Damage Potential and Feeding Preference of Halyomorpha halys (Stål), Nezara viridula (L.), and Leptoglossus zonatus (Dallas) Among Different Ripening Stages of Tomato
Simple Summary
Abstract
1. Introduction
- Determine the feeding preference of different piercing–sucking hemipteran pests (stink bugs and leaf-footed bug) among four major ripening stages (green, breaker, pink, and red) of tomato fruit, in order to find out which ripening stage is most preferred by stink bugs.
- Determine whether the feeding preferences of piercing–sucking hemipteran pests for the tomato ripening stages vary among different developmental stages (nymphs, adult males, and adult females).
- Investigate the damage potential among different piercing–sucking hemipteran pests in tomato.
2. Materials and Methods
2.1. Insect Collection and Rearing
2.2. Growing Greenhouse Tomatoes
2.3. Experimental Arenas (Figure 1)

2.4. Experiment 1: Feeding Preference Bioassay
2.5. Experiment 2: Behavioral Assay
2.6. Data Analysis
3. Results
3.1. Feeding Bioassay
3.2. Behavioral Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA ERS. Vegetables and Pulses Data. Available online: https://www.ers.usda.gov/data-products/vegetables-and-pulses-data/ (accessed on 30 January 2024).
- Cui, X.; Guan, Z.; Morgan, K.L.; Huang, K.-M.; Hammami, A.M. Multitiered Fresh Produce Supply Chain: The Case of Tomatoes. Horticulturae 2022, 8, 1204. [Google Scholar] [CrossRef]
- McPherson, J.E.; McPherson, R.M. Stink Bugs of Economic Importance in America North of Mexico; Taylor & Francis: London, UK, 2000; p. 254. ISBN 978-0-429-11502-8. [Google Scholar]
- Leppla, N.C.; Stacey, K.J.; Rooney, L.M.; Lennon, K.M.; Hodges, A.C. Stink Bug (Hemiptera: Pentatomidae) Occurrence, Reproduction, and Injury to Fruit in an Organic Tomato Crop Bordered by Sorghum. J. Econ. Entomol. 2023, 116, 144–152. [Google Scholar] [CrossRef]
- Lye, B.-H.; Story, R.N. Feeding Preference of the Southern Green Stink Bug (Hemiptera: Pentatomidae) on Tomato Fruit. J. Econ. Entomol. 1988, 81, 522–526. [Google Scholar] [CrossRef]
- Lye, B.-H.; Story, R.N. Spatial Dispersion and Sequential Sampling Plan of the Southern Green Stink Bug (Hemiptera: Pentatomidae) on Fresh Market Tomatoes. Environ. Entomol. 1989, 18, 139–144. [Google Scholar] [CrossRef]
- Siebert, M.W.; Leonard, B.R.; Gable, R.H.; Lamotte, L.R. Cotton Boll Age Influences Feeding Preference by Brown Stink Bug (Heteroptera: Pentatomidae). J. Econ. Entomol. 2005, 98, 82–87. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Hamilton, G.; Rodriguez-Saona, C.; Nielsen, A.L. Plant Stimuli and Their Impact on Brown Marmorated Stink Bug Dispersal and Host Selection. Front. Ecol. Evol. 2019, 7, 414. [Google Scholar] [CrossRef]
- Zobel, E.S.; Hooks, C.R.R.; Dively, G.P. Seasonal Abundance, Host Suitability, and Feeding Injury of the Brown Marmorated Stink Bug, Halyomorpha Halys (Heteroptera: Penatomidae), in Selected Vegetables. J. Econ. Entomol. 2016, 109, 1289–1302. [Google Scholar] [CrossRef]
- Lye, B.-H.; Story, R.N.; Wright, V.L. Southern Green Stink Bug (Hemiptera: Pentatomidae) Damage to Fresh Market Tomatoes. J. Econ. Entomol. 1988, 81, 189–194. [Google Scholar] [CrossRef]
- Kennedy, G.G.; Romanow, L.R.; Jenkins, S.F.; Sanders, D.C. Insects and Diseases Damaging Tomato Fruits in the Coastal Plain of North Carolina1. J. Econ. Entomol. 1983, 76, 168–173. [Google Scholar] [CrossRef]
- Zalom, F.G.; SmlLanick, J.M.; Ehler, L.E. Fruit Damage by Stink Bugs (Hemiptera: Pentatomidae) in Bush-Type Tomatoes. J. Econ. Entomol. 1997, 90, 1300–1306. [Google Scholar] [CrossRef]
- Tillman, P.G.; Northfield, T.D.; Mizell, R.F.; Riddle, T.C. Spatiotemporal Patterns and Dispersal of Stink Bugs (Heteroptera: Pentatomidae) in Peanut-Cotton Farmscapes. Environ. Entomol. 2009, 38, 1038–1052. [Google Scholar] [CrossRef]
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the Invasive Brown Marmorated Stink Bug, Halyomorpha Halys (Stål), in Mid-Atlantic Tree Fruit Orchards in the United States: Case Studies of Commercial Management. Psyche J. Entomol. 2012, 2012, e535062. [Google Scholar] [CrossRef]
- Lee, D.-H.; Nielsen, A.L.; Leskey, T.C. Dispersal Capacity and Behavior of Nymphal Stages of Halyomorpha Halys (Hemiptera: Pentatomidae) Evaluated Under Laboratory and Field Conditions. J. Insect. Behav. 2014, 27, 639–651. [Google Scholar] [CrossRef]
- Bae, S.; Yi, H.; Yoon, Y.; Jang, Y.; Kim, Y.; Maharjan, R. Attraction of Stink Bugs to Rocket Traps with Different Combinations of Wing and Landing Board Color. J. Asia-Pac. Entomol. 2019, 22, 243–249. [Google Scholar] [CrossRef]
- Leskey, T.C.; Hogmire, H.W. Monitoring Stink Bugs (Hemiptera: Pentatomidae) in Mid-Atlantic Apple and Peach Orchards. J. Econ. Entomol. 2005, 98, 143–153. [Google Scholar] [CrossRef]
- Brennan, S.A.; Liburd, O.E.; Eger, J.E.; Rhodes, E.M. Species Composition, Monitoring, and Feeding Injury of Stink Bugs (Heteroptera: Pentatomidae) in Blackberry. J. Econ. Entomol. 2013, 106, 912–923. [Google Scholar] [CrossRef]
- Tomato Grades and Standards|Agricultural Marketing Service. Available online: https://www.ams.usda.gov/grades-standards/tomato-grades-and-standards (accessed on 29 January 2024).
- Gonzali, S.; Perata, P. Fruit Colour and Novel Mechanisms of Genetic Regulation of Pigment Production in Tomato Fruits. Horticulturae 2021, 7, 259. [Google Scholar] [CrossRef]
- Klee, H.; Giovannoni, J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and Beyond Color: Comparative Overview of Functional Quality of Tomato and Watermelon Fruits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Ginies, C.; Gouble, B.; Bureau, S.; Causse, M. Home Conservation Strategies for Tomato (Solanum Lycopersicum): Storage Temperature vs. Duration—Is There a Compromise for Better Aroma Preservation? Food Chem. 2013, 139, 825–836. [Google Scholar] [CrossRef]
- Hayata, Y.; Chawengkijwanich, C.; Kozuka, H.; Sakamoto, K.; Ozajima, Y. Flavor Volatile Analysis of “House Momotaro” Tomato Fruit Extracts at Different Ripening Stages by Porapak Q Column. Engei Gakkai Zasshi 2002, 71, 473–479. [Google Scholar] [CrossRef]
- Cheng, G.T.; Chang, P.P.; Wang, X.Y.; Lou, Q.Q.; El-Sappah, A.H.; Zhang, F.; Liang, Y. Effect of Fruit Color and Ripeness on Volatiles Profiles in Cherry Tomato (Solanum Lycopersicum Var. Cerasiforme) Fruit. Food Sci. 2020, 42, 173–183. [Google Scholar] [CrossRef]
- Petro-Turza, M. Flavor of Tomato and Tomato Products. Food Rev. Int. 1986, 2, 309–351. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Flath, R.A.; Ling, L.C. Fresh Tomato Volatiles: Composition and Sensory Studie. ACS Symp. Ser. Am. Chem. Soc. 1989, 388, 213–222. [Google Scholar]
- Viator, H.P.; Pantoja, A.; Smith, C.M. Damage to Wheat Seed Quality and Yield by the Rice Stink Bug and Southern Green Stink Bug (Hemiptera: Pentatomidae). J. Econ. Entomol. 1983, 76, 1410–1413. [Google Scholar] [CrossRef]
- Schumann, F.W.; Todd, J.W. Population Dynamics of the Southern Green Stink Bug (Heteroptera: Pentatomidae) in Relation to Soybean Phenology. J. Econ. Entomol. 1982, 75, 748–753. [Google Scholar] [CrossRef]
- Mensah-Bonsu, M.; Dingha, B.N.; Jackai, L.E.N.; Adjei-Fremah, S.; Worku, M. Evaluation of Preference of Brown Marmorated Stink Bug, Halyomorpha Halys (Stål) for Different Colour Bell Peppers and the Role of Plant Protein. Arthropod-Plant Interact. 2020, 14, 363–372. [Google Scholar] [CrossRef]
- Michelbacher, A.E.; Middlekauff, W.W.; Bacon, O.G. Stink Bug Injury to Tomatoes in California. J. Econ. Entomol. 1952, 45, 126. [Google Scholar] [CrossRef]
- Grozea, I.; Virteiu, A.M.; Molnar, L. Southern Green Stink Bugs (Nezara viridula L.) a new pest of tomato crops in western romania. Res. J. Agric. Sci. 2012, 44, 24–27. [Google Scholar]
- Henne, D.C.; Johnson, S.J.; Bourgeois, W.J. Pest Status of Leaf-Footed Bugs (Heteroptera: Coreidae) on Citrus in Louisiana. Proc. Fla. State Hort. Soc. 2003, 116, 240–241. [Google Scholar]
- Fadamiro, H.; Xiao, Y.; Hargroder, T.; Nesbitt, M.; Umeh, V.; Childers, C. Seasonal Occurrence of Key Arthropod Pests and Associated Natural Enemies in Alabama Satsuma Citrus. Environ. Entomol. 2008, 37, 555–567. [Google Scholar] [CrossRef]
- Xiao, Y.; Fadamiro, H.Y. Host Preference and Development of Leptoglossus Zonatus (Hemiptera: Coreidae) on Satsuma Mandarin. J. Econ. Entomol. 2009, 102, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Medal, J.; Smith, T.; Fox, A.; Cruz, A.S.; Poplin, A.; Hodges, A. Rearing the Brown Marmorated Stink Bug Halyomorpha Halys (Heteroptera: Pentatomidae). Fla. Entomol. 2012, 95, 800–802. [Google Scholar] [CrossRef]
- Hochmuth, G.J.; Vavrina, C.S. Tomato Production Guide for Florida: Crop Establishment; Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1998. [Google Scholar]
- Lamptey, S.; Koomson, E. The Role of Staking and Pruning Methods on Yield and Profitability of Tomato (Solanum Lycopersicum L.) Production in the Guinea Savanna Zone of Ghana. Adv. Agric. 2021, 2021, e5570567. [Google Scholar] [CrossRef]
- Alam, M.; Islam, N.; Ahmad, S.; Hossen, M.; Islam, M. Effect of Different Staking Methods and Stem Pruning on Yield and Quality of Summer Tomato. Bangladesh J. Agric. Res 2016, 41, 419–432. [Google Scholar] [CrossRef]
- Decognet, V.; Ravetti, F.; Martin, C.; Nicot, P.C. Improved Leaf Pruning Reduces Development of Stem Cankers Caused by Grey Mould in Greenhouse Tomatoes. Agron. Sustain. Dev. 2010, 30, 465–472. [Google Scholar] [CrossRef]
- Shearer, P.W.; Jones, V.P. Diel Feeding Pattern of Adult Female Southern Green Stink Bug (Hemiptera: Pentatomidae). Environ. Entomol. 1996, 25, 599–602. [Google Scholar] [CrossRef]
- Krupke, C.H.; Jones, V.P.; Brunner, J.F. Diel Periodicity of Euschistus Conspersus (Heteroptera: Pentatomidae) Aggregation, Mating, and Feeding. Ann. Entomol. Soc. Am. 2006, 99, 169–174. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Version 4.4.0 (Patched); R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 28 April 2025).
- Stroup, W.W.; Ptukhina, M.; Garai, J. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Brooks, M.; Bolker, B.; Kristensen, K.; Maechler, M.; Magnusson, M.; McGillycuddy, H.; Skaug, A.; Nielsen, C.; Berg, K.; van Bentham, N.; et al. glmmTMB: Generalized Linear Mixed Models using Template Model Builder. Version 1.1.9. 2024. Available online: https://cran.r-project.org/web/packages/glmmTMB/index.html (accessed on 28 April 2025).
- Hartig, F.; Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Version 0.4.5. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 28 April 2025).
- Lenth, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; Riebl, H.; et al. emmeans: Estimated Marginal Means, Aka Least-Squares Means. Version 1.10.2. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 28 April 2025).
- Llorente, B.; D’Andrea, L.; Ruiz-Sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato Fruit Carotenoid Biosynthesis Is Adjusted to Actual Ripening Progression by a Light-Dependent Mechanism. Plant J. 2016, 85, 107–119. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Arikawa, K. Evolution of Color and Vision of Butterflies. Arthropod. Struct. Dev. 2006, 35, 307–318. [Google Scholar] [CrossRef]
- Heath, J.; Cipollini, D.; Stireman, J. The Role of Carotenoids and Their Derivatives in Mediating Interactions between Insects and Their Environment. Arthropod-Plant Interact. 2013, 7, 1–20. [Google Scholar] [CrossRef]
- Bowling, C.C. The Stylet Sheath as an Indicator of Feeding Activity by the Southern Green Stink Bug on Soybeans. J. Econ. Entomol. 1980, 73, 1–3. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Alves, R.M.L. Performance of Nymphs and Adults of the Southern Green Stink Bug (Heteroptera: Pentatomidae) Exposed to Soybean Pods at Different Phenological Stages of Development. J. Econ. Entomol. 1993, 86, 1088–1093. [Google Scholar] [CrossRef]
- Ewunkem, A. Nutritional and Field Ecology of Stink Bugs on Tomato and Cowpea Under Two Insecticide Regimens. Master’s Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2011. [Google Scholar]
- Lee, D.-H.; Leskey, T.C. Flight Behavior of Foraging and Overwintering Brown Marmorated Stink Bug, Halyomorpha Halys (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2015, 105, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Bosco, L.; Nardelli, M.; Tavella, L. First Insights on Early Host Plants and Dispersal Behavior of Halyomorpha Halys (Hemiptera: Pentatomidae) from Overwintering to Crop Colonization. Insects 2020, 11, 866. [Google Scholar] [CrossRef]
- Stahl, J.M.; Scaccini, D.; Pozzebon, A.; Daane, K.M. Comparing the Feeding Damage of the Invasive Brown Marmorated Stink Bug to a Native Stink Bug and Leaffooted Bug on California Pistachios. Insects 2020, 11, 688. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Greene, J.K.; Toews, M.D.; Reeves, R.B. Sampling Stink Bugs (Hemiptera: Pentatomidae) for Population Estimation and Pest Management in Southeastern Cotton Production. J. Econ. Entomol. 2009, 102, 2360–2370. [Google Scholar] [CrossRef]
- Ribeiro, A.V.; Aita, R.C.; Pezzini, D.T.; DiFonzo, C.D.; Hunt, T.E.; Knodel, J.J.; Krupke, C.H.; Marchi-Werle, L.; Michel, A.P.; Seiter, N.J.; et al. Optimization of Sample Unit Size for Sampling Stink Bugs (Hemiptera: Pentatomidae) in Soybean. Crop Prot. 2022, 157, 105986. [Google Scholar] [CrossRef]
- Liburd, O.E.; Alm, S.R.; Casagrande, R.A.; Polavarapu, S. Effect of Trap Color, Bait, Shape, and Orientation in Attraction of Blueberry Maggot (Diptera: Tephritidae) Flies. J. Econ. Entomol. 1998, 91, 243–249. [Google Scholar] [CrossRef]
- Liburd, O.E.; Polavarapu, S.; Alm, S.R.; Casagrande, R.A. Effect of Trap Size, Placement, and Age on Captures of Blueberry Maggot Flies (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 1452–1458. [Google Scholar] [CrossRef]
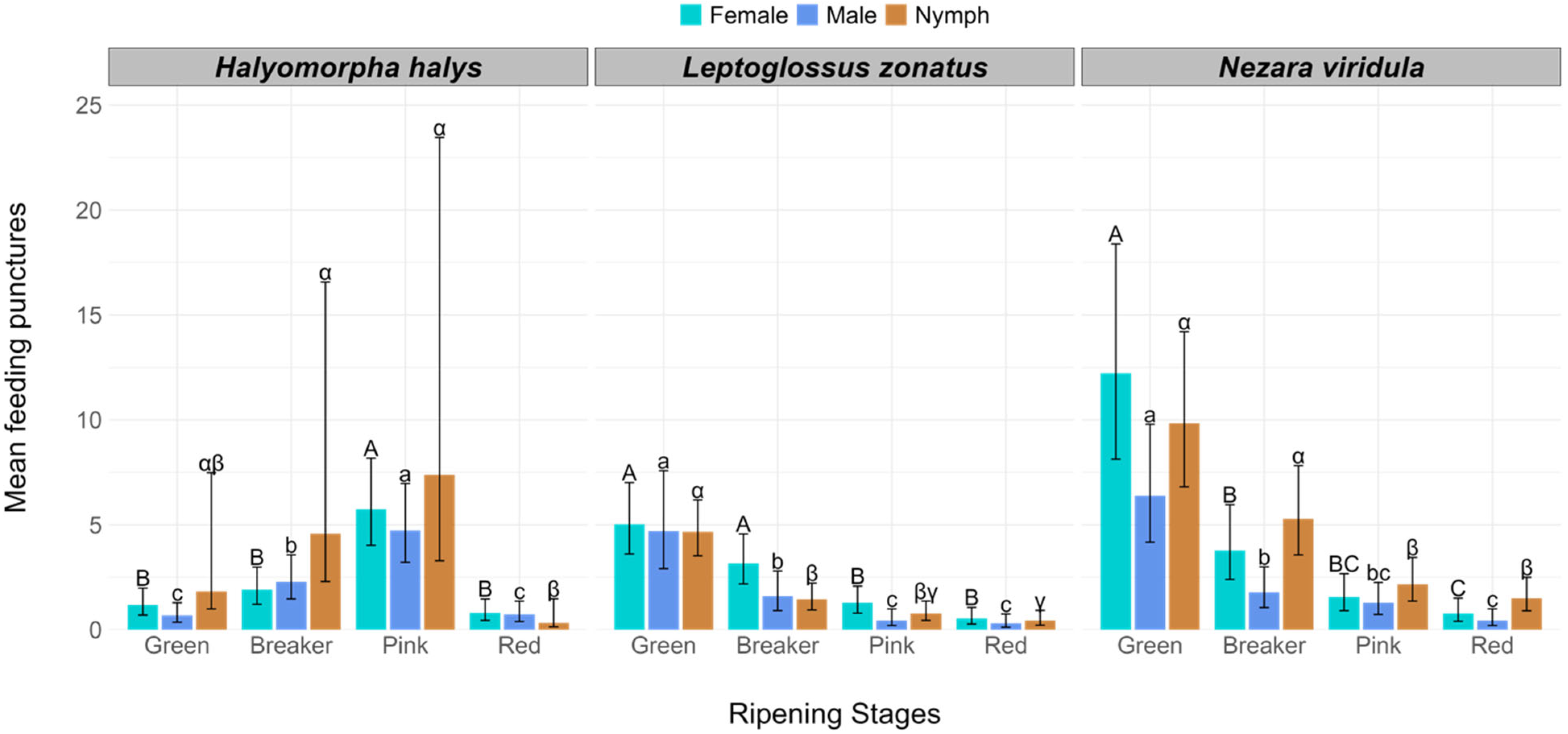
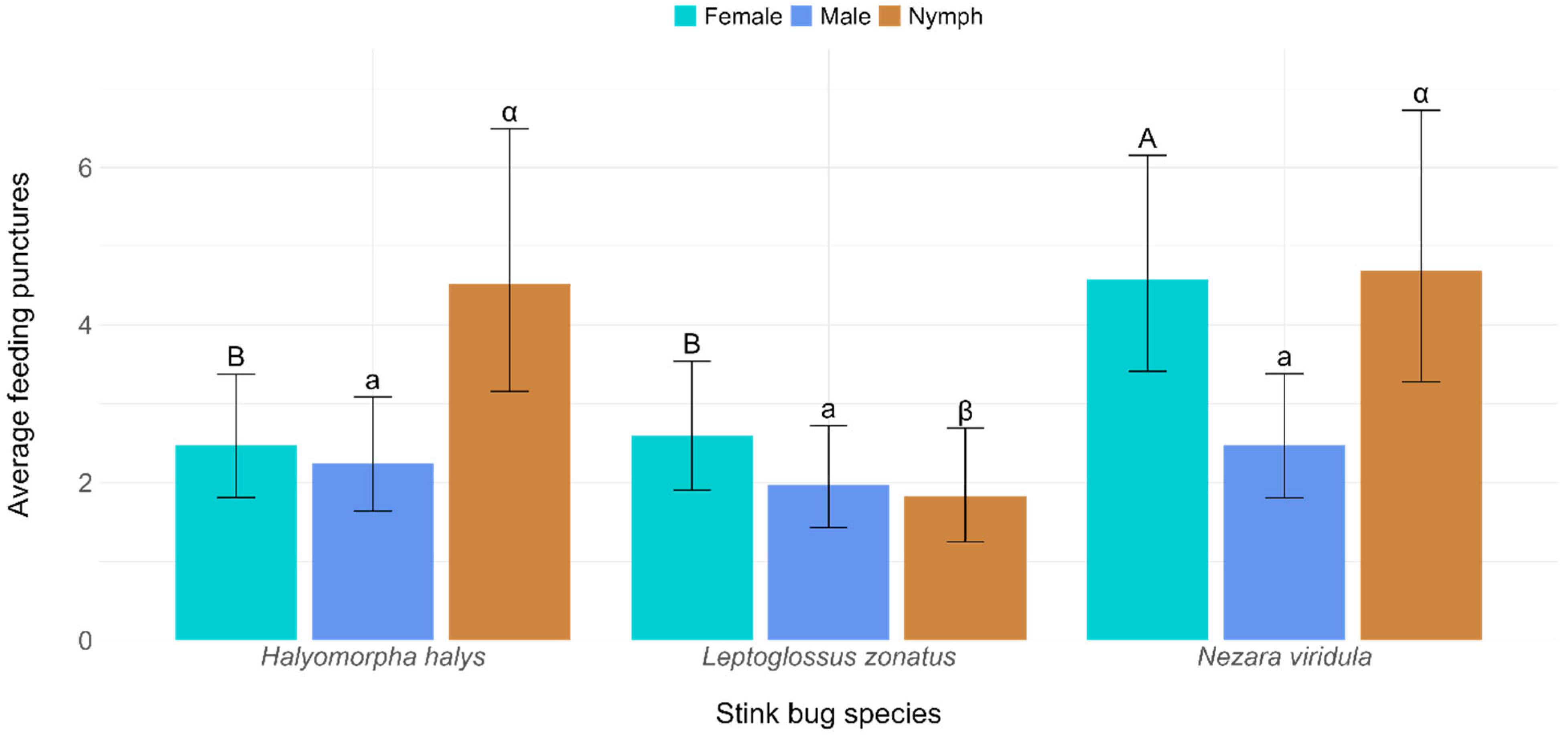
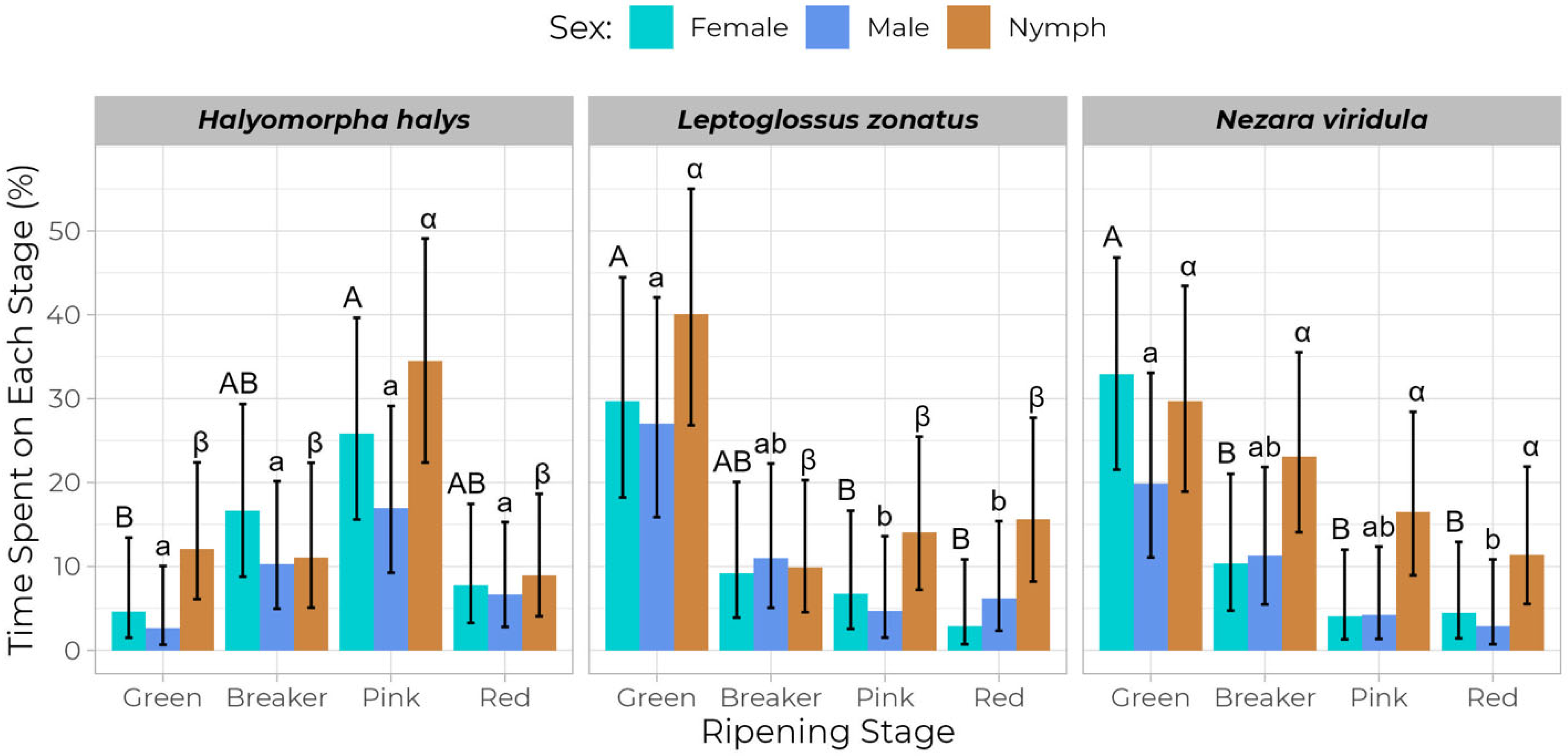
| Tomato Ripening Stage | Mean (±SE) Feeding Punctures by Halyomorpha halys | Mean (±SE) Feeding Punctures by Nezara viridula | Mean (±SE) Feeding Punctures by Leptoglossus zonatus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Nymph | Female | Male | Nymph | Female | Male | Nymph | |
| Green | 1.18 ± 0.31 | 0.68 ± 0.22 | 1.83 ± 1.40 | 12.22 ± 2.50 | 6.39 ± 1.36 | 9.83 ± 1.81 | 5.03 ± 0.84 | 4.70 ± 1.12 | 4.67 ± 0.66 |
| Breaker | 1.90 ± 0.43 | 2.28 ± 0.51 | 4.57 ± 3.34 | 3.78 ± 0.86 | 1.78 ± 0.46 | 5.28 ± 1.04 | 3.15 ± 0.58 | 1.59 ± 0.45 | 1.44 ± 0.31 |
| Pink | 5.73 ± 1.02 | 4.73 ± 0.92 | 7.38 ± 5.30 | 1.56 ± 0.42 | 1.28 ± 0.36 | 2.17 ± 0.50 | 1.28 ± 0.31 | 0.45 ± 0.18 | 0.78 ± 0.22 |
| Red | 0.81 ± 0.24 | 0.73 ± 0.23 | 0.32 ± 0.28 | 0.78 ± 0.26 | 0.44 ± 0.18 | 1.50 ± 0.38 | 0.54 ± 0.18 | 0.29 ± 0.14 | 0.44 ± 0.16 |
| Sex | Halyomorpha halys | Nezara viridula | Leptoglossus zonatus |
|---|---|---|---|
| Female | 2.47 ± 0.39 | 4.58 ± 0.69 | 2.60 ± 0.41 |
| Male | 2.25 ± 0.36 | 2.47 ± 0.39 | 1.97 ± 0.32 |
| Nymph | 4.52 ± 0.83 | 4.69 ± 0.86 | 1.83 ± 0.36 |
| Tomato Ripening Stage | Mean (±SE) Percentage (%) of Feeding Activity Time of Halyomorpha halys | Mean (±SE) Percentage (%) of Feeding Activity Time of Nezara viridula | Mean (±SE) Percentage (%) of Feeding Activity Time of Leptoglossus zonatus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Nymph | Female | Male | Nymph | Female | Male | Nymph | |
| Green | 4.63 ± 2.62 | 2.64 ± 1.86 | 12.05 ± 4.03 | 32.96 ± 6.57 | 19.88 ± 5.60 | 29.73 ± 6.35 | 29.69 ± 6.81 | 27.02 ± 6.78 | 40.11 ± 7.38 |
| Breaker | 16.67 ± 5.18 | 10.29 ± 3.72 | 11.05 ± 4.22 | 10.32 ± 3.96 | 11.28 ± 4.02 | 23.10 ± 5.50 | 9.16 ± 3.87 | 11.01 ± 4.20 | 9.90 ± 3.82 |
| Pink | 25.83 ± 6.20 | 16.99 ± 5.02 | 34.53 ± 6.96 | 4.07 ± 2.32 | 4.22 ± 2.40 | 16.49 ± 4.91 | 6.75 ± 3.26 | 4.69 ± 2.65 | 14.02 ± 4.55 |
| Red | 7.80 ± 3.36 | 6.69 ± 2.94 | 8.97 ± 3.52 | 4.43 ± 2.51 | 2.87 ± 2.01 | 11.34 ± 4.03 | 2.87 ± 2.01 | 6.19 ± 3.01 | 15.61 ± 4.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashed, M.T.N.N.; Dale, A.G.; Alake, G.; Riley, S.S.; Benda, N.; Hodges, A.C. Damage Potential and Feeding Preference of Halyomorpha halys (Stål), Nezara viridula (L.), and Leptoglossus zonatus (Dallas) Among Different Ripening Stages of Tomato. Insects 2025, 16, 740. https://doi.org/10.3390/insects16070740
Rashed MTNN, Dale AG, Alake G, Riley SS, Benda N, Hodges AC. Damage Potential and Feeding Preference of Halyomorpha halys (Stål), Nezara viridula (L.), and Leptoglossus zonatus (Dallas) Among Different Ripening Stages of Tomato. Insects. 2025; 16(7):740. https://doi.org/10.3390/insects16070740
Chicago/Turabian StyleRashed, Md Tafsir Nur Nabi, Adam G. Dale, Gideon Alake, Simon S. Riley, Nicole Benda, and Amanda C. Hodges. 2025. "Damage Potential and Feeding Preference of Halyomorpha halys (Stål), Nezara viridula (L.), and Leptoglossus zonatus (Dallas) Among Different Ripening Stages of Tomato" Insects 16, no. 7: 740. https://doi.org/10.3390/insects16070740
APA StyleRashed, M. T. N. N., Dale, A. G., Alake, G., Riley, S. S., Benda, N., & Hodges, A. C. (2025). Damage Potential and Feeding Preference of Halyomorpha halys (Stål), Nezara viridula (L.), and Leptoglossus zonatus (Dallas) Among Different Ripening Stages of Tomato. Insects, 16(7), 740. https://doi.org/10.3390/insects16070740






