Sampling Techniques Affect Mayfly Nymph Community Indices and May Bias Bioassessments
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Survey
2.2. Net Sampling
2.3. Stone Sampling
2.4. Lab Analysis
2.5. Statistical Analyses
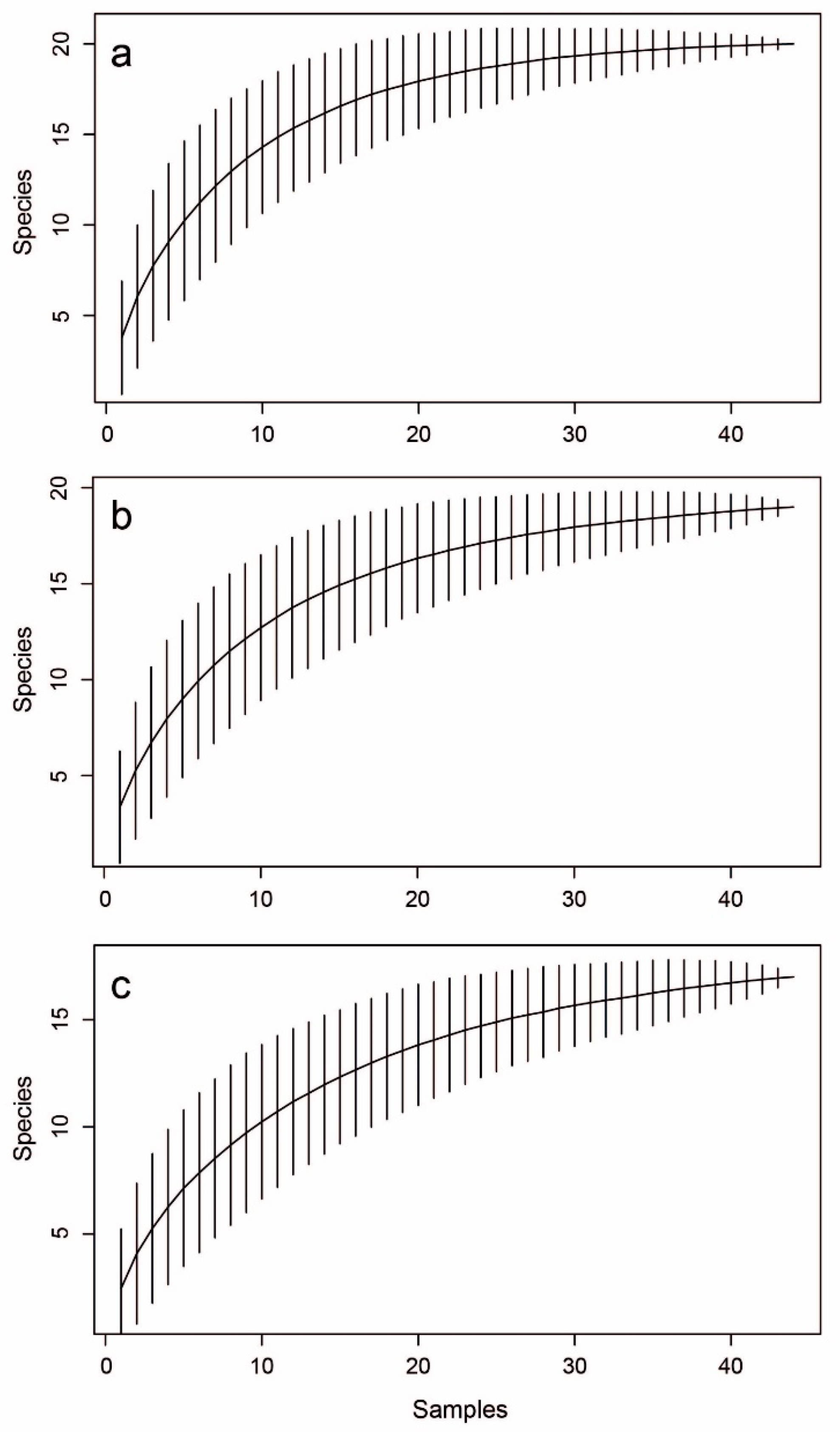
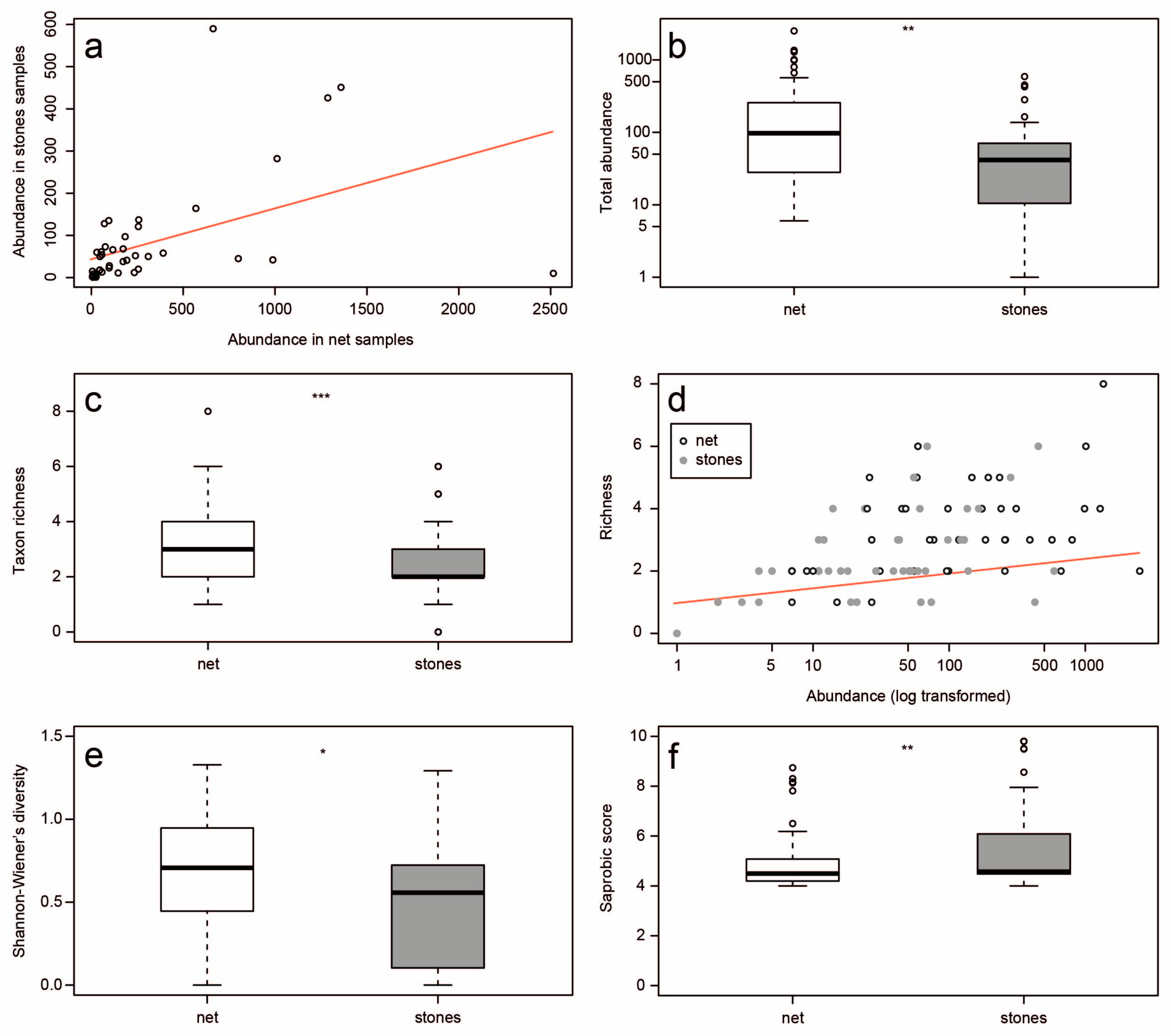
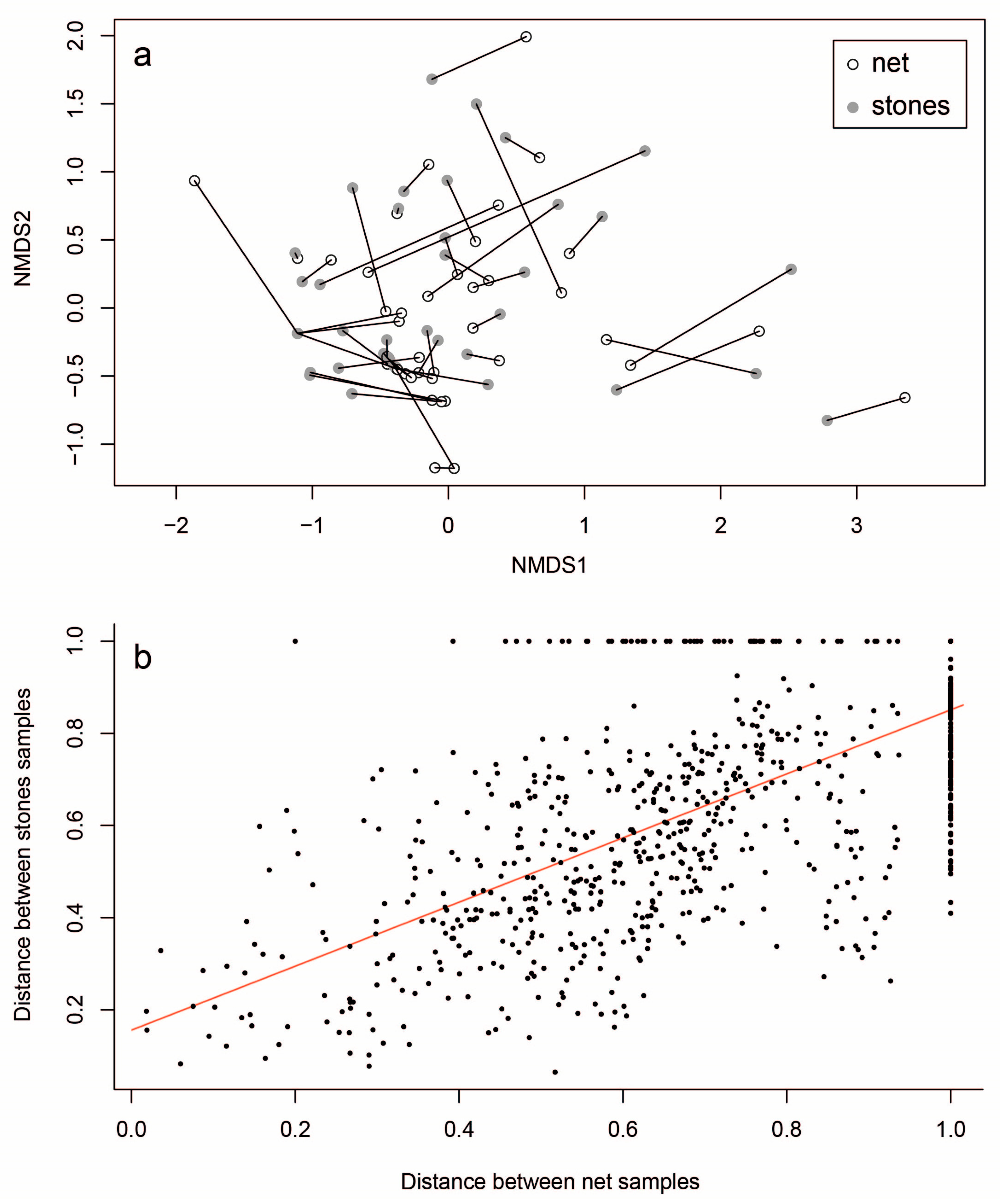
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollis, D.J.; Dallas, H.F.; Esler, K.J.; Boucher, C. Bioassessment of the ecological integrity of river ecosystems using aquatic macroinvertebrates: An overview with a focus on South Africa. Afr. J. Aquat. Sci. 2006, 31, 205–227. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- AQEM Expert Consortium. Manual for the Application of the AQEM System. A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive. Version 1.0. February 2002. Available online: www.aqem.de (accessed on 31 March 2025).
- Stoddard, J.L.; Larsen, D.P.; Hawkins, C.P.; Johnson, R.K.; Norris, R.H. Setting expectations for the ecological condition of streams: The concept of reference condition. Ecol. Appl. A Publ. Ecol. Soc. Am. 2006, 16, 1267–1276. [Google Scholar] [CrossRef]
- Hawkins, C.P.; Olson, J.R.; Hill, R.A. The reference condition: Predicting benchmarks for ecological and water-quality assessments. J. N. Am. Benthol. Soc. 2010, 29, 312–343. [Google Scholar] [CrossRef]
- Kerans, B.L.; Karr, J.R.; Ahlstedt, S.A. Aquatic invertebrate assemblages: Spatial and temporal differences among sampling protocols. J. N. Am. Benthol. Soc. 1992, 11, 377–390. [Google Scholar] [CrossRef]
- Mackey, A.P.; Cooling, D.A.; Berrie, A.D. An evaluation of sampling strategies for qualitative surveys of macro-invertebrates in rivers, using pond nets. J. Appl. Ecol. 1984, 21, 515–534. Available online: https://www.jstor.org/stable/2403426 (accessed on 31 March 2025). [CrossRef]
- Muzaffar, S.B.; Colbo, M.H. The effects of sampling technique on the ecological characterization of shallow, benthic macroinvertebrate communities in two Newfoundland ponds. Hydrobiologia 2002, 477, 31–39. [Google Scholar] [CrossRef]
- Kazila, E.; Ntislidou, C.; Voreadou, C. Comparison of quantitative and semi-quantitative sampling methodologies for biomonitoring of Mediterranean streams using benthic macroinvertebrates: A case study from Greece. Environ. Monit. Assess. 2023, 195, 53. [Google Scholar] [CrossRef]
- Shaw, B. Benthic Macroinvertebrate Survey of the Upper Susquehanna River Using Two Sampling Methods; 48th Annual Report; Biological Field Station: Coopertown, NY, USA, 2023; pp. 193–204. [Google Scholar]
- Bauernfeind, E.; Moog, O.; Weichselbaumer, P. Mayflies (Insecta: Ephemeroptera) and the assessment of ecological integrity: A methodological approach. Hydrobiologia 2000, 422, 71–83. [Google Scholar] [CrossRef]
- Buffagni, A.; Cazzola, M.; López-Rodríguez, M.J.; Alba-Tercedor, J.; Armanini, D.G. Volume 3: Ephemeroptera. In Distribution and Ecological Preferences of European Freshwater Organisms; Schmidt-Kloiber, A., Hering, D., Eds.; Pensoft Publishers: Sofia, Bulgaria; Moscow, Russia, 2009; 256p. [Google Scholar]
- Dickens, C.W.S.; Graham, P.M. The South African Scoring System (SASS) version 5 rapid assessment method for rivers. Afr. J. Aquat. Sci. 2002, 27, 1–10. [Google Scholar] [CrossRef]
- OFEV (Ed.) Méthodes d’analyse et d’appréciation des cours d’eau (IBCH_2019). In Macrozoobenthos–Niveau R. 1ère Édition Actualisée 2019; L’environnement Pratique; Office Fédéral de L’environnement: Berne, Switzerland, 2019; 58p. (In French) [Google Scholar]
- Dallas, H.F. Rapid bioassessment protocols using aquatic macroinvertebrates in Africa—Considerations for regional adaptation of existing biotic indices. Front. Water 2021, 3, 628227. [Google Scholar] [CrossRef]
- Eriksen, T.E.; Brittain, J.E.; Søli, G.; Jacobsen, D.; Goethals, P.; Friberg, N. A global perspective on the application of riverine macroinvertebrates as biological indicators in Africa, South-Central America, Mexico and Southern Asia. Ecol. Indic. 2021, 126, 107609. [Google Scholar] [CrossRef]
- Storey, A.W.; Edward, D.H.D.; Gazey, P. Surber and kick sampling: A comparison for the assessment of macroinvertebrate community structure in streams of south-western Australia. Hydrobiologia 1991, 211, 111–121. [Google Scholar] [CrossRef]
- Tronstad, L.; Wilmot, O.; Thornbrugh, D.; Hotaling, S. To composite or replicate: How sampling method and protocol differences alter collected stream invertebrates and associated bioassessment metrics. Environ. Monit. Assess. 2020, 192, 531. [Google Scholar] [CrossRef] [PubMed]
- Correa-Araneda, F.; Núñez, D.; Díaz, M.E.; Gómez-Capponi, F.; Figueroa, R.; Acuña, J.; Boyero, L.; Esse, C. Comparison of sampling methods for benthic macroinvertebrates in forested wetland. Ecol. Indic. 2021, 125, 107551. [Google Scholar] [CrossRef]
- Marsh, H.; Storey, R.; Kusabs, I.; Smith, B. Adaption of a traditional Māori fishing method for biomonitoring: Using whakaweku for sampling benthic macroinvertebrates in streams. N. Z. J. Mar. Freshw. Res. 2024. [Google Scholar] [CrossRef]
- Sartori, M. Mayflies from Israel (Insecta; Ephemeroptera). I. Heptageniidae, Ephemerellidae, Leptophlebiidae, Palingeniidae. Rev. Suisse Zool. 1992, 99, 835–858. [Google Scholar] [CrossRef]
- Yanai, Z.; Gattolliat, J.-L.; Dorchin, N. Taxonomy of Baetis Leach in Israel (Ephemeroptera, Baetidae). ZooKeys 2018, 794, 45–84. [Google Scholar] [CrossRef]
- Yanai, Z.; Sartori, M.; Gattolliat, J.-L. Contribution to the mayflies (Insecta, Ephemeroptera) of Israel and the Palestinian Authority. Check List 2020, 16, 229–236. [Google Scholar] [CrossRef]
- Malzacher, P. Mayflies from Israel (Insecta, Ephemeroptera) II.—Caenidae. Bull. Société Entomol. Suisse 1992, 65, 385–394. [Google Scholar]
- Schmidt-Kloiber, A.; Hering, D. www.freshwaterecology.info—An online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- Schmedtje, U.; Colling, M. Ökologische Typisierung der Aquatischen Makrofauna; Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft: München, Germany, 1996; 543p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 31 March 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.5–7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 31 March 2025).
- Bonada, N.; Dallas, H.; Rieradevall, M.; Prat, N.; Day, J. A comparison of rapid bioassessment protocols used in 2 regions with Mediterranean climates, the Iberian Peninsula and South Africa. J. N. Am. Benthol. Soc. 2006, 25, 487–500. [Google Scholar] [CrossRef]
- Bauernfeind, E.; Moog, O.; Weichselbaumer, P. Ephemeroptera. In Fauna Aquatica Austriaca; Moog, O., Ed.; Lieferung Wasserwirtschaftskataster, Bundesministerium für Land-und Forstwirtschaft, Umwelt und Wasserwirtschaft: Wien, Austria, 2002. (In German) [Google Scholar]
- Sgarbi, L.F.; Bini, L.M.; Heino, J.; Jyrkänkallio-Mikkola, J.; Landeiro, V.L.; Santos, E.P.; Schneck, F.; Siqueira, T.; Soininen, J.; Tolonen, K.T.; et al. Sampling effort and information quality provided by rare and common species in estimating assemblage structure. Ecol. Indic. 2020, 110, 105937. [Google Scholar] [CrossRef]

| Species | Occurrence | P | V | Microhabitat Specialisation | Locomotion Type | ||
|---|---|---|---|---|---|---|---|
| (Total) | (Net) | (Stones) | |||||
| BAETIDAE | |||||||
| Cloeon sp.1 | 4 | 4 | 0 | mainly M | W, some P | ||
| Cloeon sp.2 | 7 | 7 | 1 | 0.02 | 28 | mainly M | W, some P |
| Procloeon sp. | 2 | 2 | 0 | diverse | W, some P | ||
| Baetis golanensis | 14 | 12 | 11 | 0.58 | 22 | M (?) | W, some P |
| Baetis monnerati | 35 | 33 | 32 | 0.11 | 217 | M (?) | W, some P |
| Baetis aureus | 8 | 7 | 3 | 0.31 | 26 | M (?) | W, some P |
| Baetis pacis | 5 | 1 | 4 | M (?) | W, some P | ||
| Baetis samochai | 3 | 2 | 2 | M (?) | W, some P | ||
| Baetis (Rhodobaetis) noa | 6 | 6 | 2 | 0.03 | 21 | M (?) | W, P |
| Alainites gasithi | 5 | 5 | 0 | mainly M | B, P | ||
| HEPTAGENIIDAE | |||||||
| Anapos kugleri | 12 | 11 | 8 | 0.62 | 32 | ||
| Electrogena galileae | 5 | 4 | 5 | C, little W | P | ||
| Rhithrogena znojkoi | 5 | 4 | 4 | C | P | ||
| Ecdyonurus asiaeminoris | 1 | 0 | 1 | C | P | ||
| Epeorus zaitzevi | 3 | 1 | 3 | B, C | P | ||
| PROSOPISTOMATIDAE | |||||||
| Prosopistoma oronti | 5 | 4 | 4 | B, C | |||
| CAENIDAE | |||||||
| Caenis spp. | 35 | 34 | 20 | <0.01 | 576 | diverse | P, other types |
| LEPTOPHLEBIIDAE | |||||||
| Choroterpes ortali | 5 | 5 | 4 | C, F | mostly P | ||
| Choroterpes picteti | 2 | 2 | 1 | C, little B | mostly P | ||
| OLIGONEURIIDAE | |||||||
| Oligoneuriopsis orontensis | 2 | 2 | 2 | diverse | |||
| Net Technique | Stone Technique | |
|---|---|---|
| Community structure | Well reflected in the collected assemblage. | Well reflected in the collected assemblage. |
| Taxon richness | High. | Low. |
| Number of collected individuals | Large numbers—optimal for abundance-based analyses or for studies that require large samples of a population (e.g., for genetic study) or for rearing. | Smaller numbers. |
| Nymph locomotion type | Ideal for free-swimmers (e.g., Baetidae) and mud-dwellers (e.g., Caenidae). | Ideal for clingers (e.g., Heptageniidae). |
| Focus | Unnecessary overkill. | Only focal species. |
| State of collected specimens | Often damaged during transfer. | Specimens picked individually and kept in good physical state. |
| Required field skills | Experience in standard aquatic net sampling. | Dexterity necessary; individuals may drop from stones before they are handpicked. Taxa may be overlooked in the field. |
| Fieldwork | Little time spent on collecting and sorting in the field. Requires large amounts of ethanol. | Time-consuming fieldwork. Requires small amounts of ethanol. |
| Lab work | Time-consuming lab sorting. | No lab time spent on sorting and removing unwanted organisms and debris. |
| Resulting data | Individuals of lower quality for identification and for taxonomic studies. | Usually does not result in measurable and comparable data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanai, Z.; Dorchin, N. Sampling Techniques Affect Mayfly Nymph Community Indices and May Bias Bioassessments. Insects 2025, 16, 723. https://doi.org/10.3390/insects16070723
Yanai Z, Dorchin N. Sampling Techniques Affect Mayfly Nymph Community Indices and May Bias Bioassessments. Insects. 2025; 16(7):723. https://doi.org/10.3390/insects16070723
Chicago/Turabian StyleYanai, Zohar, and Netta Dorchin. 2025. "Sampling Techniques Affect Mayfly Nymph Community Indices and May Bias Bioassessments" Insects 16, no. 7: 723. https://doi.org/10.3390/insects16070723
APA StyleYanai, Z., & Dorchin, N. (2025). Sampling Techniques Affect Mayfly Nymph Community Indices and May Bias Bioassessments. Insects, 16(7), 723. https://doi.org/10.3390/insects16070723






