu, Hawaii. J. Forensic Sci. 2000, 45, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; King, E.W.; Beinhart, G. Arthropod succession and decomposition of buried pigs. Nature 1968, 219, 1180–1181. [Google Scholar] [CrossRef] [PubMed]
- Zuha, R.M.; See, H.W.; Disney, R.H.; Omar, B. First record of genus Puliciphora Dahl (Diptera: Phoridae) associated with rabbit carcasses placed in concealed environments in Malaysia. Forensic Sci. Int. 2014, 245, e36–e37. [Google Scholar] [CrossRef] [PubMed]
- Disney, R.H. Duration of development of two species of carrion-breeding scuttle flies and forensic implications. Med. Vet. Entomol. 2005, 19, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.L.; Feng, D.X.; Huang, G.Y.; Sun, D.P.; Dai, S.T. Species composition and succession of necrophagous insects on small buried baits in China. J. Med. Entomol. 2022, 59, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Zuha, R.M.; Omar, B. Developmental rate, size, and sexual dimorphism of Megaselia scalaris (Loew) (Diptera:Phoridae): Its possible implications in forensic entomology. Parasitol. Res. 2014, 113, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.X.; Liu, G.C. Pupal age estimation of forensically important Megaselia scalaris (Loew) (Diptera: Phoridae). Forensic Sci. Int. 2014, 236, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Li, L.L.; Liao, M.Q.; Kang, C.T.; Hu, G.W.; Guo, Y.; Wang, Y.; Wang, J.F. Development of Megaselia scalaris at constant temperatures and its significance in estimating the time of death. Int. J. Legal. Med. 2024, 138, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.X.; Liu, G.C. Pupal age estimation of forensically important Megaselia spiracularis Schmitz (Diptera: Phoridae). Forensic Sci. Int. 2013, 231, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, D.X.; Zou, T.L. Effect of temperature on growth and development of Megaselia spiracularis Schmitz. J. Shenyang Univ. (Nat. Sci.) 2019, 31, 18–21. [Google Scholar]
- Wang, Y.; Zhang, Y.N.; Hu, G.L.; Wang, M.; Zhu, R.; Zhai, Y.S.; Sun, J.; Li, X.F.; Wang, L.H.; Wu, M.W.; et al. Development of Megaselia spiracularis (Diptera: Phoridae) at different constant temperatures. J. Therm. Biol. 2020, 93, 102722. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, D.X.; Zou, T.L.; Wang, X.H. Larval growth and development of Doprniphora cornuta (bigot) under different temperature conditions. J. Shenyang Univ. (Nat. Sci.) 2017, 29, 457–460. [Google Scholar]
- Feng, D.X.; Yue, Y.; Huang, G.; Sun, D. Larval growth and development of Diplonevra funebris (Meigen) under different temperature conditions. J. Shenyang Univ. (Nat. Sci.) 2020, 32, 132–135. [Google Scholar]
- Li, L.; Feng, D.X.; Wu, J. Developmental rate and effective accumulated temperature of Diplonevra peregrina (Wiedemann) under indoor natural temperature. J. Anhui Agric. Sci. 2016, 44, 1–2+32. [Google Scholar]
- Hertl, P.T.; Brandenburg, R.L.; Barbercheck, M.E. Effect of soil moisture on ovipositional behavior in the southern mole cricket (Orthoptera: Gryllotalpidae). Environ. Entomol. 2001, 30, 466–473. [Google Scholar] [CrossRef]
- Simelane, D.O. Influence of soil texture, moisture, and surface cracks on the performance of a root-feeding flea beetle, Longitarsus bethae (Coleoptera: Chrysomelidae), a biological control. Environ. Entomol. 2007, 36, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lepage, M.P.; Bourgeois, G.; Brodeur, J.; Boivin, G. Effect of soil temperature and moisture on survival of eggs and first-instar larvae of Delia radicum. Environ. Entomol. 2012, 41, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, L.Y.; Shahid, S.; Smagghe, G.; Liu, T.X. Effect of soil moisture on pupation behavior and inhabitation of Spodoptera frugiperda (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2021, 56, 69–74. [Google Scholar] [CrossRef]
- Pan, R.D.; Li, P.Z.; Han, D.Y.; Fu, Y.G.; Zhan, C.L.; Li, L. Effect of soil and water content on the development and eclosion of Frankliniella Intonsa Pseudopupa. Chin. Agric. Sci. Bull. 2023, 39, 138–143. [Google Scholar]
- Kökdener, M.; Şahin Yurtgan, M. The Effect of soil type and moisture level on the development of Lucilia sericata (Diptera: Calliphoridae). J. Med. Entomol. 2022, 59, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Feng, D.X.; Tang, Y.N. The effect of soil type and moisture on the development of forensically important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae). Insects 2024, 15, 666. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shelton, A.M. Impact of soil type, moisture, and depth on Swede Midge (Diptera: Cecidomyiidae) pupation and emergence. Environ. Entomol. 2007, 36, 1349–1355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, Y.Z.; Jin, X.F.; Zhu, C.Q.; Chen, X.; Ma, T.; Zhang, S.N.; Zhang, Y.; Zeng, S.C.; Chen, X.Y.; Sun, Z.H.; et al. Effect of substrate type and moisture on pupation and emergence of Heortia vitessoides (Lepidoptera: Crambidae): Choice and no-choice studies. J. Insect. Behav. 2016, 29, 473–489. [Google Scholar] [CrossRef]
- Amaral, E.J.; Sousa, M.D.; Santos, L.M.; Costa, L.M.; Melem Junior, N.J.; Toledo, J.J.; Adaime, R. Effect of soil class and moisture on the depth of pupation and pupal viability of Bactrocera carambolae Drew & Hancock (1994). Rev. Bras. Entomol. 2021, 65, e20200075. [Google Scholar]
- Clark, K.; Evans, L.; Wall, R. Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci. Int. 2006, 156, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Bambaradeniya, T.B.; Magni, P.A.; Dadour, I.R. Morphological changes of larvae and pupae of Lucilia sericata (Diptera: Calliphoridae) reared at two temperatures and on three food types. J. Med. Entomol. 2024, 61, 521–529. [Google Scholar] [CrossRef] [PubMed]
- El-Moaty, Z.A.; Kheirallah, A.E.M. Developmental variation of the blow fly Lucilia sericata (Meigen, 1826) (Diptera: Calliphoridae) by different substrate tissue types. J. Asia-Pacific Entomol. 2013, 16, 297–300. [Google Scholar] [CrossRef]
- Gallagher, M.B.; Sandhu, S.; Kimsey, R. Variation in developmental time for geographically distinct populations of the common green bottle fly, Lucilia sericata (Meigen). J. Forensic Sci. 2010, 55, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yuan, X.; Zhu, F.; Lei, C. Development time and size-related traits in the oriental blowfly, Chrysomya megacephala along a latitudinal gradient from China. J. Thermal Bio. 2010, 35, 366–371. [Google Scholar] [CrossRef]
- Pereira, A.J.; Centeno, N.D.; Nuñez-Vázquez, C. Effects of population variations and temperature on Chrysomya megacephala (Diptera: Calliphoridae) development: Implications for estimating the postmortem interval. Int. J. Legal Med. 2024, 138, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Owings, C.G.; Spiegelman, C.; Tarone, A.M.; Tomberlin, J.K. Developmental variation among Cochliomyia macellaria Fabricius (Diptera: Calliphoridae) populations from three ecoregions of Texas, USA. Int. J. Legal Med. 2014, 128, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Defilippo, F.; Bonilauri, P.; Dottori, M. Effect of temperature on six different developmental landmarks within the pupal stage of the forensically important blowfly Calliphora vicina (Robineau-Desvoidy) (Diptera: Calliphoridae). J. Forensic Sci. 2013, 58, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.M.; Moura, M.O. Intrapuparial development of Hemilucilia semidiaphana (Diptera: Calliphoridae) and its use in forensic entomology. J. Med. Entomol. 2019, 56, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.Y.; Xia, S.X.; Wang, J.F.; Zhang, Y.N.; Tao, L.Y. Estimating the age of Lucilia illustris during the intrapuparial period using two approaches: Morphological changes and differential gene expression. Forensic Sci. Int. 2018, 287, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sert, O.; Örsel, G.M.; Şabanoğlu, B.; Özdemir, S. A Study of the pupal developments of Sarcophaga argyrostoma (Robineau-Desvoidy, 1830). Forensic Sci. Med. Pathol. 2020, 16, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zajac, B.K.; Amendt, J.; Verhoff, M.A.; Zehner, R. Dating pupae of the blow fly Calliphora vicina robineau–desvoidy 1830 (Diptera: Calliphoridae) for post mortem interval estimation: Validation of molecular age markers. Genes 2018, 3, 153. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Ren, L.; Yang, L.; Wang, S.; Chen, W.; Dong, J.; Ma, H.; Qi, X.; Guo, Y. Differential gene expression for age estimation of forensically important Sarcophaga peregrina (Diptera: Sarcophagidae) intrapuparial. J. Med. Entomol. 2020, 57, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Feng, Y.; Ren, L.; Zhang, X.; Yang, F.; Zhang, C.; Guo, Y. Pupal age estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at different constant temperatures utilizing ATR-FTIR spectroscopy and cuticular hydrocarbons. Insects 2023, 14, 143. [Google Scholar] [CrossRef] [PubMed]


| Corpse Types | Species | Collection Environments | References |
|---|---|---|---|
| Human remains | Conicera tibialis Schmitz, 1925 | indoors | [6] |
| burial | [7] | ||
| Conicera similis Haliday, 1833 | burial | [8] | |
| Dohrniphora cornuta (Bigot, 1857) | indoors | [9] | |
| burial | [9] | ||
| Megaselia scalaris (Loew, 1866) | indoors | [6,10,11,12,13] | |
| burial | [14,15,16] | ||
| Megaselia abdita Schmitz, 1959 | indoors | [6,10,17] | |
| burial | [18] | ||
| Megaselia rufipes (Meigen, 1804) | indoors | [18] | |
| Megaselia spiracularis Schmitz, 1938 | indoors | [13] | |
| Megaselia curtineura (Brues, 1909) | indoors | [13] | |
| Triphleba opaca (Meigen, 1830) | burial | [5] | |
| Triphleba nudipalpis (Schmitz, 1922) | burial | [19] | |
| Animal remains | Dahliphora sigmoides Schmitz, 1923 | waste bin | [20] |
| Diplonevra funebris (Meigen, 1830) | outdoor | [21] | |
| Diplonevra peregrina (Wiedemann, 1830) | outdoor | [22,23] | |
| Diplonevra florea (Fabricius, 1794) | outdoor | [5] | |
| Dohrniphora incisuralis (Loew, 1866) | burial | [24] | |
| Gymnoptera simplex (Brues, 1905) | waste bin | [20] | |
| Metopina subarcuata Borgmeier, 1963 | burial | [24] | |
| Puliciphora borinquenensis Wheeler, 1906 | luggage and garbage bin | [25] | |
| Puliciphora beckeri Meijere, 1907 | luggage and garbage bin | [25] | |
| Puliciphora obtecta Meijere, 1912 | luggage and garbage bin | [25] | |
| Spiniphora sp. | waste bin | [20] | |
| Animal muscle tissues | Megaselia giraudii (Egger, 1862) | outdoor | [26] |
| Metopina sagittata Liu, 1995 | burial | [27] |
| Temperature (°C) | Feeding Period | Larval Period | Intra-Puparial Period | Pupation Rate (%) | Emergence Rate (%) |
|---|---|---|---|---|---|
| 18 | 124.74 ± 0.88 d | 165.18 ± 2.96 d | 606.67 ± 3.38 d | 87.67 ± 0.04 a | 93.50 ± 0.04 b |
| 21 | 86.37 ± 3.86 c | 119.72 ± 2.74 c | 404.62 ± 3.28 c | 97.00 ± 0.03 b | 96.00 ± 0.04 b |
| 24 | 67.44 ± 3.50 b | 90.28 ± 3.54 b | 269.22 ± 6.04 b | 90.00 ± 0.04 a | 93.00 ± 0.05 b |
| 27 | 45.47 ± 1.74 a | 63.04 ± 3.45 a | 237.57 ± 3.41 a | 92.01 ± 0.04 a | 81.50 ± 0.09 a |
| Temperature (°C) | Feeding Period | Larval Period | Intra-Puparial Period | Pupation Rate (%) | Emergence Rate (%) |
|---|---|---|---|---|---|
| 18 | 169.03 ± 3.39 d | 249.37 ± 4.88 d | 593.37 ± 4.75 d | 89.68 ± 0.03 a | 87.00 ± 0.04 a |
| 21 | 116.77 ± 2.52 c | 154.59 ± 1.81 c | 414.23 ± 3.51 c | 92.67 ± 0.05 a | 95.00 ± 0.03 b |
| 24 | 80.04 ± 3.01 b | 108.80 ± 2.65 b | 261.02 ± 3.16 b | 92.00 ± 0.04 a | 91.00 ± 0.05 ab |
| 27 | 62.53 ± 5.80 a | 86.04 ± 3.91 a | 236.52 ± 2.66 a | 90.00 ± 0.05 a | 88.00 ± 0.08 a |
| Temperature (°C) | Equation | R2 | F | p |
|---|---|---|---|---|
| 18 | Y = −0.415X3 + 4.225X2 + 11.742X − 4.352 | 0.807 | 454.996 | <0.001 |
| 21 | Y = −0.616X3 + 7.255X2 − 7.836X + 10.108 | 0.769 | 262.054 | <0.001 |
| 24 | Y = −0.393X3 + 4.747X2 − 3.263X + 5.675 | 0.869 | 388.120 | <0.001 |
| 27 | Y = −0.127X3 + 1.772X2 + 3.969X − 2.196 | 0.844 | 264.044 | <0.001 |
| Temperature (°C) | Equation | R2 | F | p |
|---|---|---|---|---|
| 18 | Y = −15.262X3 + 122.161X2 − 224.632X + 134.277 | 0.770 | 285.720 | <0.001 |
| 21 | Y = −6.136X3 + 48.402X2 − 76.542X + 41.802 | 0.838 | 235.351 | <0.001 |
| 24 | Y = −5.562X3 + 42.510X2 − 65.585X + 33.472 | 0.754 | 118.426 | <0.001 |
| 27 | Y = −0.456X3 + 4.212X2 + 9.413X − 8.719 | 0.917 | 278.067 | <0.001 |
| Temperature (°C) | Time of Distinctive Features Appearance (h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | |
| 18 | 82 | 126 | 154 | 204 | 324 | 350 | 412 | 432 | 450 | 464 | 490 | 514 |
| 21 | 54 | 90 | 138 | 180 | 228 | 252 | 286 | 306 | 316 | 324 | 332 | 340 |
| 24 | 40 | 64 | 96 | 116 | 166 | 176 | 192 | 202 | 208 | 212 | 228 | 232 |
| 27 | 33 | 42 | 76 | 116 | 138 | 148 | 170 | 176 | 180 | 188 | 198 | 204 |
| Temperature (°C) | Time of Distinctive Features Appearance (h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | |
| 18 | 84 | 120 | 176 | 240 | 330 | 382 | 402 | 452 | 466 | 480 | 494 | 512 |
| 21 | 59 | 90 | 114 | 168 | 230 | 262 | 278 | 312 | 320 | 328 | 336 | 344 |
| 24 | 43 | 78 | 98 | 120 | 180 | 192 | 210 | 220 | 224 | 230 | 234 | 248 |
| 27 | 38 | 46 | 60 | 106 | 148 | 154 | 164 | 176 | 180 | 182 | 194 | 204 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Feng, D.; Tang, Y. Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae) in Sandy Loam Under Constant Moisture and Different Temperature Regimes. Insects 2025, 16, 760. https://doi.org/10.3390/insects16080760
Han W, Feng D, Tang Y. Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae) in Sandy Loam Under Constant Moisture and Different Temperature Regimes. Insects. 2025; 16(8):760. https://doi.org/10.3390/insects16080760
Chicago/Turabian StyleHan, Wei, Dianxing Feng, and Yanan Tang. 2025. "Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae) in Sandy Loam Under Constant Moisture and Different Temperature Regimes" Insects 16, no. 8: 760. https://doi.org/10.3390/insects16080760
APA StyleHan, W., Feng, D., & Tang, Y. (2025). Development of Forensically Important Megaselia scalaris and Dohrniphora cornuta (Diptera: Phoridae) in Sandy Loam Under Constant Moisture and Different Temperature Regimes. Insects, 16(8), 760. https://doi.org/10.3390/insects16080760





