Hydroxylated vs. Carboxylated Nanotubes: Differential Impacts on Fall Armyworm Development, Reproduction, and Population Dynamics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Mass Culture
2.2. Preparation of Artificial Diet
2.3. Materials and Reagents
2.4. Preparation of MWCNT Solutions
2.5. Characterization of MWCNTs
2.6. Development Time, Reproduction Period, Fecundity, and Longevity
2.7. Life Table Analysis
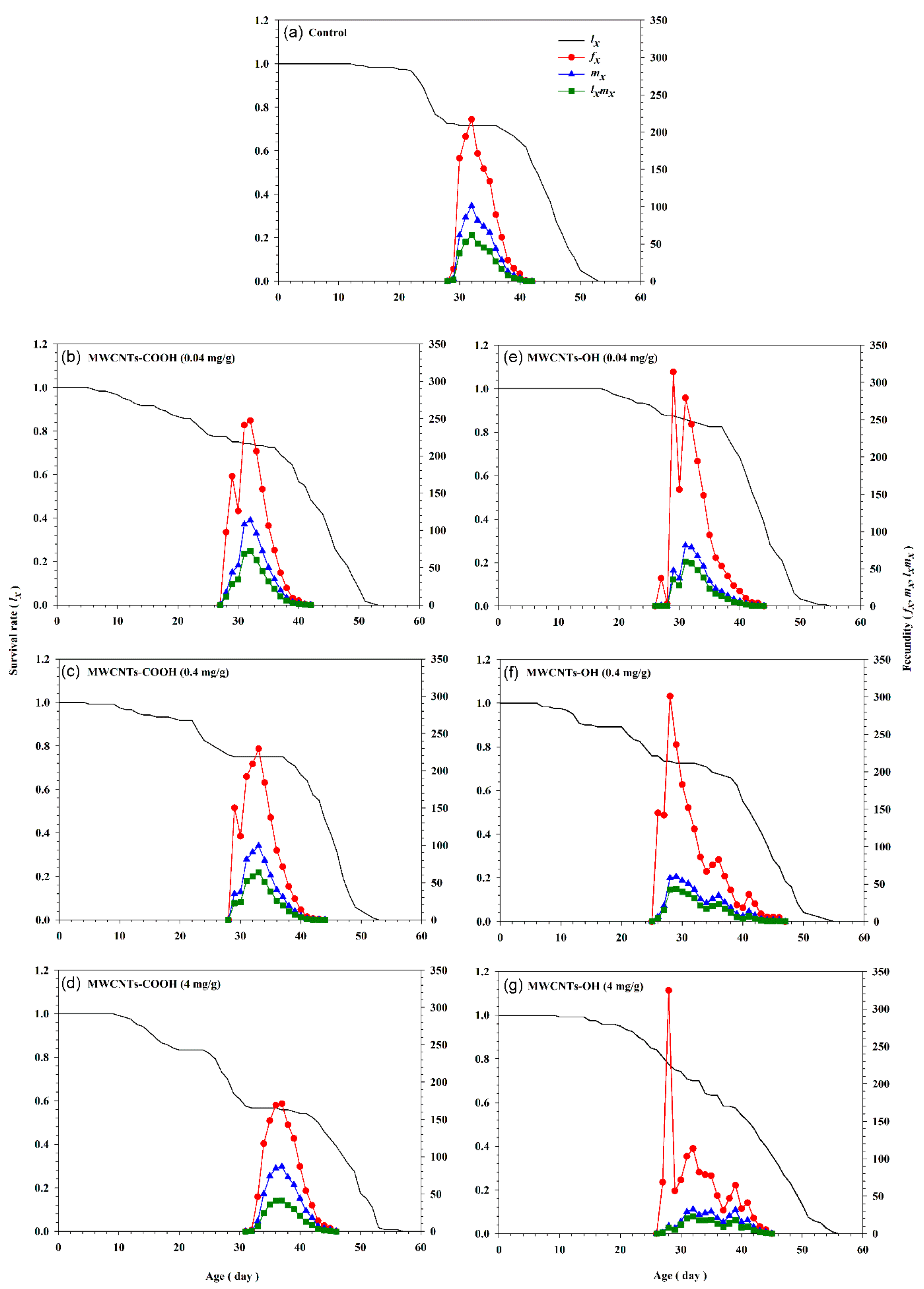
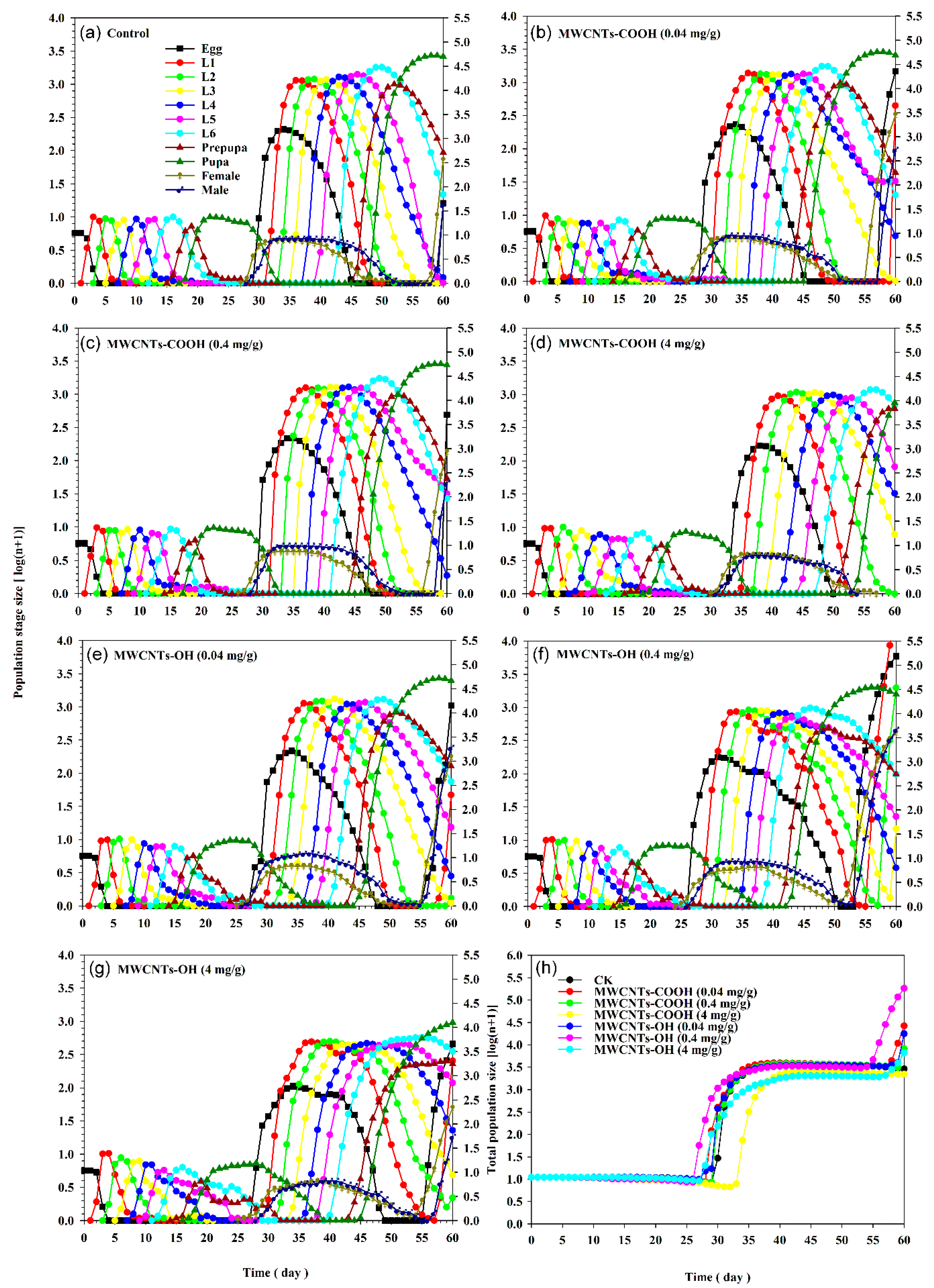
3. Results
3.1. Characterization of MWCNTs
3.2. Impact of MWCNTs on Development, Longevity, and Reproduction of S. frugiperda
3.3. Impact of MWCNTs on Population Parameters of S. frugiperda
3.4. Impact of MWCNTs on Life Table Parameters of S. frugiperda
3.5. Impact of MWCNTs on S. frugiperda Population Projection
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Huang, W.; Dong, Y.; Huang, Y.; Zhang, B.; Sun, G.; Elkahky, M.; Huang, C. Temporal analyses of global suitability distribution for fall armyworm based on Multiple factors. Ecol. Indic. 2025, 171, 113181. [Google Scholar] [CrossRef]
- Chimweta, M.; Nyakudya, I.W.; Jimu, L.; Mashingaidze, A.B. Fall armyworm [Spodoptera frugiperda (JE Smith)] damage in maize: Management options for flood-recession cropping smallholder farmers. Int. J. Pest. Manag. 2020, 66, 142–154. [Google Scholar] [CrossRef]
- Dilip, D.; Modupalli, N.; Rahman, M.M.; Kariyat, R. Atmospheric cold plasma alters plant traits and negatively affects the growth and development of fall armyworm in rice. Sci. Rep. 2025, 15, 3680. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Deng, X.; Tao, W.; Zhang, Z.; Zhang, H.; Li, Q.; Jiang, C. Sublethal effects of chlorantraniliprole on immunity in Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae): Promote encapsulation by upregulating a heat shock protein 70 family gene SfHSP68.1. Pestic. Biochem. Physiol. 2024, 201, 105892. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, F.; Jia, W.; Jiang, Y.; Wu, X.; Song, S.; Shen, H.; Shen, J. Nanomaterials and nanotechnology in agricultural pesticide delivery: A review. Langmuir 2024, 40, 18806–18820. [Google Scholar] [CrossRef] [PubMed]
- Zainab, R.; Hasnain, M.; Ali, F.; Abideen, Z.; Siddiqui, Z.S.; Jamil, F.; Hussain, M.; Park, Y.-K. Prospects and challenges of nanopesticides in advancing pest management for sustainable agricultural and environmental service. Environ. Res. 2024, 261, 119722. [Google Scholar] [CrossRef] [PubMed]
- Husen, A. Emerging Carbon Nanomaterials for Sustainable Agricultural Practices, 1st ed.; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kushwah, A.; Satpute, A.N.; Singh, S.K.; Patil, A.K. A comprehensive review on potential application of nanomaterials in the field of agricultural engineering. J. Biosyst. Eng. 2023, 48, 457–477. [Google Scholar] [CrossRef]
- Sakthivel, A.; Chandrasekaran, R.; Balasubramaniam, S.; Sathyanarayanan, H.; Gnanajothi, K.; Selvakumar, T. Nanomaterials as potential plant growth modulators: Applications, mechanism of uptake, and toxicity: A comprehensive review. BioNanoScience 2025, 15, 53. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, J.; Liu, Y.; Jiang, R.; Cai, S.; Liu, Y.; Zhu, F.; Zeng, F.; Luan, T.; Ouyang, G. Carbon nanotubes act as contaminant carriers and translocate within plants. Sci. Rep. 2015, 5, 15682. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-L.; Zhang, Y.-H.; Xing, R.; Zhou, Y.-F.; Chen, X.-D.; Wang, H.; Song, B.; Sima, Y.-H.; He, Y.; Xu, S.-Q. Different toxicity of cadmium telluride, silicon, and carbon nanomaterials against hemocytes in silkworm, Bombyx mori. RSC Adv. 2017, 7, 50317–50327. [Google Scholar] [CrossRef]
- Yu, F.-X.; Mobarak, S.H.; Yang, M.-F.; Hu, C.-X.; Liu, T.-X. Influence of carboxylated and hydroxylated multi-walled carbon nanotubes on the development and reproductive abilities of Myzus persicae. Entomol. Gen. 2024, 44, 1213–1223. [Google Scholar] [CrossRef]
- Chi, H.; Ali, G.; Aurang, K.; Gholamhossein, G.; Remzi, A.; Mehmet, S.Ö.; Jalal, S.; Masood, A.-M.; Mostafa, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Karmakar, A.; Mobarak, S.H.; Koner, A.; Mitra, P.; Barik, A. Effects of photoperiods on demography and population growth of Aulacophora foveicollis Lucas reared on Solena amplexicaulis plant. Int. J. Trop. Insect Sci. 2021, 41, 1407–1418. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.S.; Mukherjee, S. Carbon Nanotubes in Agriculture; Elsevier Inc.: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Kumar, V.; Dasgupta, N.; Ranjan, S. Environmental Toxicity of Nanomaterials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–537. [Google Scholar] [CrossRef]
- Huang, B. Carbon nanotubes and their polymeric composites: The applications in tissue engineering. Biomanufact. Rev. 2020, 5, 3. [Google Scholar] [CrossRef]
- Li, C.; Hu, C.; Zhi, J.; Yue, W.; Li, H. Effects of nano-graphene oxide on the growth and reproductive dynamics of Spodoptera frugiperda based on an age-stage, two-sex life table. Insects 2022, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.H.Z.; de Sousa, M.; Fonseca, L.C.; Martinez, D.S.T.; Alves, O.L. Biological effects of oxidized carbon nanomaterials (1D versus 2D) on Spodoptera frugiperda: Material dimensionality influences on the insect development, performance and nutritional physiology. Chemosphere 2019, 215, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, M.; Li, W.; Luo, S.; Wu, Y.; Ma, C.; Liu, S. Adsorption separation of Cr(VI) from a water phase using multiwalled carbon nanotube-immobilized ionic liquids. ACS Omega 2020, 5, 22827–22839. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for Age Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2024; Available online: https://lifetablechi.com/software/ (accessed on 13 November 2024).
- Mobarak, S.H.; Roy, N.; Barik, A. Two-sex life table and feeding dynamics of Spilosoma obliqua Walker (Lepidoptera: Arctiidae) on three green gram cultivars. Bull. Entomol. Res. 2020, 110, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. TIMING-MSChart: A Computer Program for Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2024; Available online: https://lifetablechi.com/software/ (accessed on 13 November 2024).
- Cui, H.; Yan, X.; Monasterio, M.; Xing, F. Effects of various surfactants on the dispersion of MWCNTs–OH in aqueous solution. Nanomaterials 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyar, N.K.; Esmaeili, S.; Javadpour, R.; Heris, S.Z. Experimental investigation of indirect heat transfer through a novel designed lab-scale setup using functionalized MWCNTs nanofluids (MWCNTs-COOH/water and MWCNTs- OH/water). Case Stud. Therm. Eng. 2023, 45, 102951. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Cristóvan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Mobarak, S.H.; Debnath, R.; Koner, A.; Barik, A. Effect of temperature for mass rearing of Spilosoma obliqua on an artificial diet using age-stage, two-sex life table approach. Biologia 2022, 77, 1327–1335. [Google Scholar] [CrossRef]
- Chi, H.; Yang, T.C. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2003, 32, 327–333. [Google Scholar] [CrossRef]
- Tripathi, S.; Mahra, S.; Victoria, J.; Tiwari, K.; Rana, S.; Tripathi, D.K.; Sharma, S.; Sahi, S. Recent advances and perspectives of nanomaterials in agricultural management and associated environmental risk: A review. Nanomaterials 2023, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Garg, V.K.; Chhillar, A.K.; Rana, J.S. Recent advances in nanotechnology for the improvement of conventional agricultural systems: A review. Plant Nano. Biol. 2023, 4, 100032. [Google Scholar] [CrossRef]
- Cañete-Rosales, P.; Ortega, V.; Álvarez-Lueje, A.; Bollo, S.; González, M.; Ansón, A.; Martínez, M.T. Influence of size and oxidative treatments of multi-walled carbon nanotubes on their electrocatalytic properties. Electrochim. Acta 2012, 62, 163–171. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Kokotos, C.G.; Karousis, N.; Kokotos, G.; Tagmatarchis, N. Functionalized multi-walled carbon nanotubes in an aldol reaction. Nanoscale 2015, 7, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Coll. Int. Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, N.; Mohebbi, M. Anti-infective and toxicity properties of carbon based materials: Graphene and functionalized carbon nanotubes. Microorganisms 2022, 10, 2439. [Google Scholar] [CrossRef] [PubMed]
- McShan, D.; Yu, H. DNA damage in human skin keratinocytes caused by multiwalled carbon nanotubes with carboxylate functionalization. Toxicol. Ind. Health 2014, 30, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Solorio-Rodriguez, S.A.; Williams, A.; Poulsen, S.S.; Knudsen, K.B.; Jensen, K.A.; Clausen, P.A.; Danielsen, P.H.; Wallin, H.; Vogel, U.; Halappanavar, S. Single-walled vs. multi-walled carbon nanotubes: Influence of physico-chemical properties on toxicogenomics responses in mouse lungs. Nanomaterials 2023, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Toscano, F.; Torres-Arias, M. Nanoparticles cellular uptake, trafficking, activation, toxicity and in vitro evaluation. Curr. Res. Immunol. 2023, 4, 100073. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chauhan, R.; Saurabh, S.; Shukla, A.K.; Jamal, F.; Singh, S.P.; Singh, P.K.; Mishra, M. The impact of carbon NPs on the accumulation of storage proteins and the generation advancement of the polyphagous insect pest tobacco cutworm Spodoptera litura (Fabricius). Environ. Sci. Nano. 2024, 11, 2428–2446. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Witas, P.; Karpeta-Kaczmarek, J.; Kwaśniewska, J.; Flasz, B.; Balin, K.; Augustyniak, M. Reduced fecundity and cellular changes in Acheta domesticus after multigenerational exposure to graphene oxide nanoparticles in food. Sci. Total Environ. 2018, 635, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, B.; Feng, W.; Du, W.; Ouyang, H.; Chai, Z.; Bi, X. Oral magnetite nanoparticles disturb the development of Drosophila melanogaster from oogenesis to adult emergence. Nanotoxicology 2015, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Tavabe, K.R.; Kuchaksaraei, B.S.; Javanmardi, S. Impacts of ZnO and multi-walled carbon nanotubes (MWCNTs) on biological parameters of the Oriental river prawn Macrobrachium nipponense De Haan, 1849 (Decapoda: Caridea: Palaemonidae). J. Crustac. Biol. 2023, 43, ruad019. [Google Scholar] [CrossRef]
- Asghar, M.S.; Sarwar, Z.M.; Almadiy, A.A.; Shami, A.; Mohamed, R.A.E.H.; Ahmed, N.; Waghulade, M.S.; Alam, P.; Galil, F.M.A.A. Toxicological effects of silver and zinc oxide nanoparticles on the biological and life table parameters of Helicoverpa armigera (Noctuidae: Lepidoptera). Agriculture 2022, 12, 1744. [Google Scholar] [CrossRef]
- Korghond, G.T.; Sahebzadeh, N.; Allahyari, H.; Ramroodi, S. Acute toxicity and sublethal effects of metal oxide nanoparticles against the bulb mite. Syst. Appl. Acarol. 2021, 26, 788–800. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Walker, V.K.; Afrooz, A.R.M.N.; Saleh, N.B.; Winn, L.M. Investigating the effects of functionalized carbon nanotubes on reproduction and development in Drosophila melanogaster and CD-1 mice. Reprod. Toxicol. 2011, 32, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Xie, H.; Wang, Z.; Jing, D.; He, K.; Gao, X. Phenotypic responses and potential genetic mechanism of lepidopteran insects under exposure to graphene oxide. Ecotoxicol. Environ. Saf. 2021, 228, 113008. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vinson, D.; Abt, D.; Hurt, R.H.; Rand, D.M. Differential toxicity of carbon nanomaterials in Drosophila: Larval dietary uptake is benign, but adult exposure causes locomotor impairment and mortality. Environ. Sci. Technol. 2009, 43, 6357–6363. [Google Scholar] [CrossRef] [PubMed]
- Marano, F.; Hussain, S.; Rodrigues-Lima, F.; Baeza-Squiban, A.; Boland, S. Nanoparticles: Molecular targets and cell signalling. Arch. Toxicol. 2011, 85, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, M.S.; Reiter, K.E.; Bennett, A.; Gerard, P.D.; Wei, Q.H.; Byler, M.; Yan, H.; Lee, W.K. The ingestion of fluorescent, magnetic nanoparticles for determining fluid-uptake abilities in insects. J. Vis. Exp. 2017, 130, e56619. [Google Scholar] [CrossRef] [PubMed]
- Pidgeon, N.; Harthorn, B.H.; Bryant, K.; Rogers-Hayden, T. Deliberating the risks of nanotechnologies for energy and health applications in the United States and United Kingdom. Nat. Nanotechnol. 2008, 4, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Son, Y.; Yoon, T.K.; Kim, S.; Kim, W. The effect of multi-walled carbon nanotubes on soil microbial activity. Ecotoxicol. Environ. Saf. 2011, 74, 569–575. [Google Scholar] [CrossRef] [PubMed]


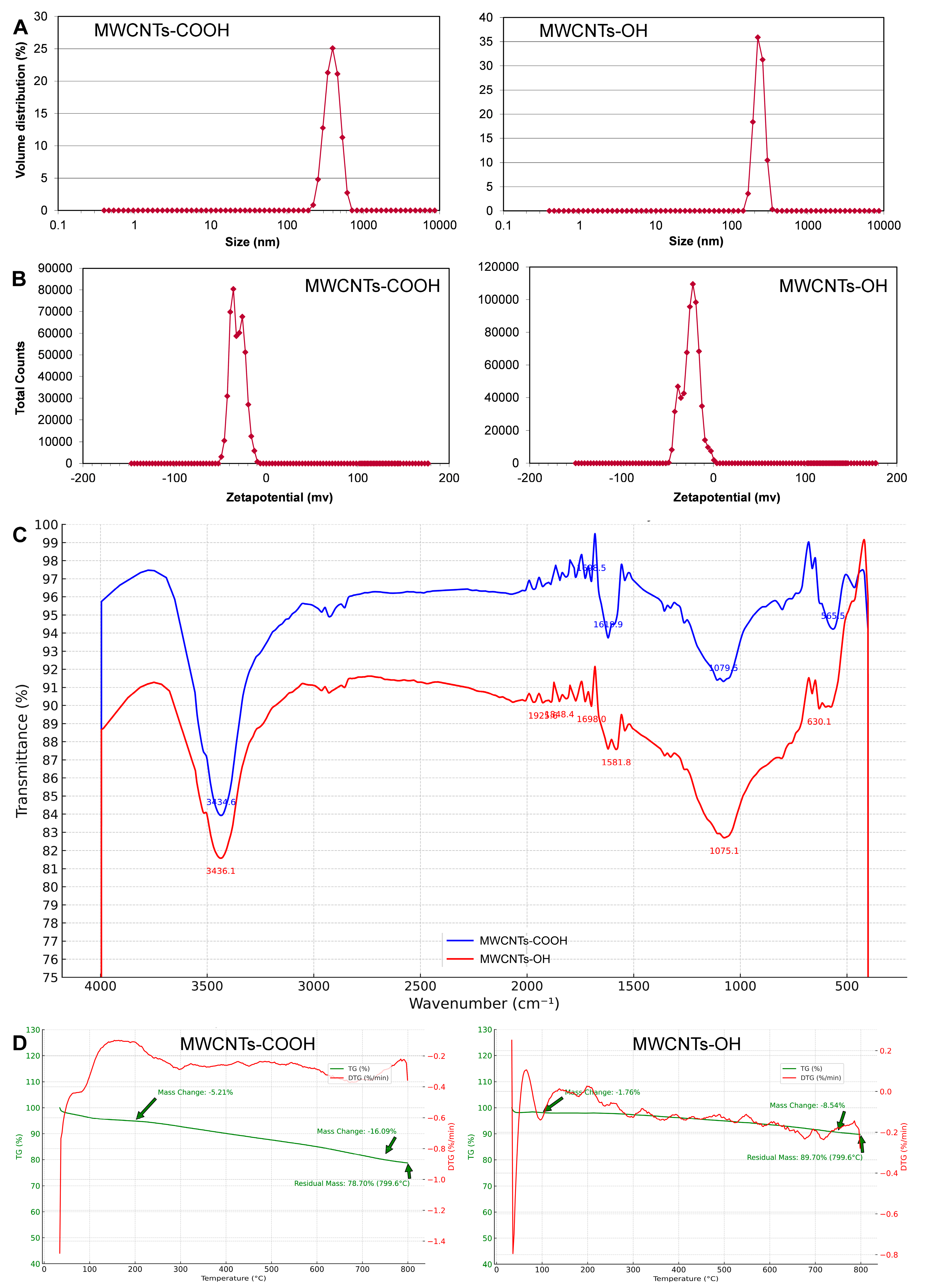
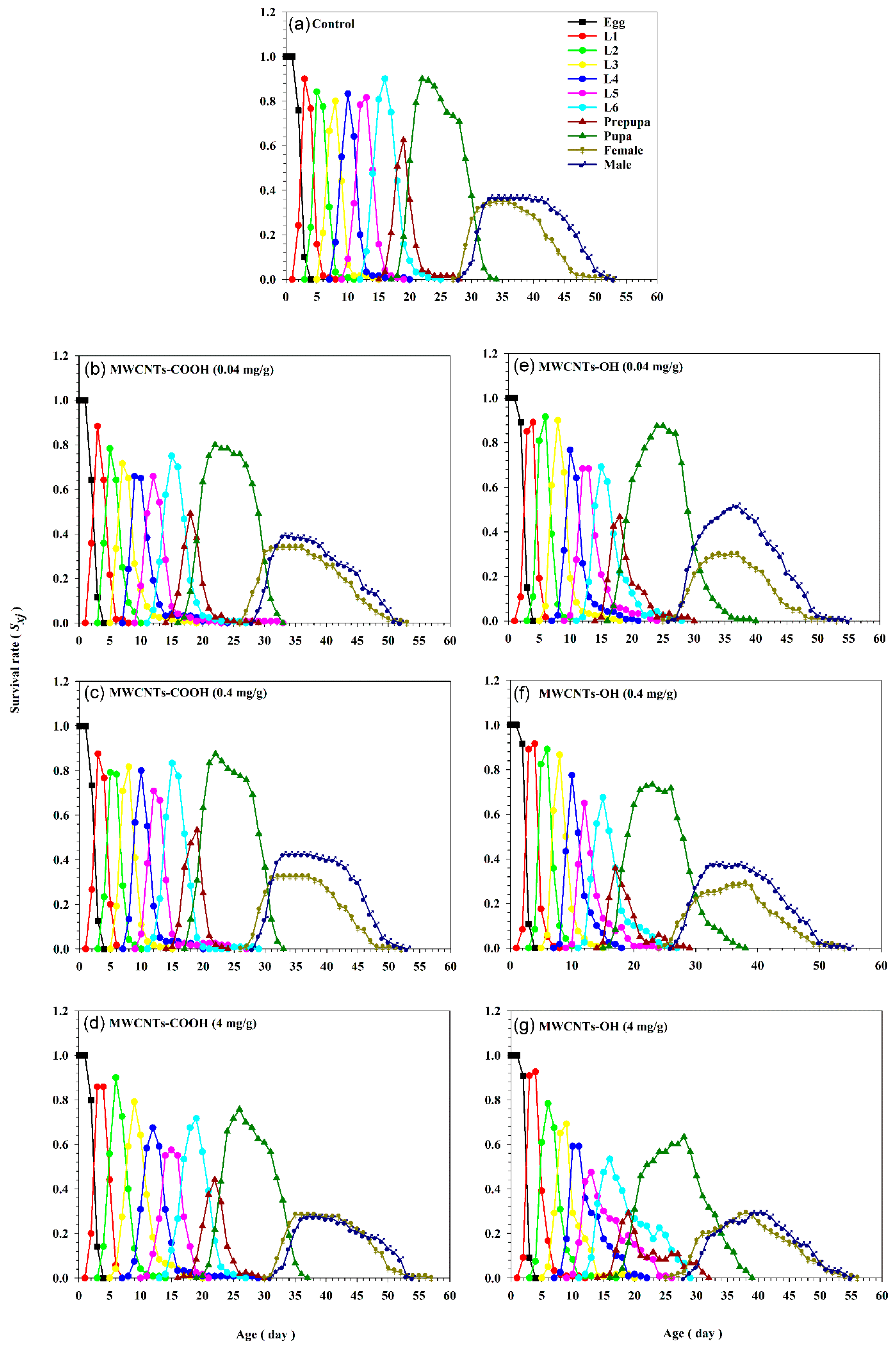


| Parameters | N | Control | MWCNTs-COOH (mg/g) | MWCNTs-OH (mg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 0.04 | N | 0.4 | N | 4 | N | 0.04 | N | 0.4 | N | 4 | |||
| Egg (d) | 120 | 2.86 ± 0.05 bc | 120 | 2.76 ± 0.06 c | 120 | 2.86 ± 0.06 bc | 120 | 2.94 ± 0.05 ab | 120 | 3.04 ± 0.05 a | 120 | 3.03 ± 0.04 a | 120 | 3.00 ± 0.04 a |
| 1st instar (d) | 120 | 2.09 ± 0.03 b | 120 | 2.13 ± 0.04 b | 120 | 2.13 ± 0.03 b | 120 | 2.42 ± 0.05 a | 120 | 2.06 ± 0.02 b | 120 | 2.13 ± 0.04 b | 120 | 2.56 ± 0.09 a |
| 2nd instar (d) | 120 | 2.23 ± 0.04 bcd | 119 | 2.18 ± 0.04 cd | 119 | 2.16 ± 0.03 d | 116 | 2.84 ± 0.06 a | 120 | 2.33 ± 0.05 b | 118 | 2.29 ± 0.04 bc | 119 | 2.68 ± 0.06 a |
| 3rd instar (d) | 119 | 2.19 ± 0.04 e | 115 | 2.29 ± 0.06 de | 117 | 2.32 ± 0.05 de | 106 | 2.98 ± 0.08 a | 120 | 2.66 ± 0.10 b | 116 | 2.42 ± 0.06 cd | 118 | 2.54 ± 0.07 bc |
| 4th instar (d) | 117 | 2.46 ± 0.05 b | 108 | 2.48 ± 0.08 b | 113 | 2.48 ± 0.08 b | 100 | 3.10 ± 0.06 a | 120 | 2.38 ± 0.08 b | 115 | 2.43 ± 0.07 b | 116 | 2.88 ± 0.10 a |
| 5th instar (d) | 117 | 2.82 ± 0.05 b | 104 | 2.62 ± 0.07 c | 109 | 2.44 ± 0.05 d | 99 | 3.02 ± 0.07 a | 120 | 2.68 ± 0.09 bc | 107 | 2.37 ± 0.07 d | 113 | 3.19 ± 0.13 a |
| 6th instar (d) | 117 | 3.91 ± 0.07 b | 101 | 3.75 ± 0.07 bc | 107 | 3.73 ± 0.05 c | 99 | 4.31 ± 0.05 a | 119 | 3.15 ± 0.05 e | 107 | 3.47 ± 0.06 d | 111 | 4.47 ± 0.10 a |
| Prepupa (d) | 115 | 2.04 ± 0.05 a | 99 | 2.02 ± 0.05 ab | 107 | 2.05 ± 0.05 a | 96 | 2.13 ± 0.06 a | 111 | 1.96 ± 0.06 ab | 102 | 1.60 ± 0.05 c | 108 | 1.92 ± 0.04 b |
| Pupa (d) | 86 | 10.30 ± 0.09 bc | 88 | 10.44 ± 0.11 ab | 90 | 10.59 ± 0.11 a | 68 | 10.72 ± 0.11 a | 102 | 10.42 ± 0.08 b | 87 | 10.17 ± 0.10 bc | 72 | 10.14 ± 0.10 c |
| Preadult (d) | 86 | 30.58 ± 0.14 c | 88 | 29.98 ± 0.16 d | 90 | 30.31± 0.14 cd | 68 | 33.88 ± 0.17 a | 102 | 30.43 ± 0.24 cd | 87 | 29.92 ± 0.30 d | 72 | 32.22 ± 0.38 b |
| Adult (d) | 86 | 14.77 ± 0.37 a | 88 | 14.76 ± 0.43 a | 90 | 14.98 ± 0.31 a | 68 | 14.51 ± 0.46 ab | 102 | 13.53 ± 0.35 b | 87 | 14.24 ± 0.38 ab | 72 | 14.49 ± 0.47 ab |
| Total longevity (d) | 120 | 39.43 ± 0.92 ab | 120 | 38.06 ± 1.12 b | 120 | 39.22 ± 1.04 ab | 120 | 37.53 ± 1.23 b | 120 | 41.08 ± 0.73 a | 120 | 37.28 ± 1.13 b | 120 | 38.98 ± 0.99 ab |
| Female:Male | 42:44 | 41:47 | 39:51 | 34:34 | 37:65 | 36:51 | 35:37 | |||||||
| Population Parameters | N | Control | MWCNTs-COOH (mg/g) | MWCNTs-OH (mg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 0.04 | N | 0.4 | N | 4 | N | 0.04 | N | 0.4 | N | 4 | |||
| Female total longevity (d) | 42 | 43.24 ± 0.51 b | 41 | 44.07 ± 0.64 b | 39 | 43.77 ± 0.51 b | 34 | 47.97 ± 0.59 a | 37 | 43.08 ± 0.60 b | 36 | 43.81 ± 0.69 b | 35 | 46.11 ± 0.78 a |
| Male total longevity (d) | 44 | 47.36 ± 0.44 ab | 47 | 45.32 ± 0.65 bc | 51 | 46.45 ± 0.43 b | 34 | 48.82 ± 0.77 a | 65 | 44.46 ± 0.54 c | 51 | 44.41 ± 0.70 c | 37 | 47.27 ± 0.73 ab |
| APOP (d) | 42 | 1.52 ± 0.15 bc | 41 | 1.32 ± 0.09 cd | 39 | 1.56 ± 0.14 bc | 34 | 2.21 ± 0.26 a | 37 | 1.54 ± 0.17 bc | 36 | 1.14 ± 0.07 d | 35 | 1.80 ± 0.17 ab |
| TPOP (d) | 42 | 31.45 ± 0.25 c | 41 | 30.32 ± 0.22 d | 39 | 31.08 ± 0.20 c | 34 | 35.44 ± 0.29 a | 37 | 31.51 ± 0.37 c | 36 | 30.61 ± 0.57 cd | 35 | 33.17 ± 0.58 b |
| Oviposition Period (d) | 42 | 7.00 ± 1.40 a | 41 | 7.00 ± 1.43 a | 39 | 8.00 ± 1.34 a | 34 | 6.00 ± 1.74 a | 37 | 7.00 ± 1.64 a | 36 | 7.00 ± 1.88 a | 35 | 6.00 ± 2.05 a |
| Fecundity (eggs/female) | 42 | 995.98 ± 74.45 ab | 41 | 1156.10 ± 70.59 ab | 39 | 1168.72 ± 63.42 a | 34 | 918.88 ± 105.50 bc | 37 | 1120.70 ± 67.38 ab | 36 | 1164.81 ± 66.48 a | 35 | 693.71 ± 49.08 c |
| Life Table Parameters | Control | MWCNTs-COOH (mg/g) | MWCNTs-OH (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| 0.04 | 0.4 | 4 | 0.04 | 0.4 | 4 | ||
| Net reproductive rate (R0) | 348.59 ± 50.53 a | 395.00 ± 55.54 a | 379.83 ± 54.13 a | 260.35 ± 48.12 ab | 345.55 ± 51.46 a | 349.44 ± 52.60 a | 202.33 ± 31.99 b |
| Intrinsic rate of increase (r) | 0.1733 ± 0.0046 a | 0.1811 ± 0.0044 a | 0.1760 ± 0.0046 a | 0.1469 ± 0.0051 b | 0.1760 ± 0.0052 a | 0.1828 ± 0.0058 a | 0.1534 ± 0.0058 b |
| Finite rate of increase (λ) | 1.1893 ± 0.0055 a | 1.1986 ± 0.0053 a | 1.1925 ± 0.0054 a | 1.1582 ± 0.0058 b | 1.1925 ± 0.0061 a | 1.2006 ± 0.0069 a | 1.1658 ± 0.0068 b |
| Mean generation time (T) | 33.77 ± 0.20 b | 33.01 ± 0.23 c | 33.74 ± 0.25 b | 37.87 ± 0.27 a | 33.20 ± 0.32 bc | 32.03 ± 0.52 c | 34.62 ± 0.67 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Mobarak, S.H.; Lu, F.-X.; Ai, J.; Bai, X.-Y.; Wu, L.; Qin, S.-Z.; Hu, C.-X. Hydroxylated vs. Carboxylated Nanotubes: Differential Impacts on Fall Armyworm Development, Reproduction, and Population Dynamics. Insects 2025, 16, 748. https://doi.org/10.3390/insects16080748
Wang Z, Mobarak SH, Lu F-X, Ai J, Bai X-Y, Wu L, Qin S-Z, Hu C-X. Hydroxylated vs. Carboxylated Nanotubes: Differential Impacts on Fall Armyworm Development, Reproduction, and Population Dynamics. Insects. 2025; 16(8):748. https://doi.org/10.3390/insects16080748
Chicago/Turabian StyleWang, Zhao, Syed Husne Mobarak, Fa-Xu Lu, Jing Ai, Xie-Yuan Bai, Lei Wu, Shao-Zhao Qin, and Chao-Xing Hu. 2025. "Hydroxylated vs. Carboxylated Nanotubes: Differential Impacts on Fall Armyworm Development, Reproduction, and Population Dynamics" Insects 16, no. 8: 748. https://doi.org/10.3390/insects16080748
APA StyleWang, Z., Mobarak, S. H., Lu, F.-X., Ai, J., Bai, X.-Y., Wu, L., Qin, S.-Z., & Hu, C.-X. (2025). Hydroxylated vs. Carboxylated Nanotubes: Differential Impacts on Fall Armyworm Development, Reproduction, and Population Dynamics. Insects, 16(8), 748. https://doi.org/10.3390/insects16080748








