Effects of Endosymbionts on the Nutritional Physiology and Biological Characteristics of Whitefly Bemisia tabaci
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Hosts
2.2. Insects
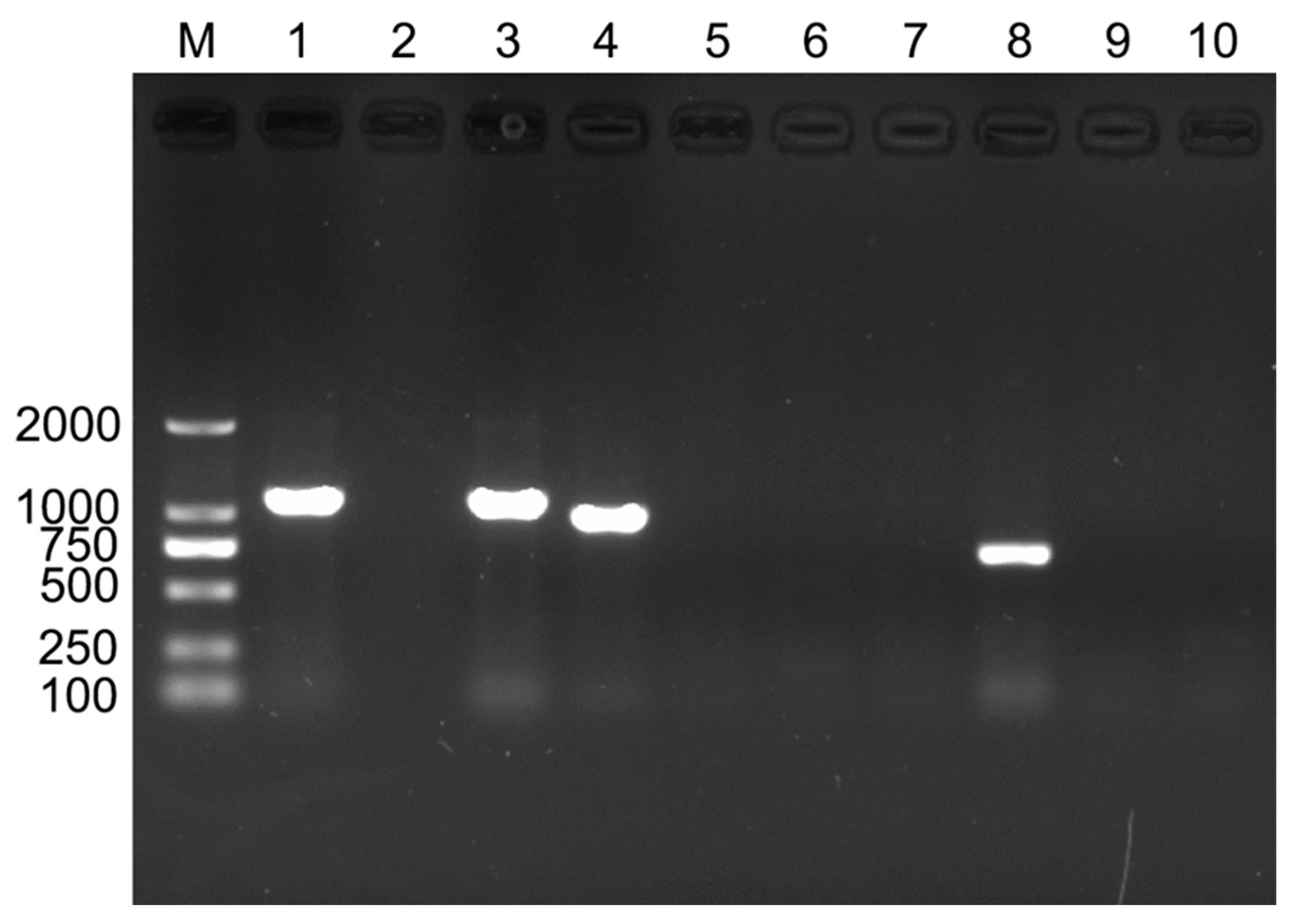
2.3. Endosymbionts Species and Their Infection in Different MEAM1 Populations Reared on Each Tested Host Plant
2.4. Quantitative PCR Detection of Endosymbionts in Different B. tabaci Populations Reared on Each Tested Host Plant
| Symbiont | Gene | Amplicon Size (bp) | Primer Sequence (5′-3′) | Reference |
|---|---|---|---|---|
| Portiera | 16S r DNA | 229 | Port-F 5′-TAGTCCACGCTGTAAACG-3′ Port-R 5′-AGGCACCCTTCCATCT-3′ | [44] |
| Hamiltonella | 16S r DNA | 243 | Ham-F 5′-GCATCGAGTGAGCACAGTT-3′ Ham-R 5′-TATCCTCTCAGACCCGCTAA-3′ | [45] |
| Rickettsia | Citrate synthase (gltA) | 154 | Glta-F 5′-CGGATTGCTTTACTTAC-3′ Glta-R 5′-AAATACGCCACCTCTA-3′ | [44] |
| β-actin | B. tabaci β-actin | 130 | Actin-F 5′-TCTTCCAGCCATCCTTCTTG-3′, Actin-R 5′-CGGTGATTTCCTTCTGCATT-3′ | [46] |
2.5. Analysis of FAAs in Different B. tabaci Populations Reared on Each Tested Host Plant
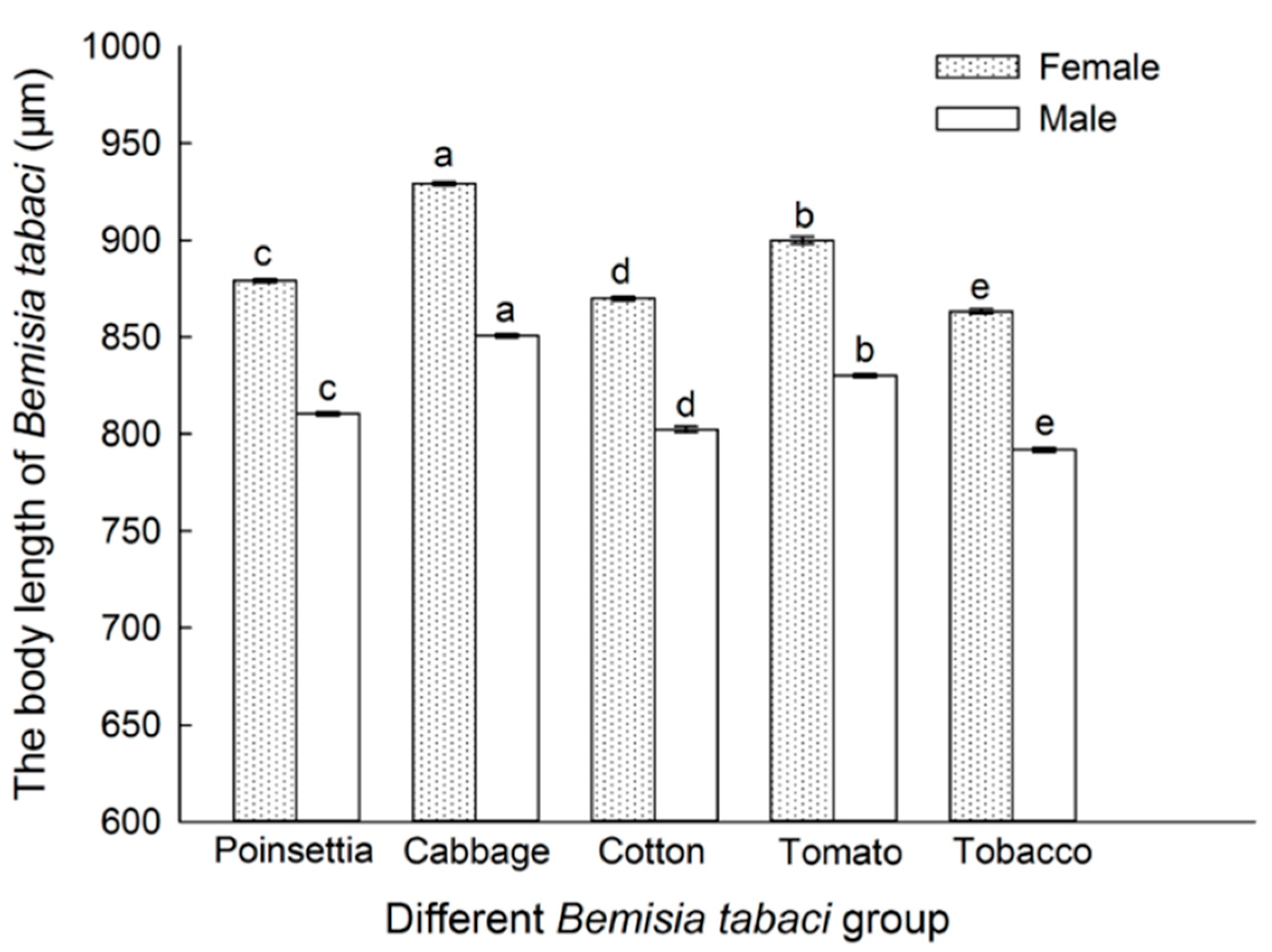
2.6. Body Size Measurement of B. tabaci from Different MEAM1 Populations Reared on Each Tested Host Plant
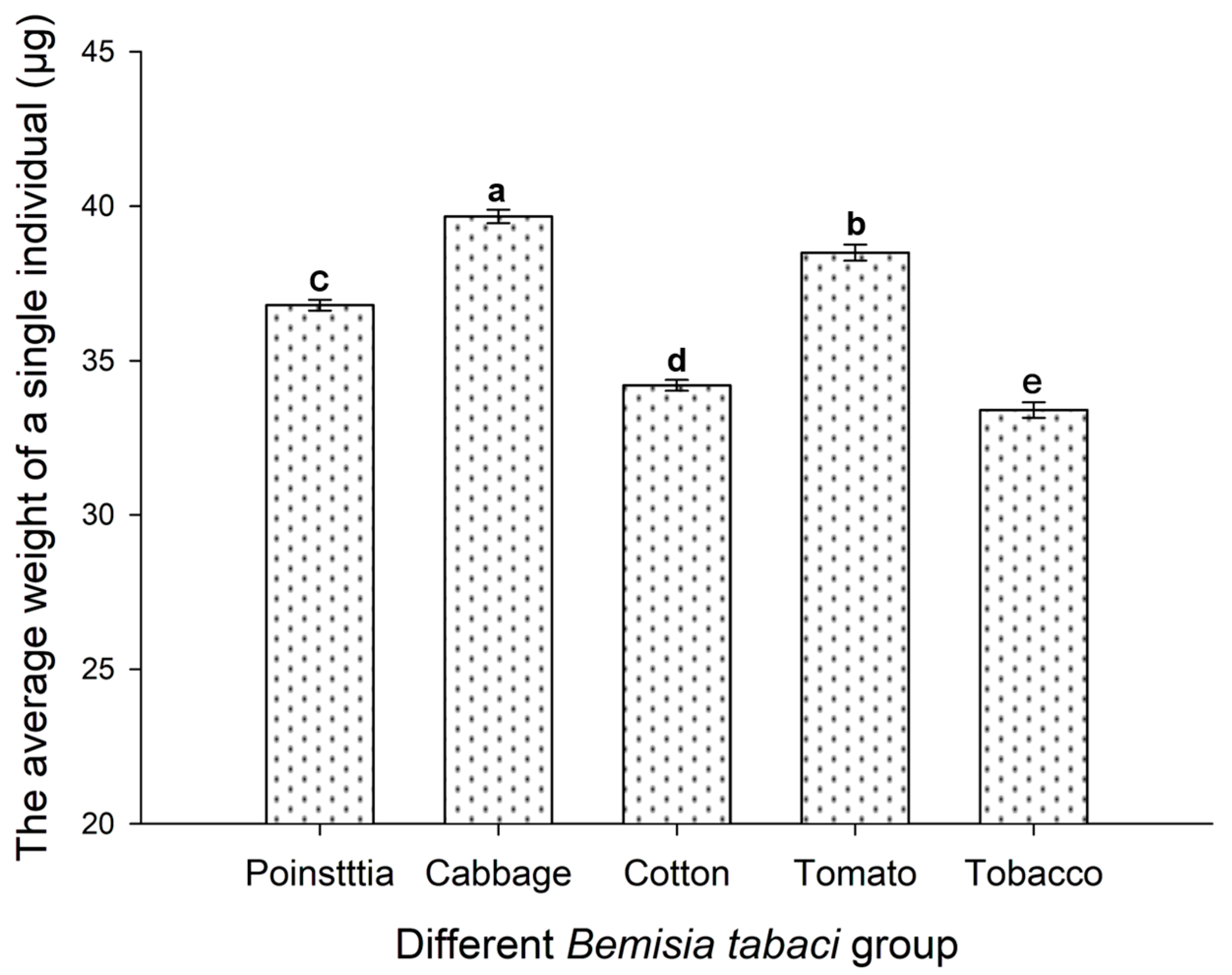
2.7. Weight Determination of Different B. tabaci Populations Reared on Each Tested Host Plant
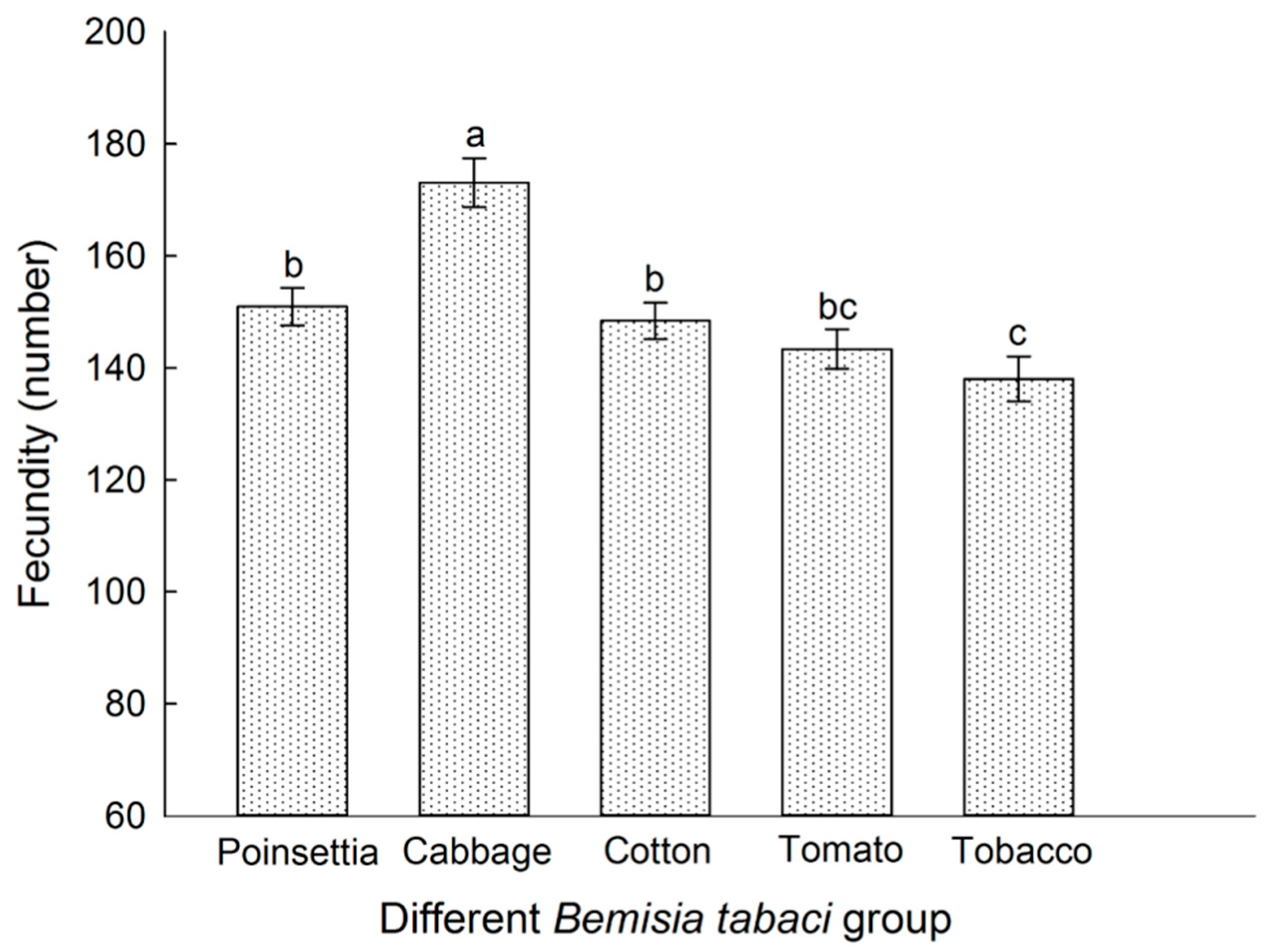
2.8. Fecundity Determination of Different B. tabaci Populations Reared on Each Tested Host Plant
2.9. Statistical Analyses
3. Results
3.1. Detection of Endosymbionts in B. tabaci Cryptic Species
3.2. Endosymbiont Titers in Different B. tabaci Populations Reared on Each Tested Host Plant
3.3. Host Plant Effects on the Body Size of B. tabaci Adults
3.4. Host Plant Effects on the Weight of B. tabaci Adults
3.5. Host Plant Effects on the Fecundity Different of B. tabaci Populations Reared on Each Tested Host Plant
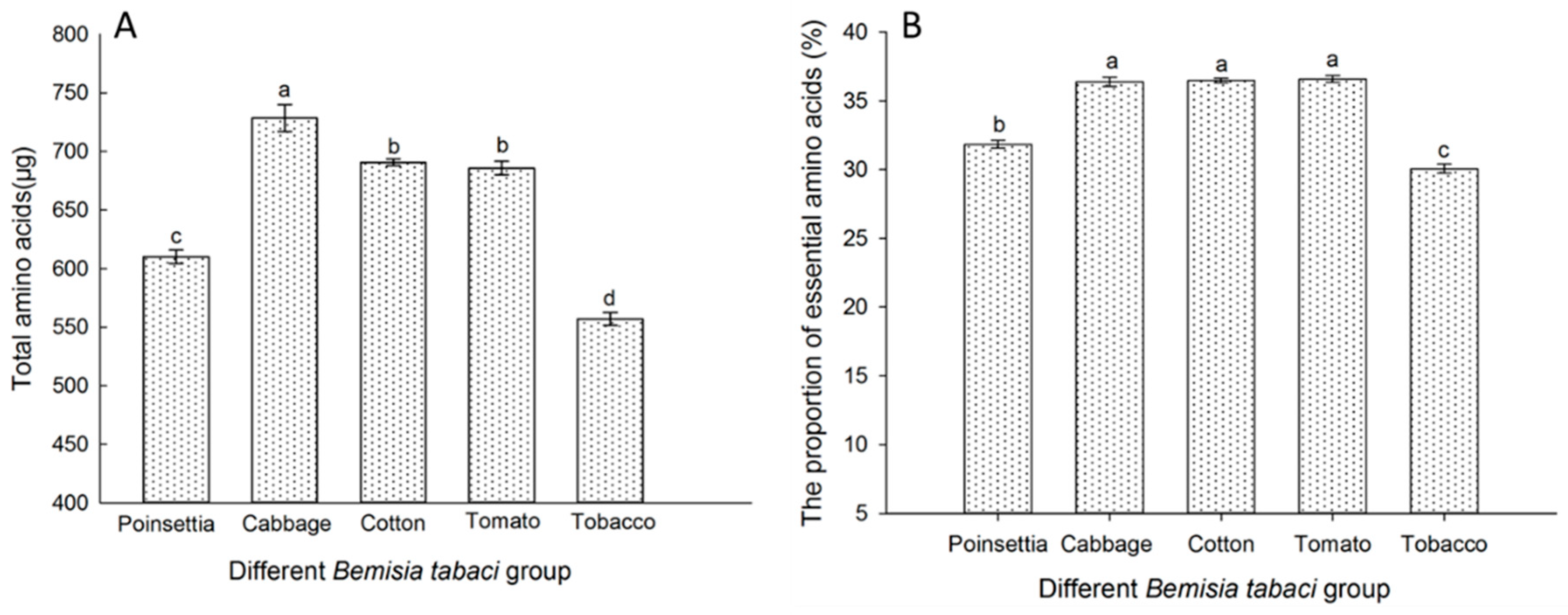
3.6. The FAAs in the Different B. tabaci Populations Reared on Each Tested Host Plant
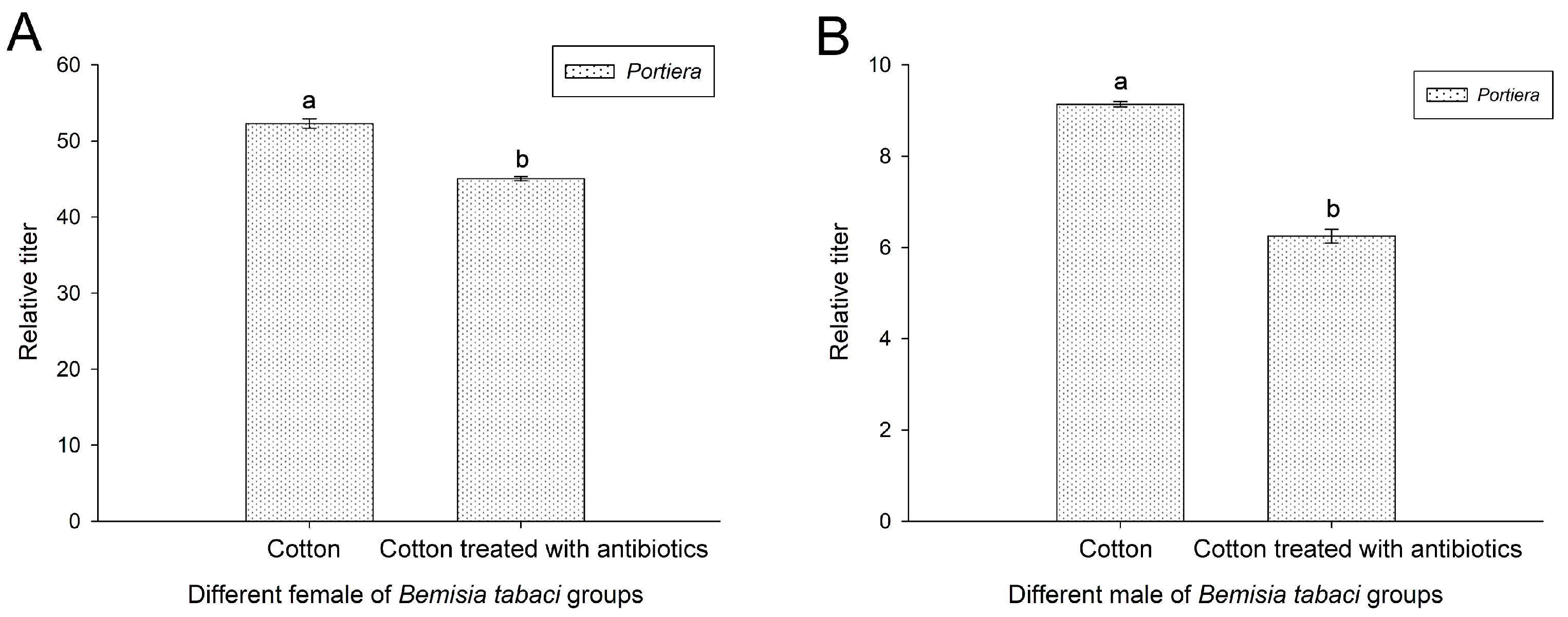
3.7. Effect of Antibiotic Treatment on Portiera of Bemisia tabaci
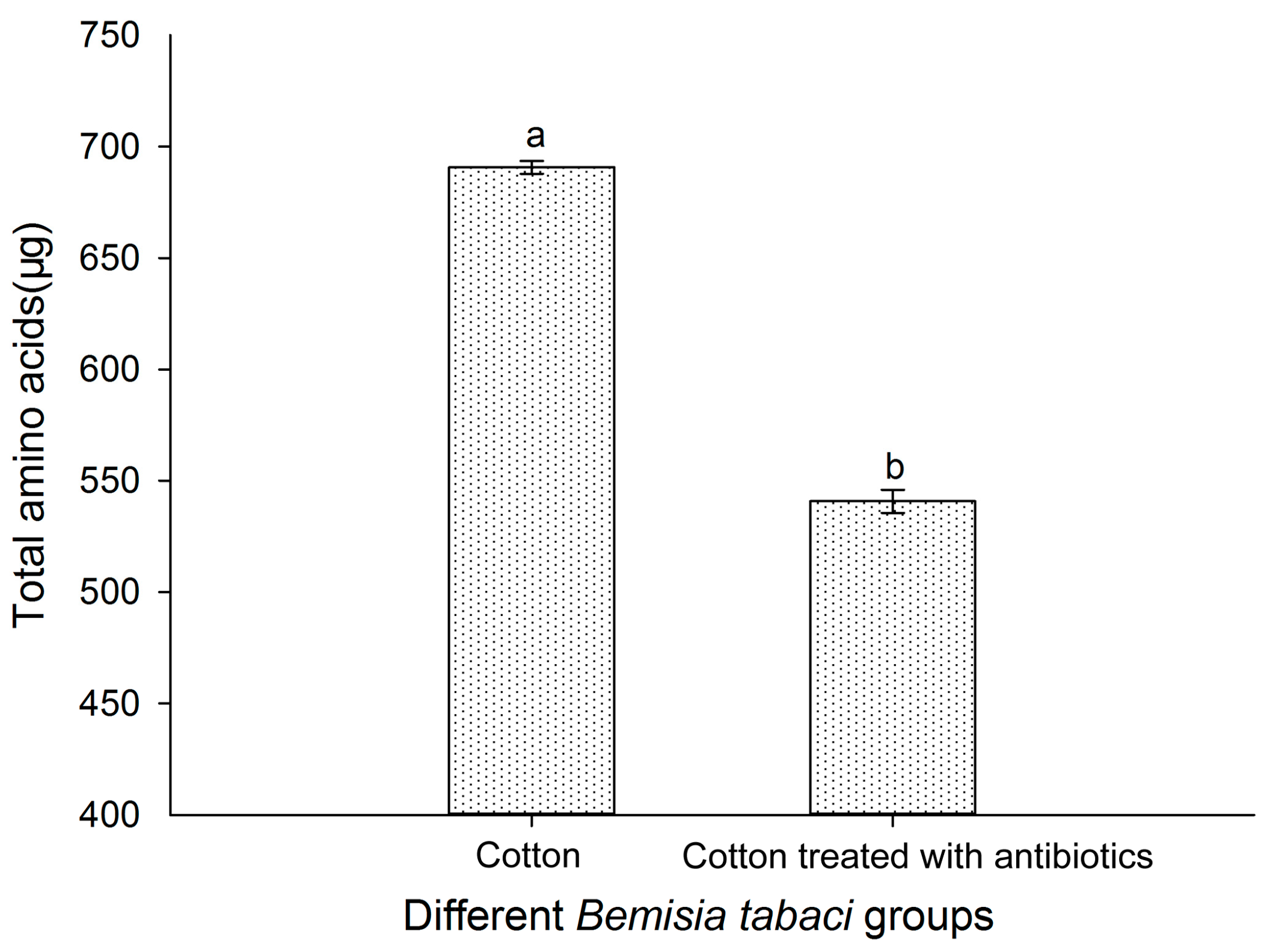
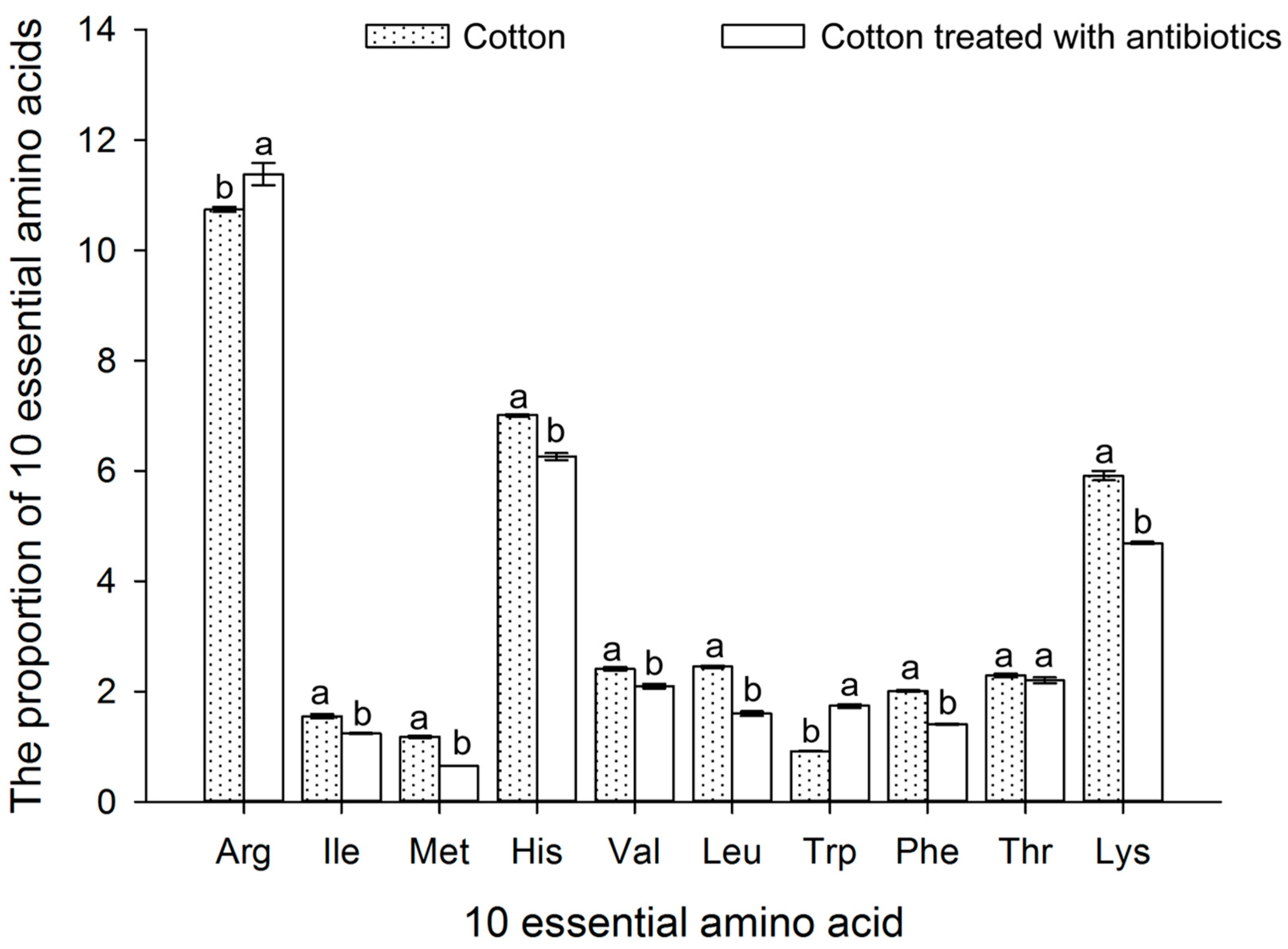
3.8. Effect of Antibiotic Treatment on the FAAs of Bemisia tabaci
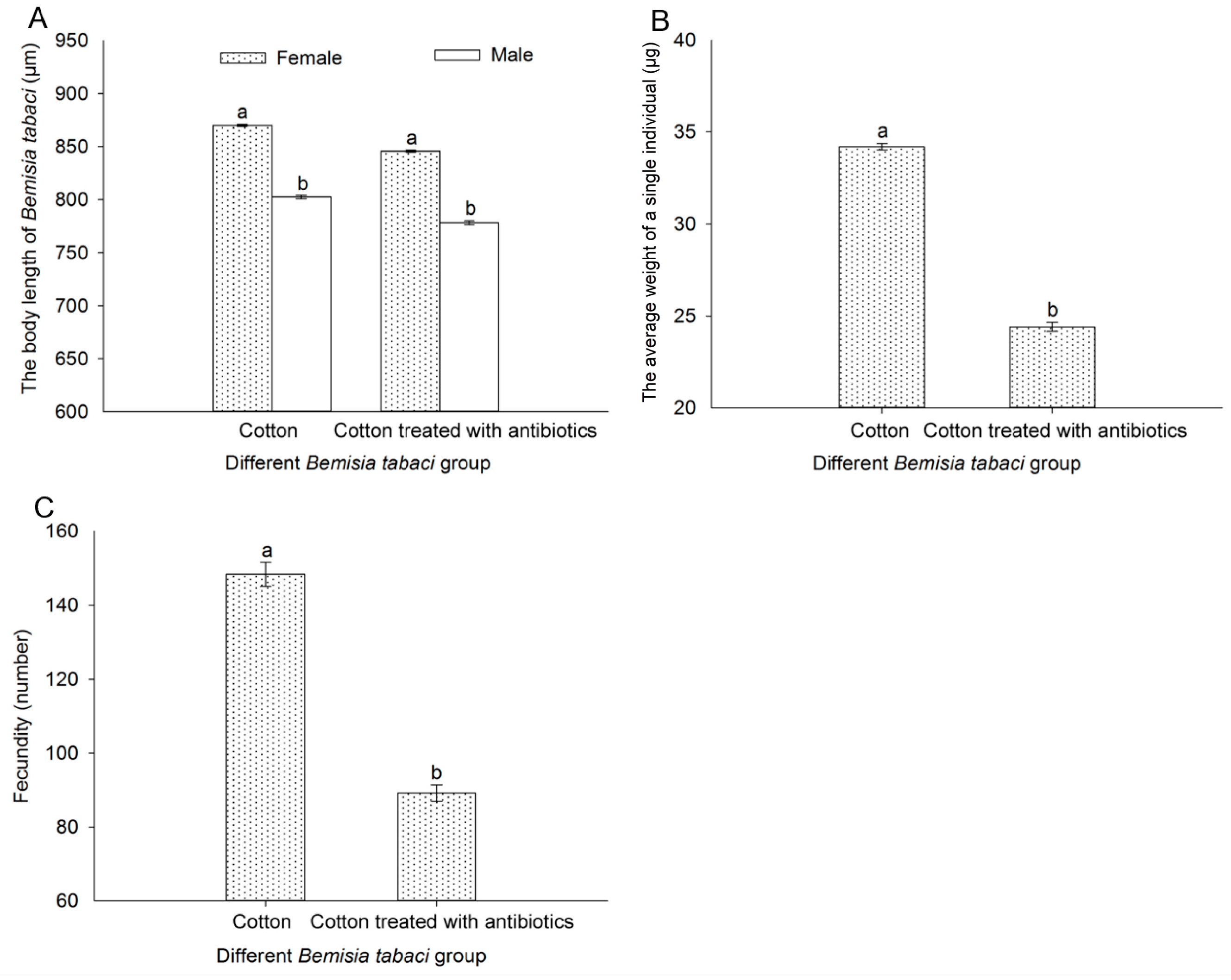
3.9. Effect of Antibiotic Treatment on the Body Length, Weight of Thousand Individuals, and Fecundity of Bemisia tabaci
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shamjana, U.; Vasu, D.A.; Hembrom, P.S.; Nayak, K.; Grace, T. The role of insect gut microbiota in host fitness, detoxification and nutrient supplementation. Antonie Van Leeuwenhoek 2024, 117, 71. [Google Scholar] [PubMed]
- Hoffmann, A.A.; Cooper, B.S. Describing endosymbiont–host interactions within the parasitism–mutualism continuum. Ecol. Evol. 2024, 14, e11705. [Google Scholar] [PubMed]
- Michalik, A.; Franco, D.C.; Szklarzewicz, T.; Stroiński, A.; Łukasik, P. Facultatively intrabacterial localization of a planthopper endosymbiont as an adaptation to its vertical transmission. Msystems 2024, 9, e00634-24. [Google Scholar]
- Liu, Y.; Fan, Z.-Y.; An, X.; Shi, P.-Q.; Ahmed, M.Z.; Qiu, B.L. A single-pair method to screen Rickettsia-infected and uninfected whitefly Bemisia tabaci populations. J. Microbiol. Methods 2020, 168, 105797. [Google Scholar]
- Bing, X.L.; Ruan, Y.M.; Rao, Q.; Wang, X.W.; Liu, S.S. Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci. 2013, 20, 194–206. [Google Scholar]
- Bing, X.L.; Yang, J.; Zchori-Fein, E.; Wang, X.W.; Liu, S.S. Characterization of a Newly Discovered Symbiont of the Whitefly Bemisia tabaci (Hemiptera: Aleyrodidae). Appl. Environ. Microb. 2013, 79, 569–575. [Google Scholar]
- Baumann, P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar]
- Pan, H.P.; Li, X.C.; Ge, D.Q.; Wang, S.L.; Wu, Q.J.; Xie, W.; Jiao, X.G.; Chu, D.; Liu, B.M.; Xu, B.Y.; et al. Factors Affecting Population Dynamics of Maternally Transmitted Endosymbionts in Bemisia tabaci. PLoS ONE 2012, 7, e30760. [Google Scholar]
- Santos-Garcia, D.; Silva, F.J.; Moya, A.; Latorre, A. No exception to the rule: Candidatus Portiera aleyrodidarum cell wall revisited. FEMS Microbiol. Lett. 2014, 360, 132–136. [Google Scholar]
- Chen, W.; Hasegawa, D.K.; Kaur, N.; Kliot, A.; Pinheiro, P.V.; Luan, J.; Stensmyr, M.C.; Zheng, Y.; Liu, W.; Sun, H.; et al. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 2016, 14, 110. [Google Scholar]
- Li, Y.; Mbata, G.N.; Simmons, A.M.; Shapiro-Ilan, D.I.; Wu, S. Management of Bemisia tabaci on vegetable crops using entomopathogens. Crop Prot. 2024, 180, 106638. [Google Scholar]
- Kumaraswamy, S.; Sotelo-Cardona, P.; Shivanna, A.; Mohan, M.; Srinivasan, R. Evaluation of resistance in wild tomato accessions to the whitefly Bemisia tabaci and the invasive tomato leafminer Tuta absoluta. Entomol. Gen. 2024, 44, 307–314. [Google Scholar]
- Mbata, G.N.; Li, Y.; Warsi, S.; Simmons, A.M. Susceptibility of Yellow Squash and Zucchini Cultivars to the Sweetpotato Whitefly, Bemisia tabaci (Gennadius) (MEAM1), in the Southeastern United States. Insects 2024, 15, 429. [Google Scholar] [CrossRef]
- Li, Y.-H.; Peng, J.; Wu, Q.-J.; Sun, J.-C.; Zhang, P.-J.; Qiu, B.L. Transcriptome data reveal beneficial effects of Rickettsia (Rickettsiales: Rickettsiaceae) on Bemisia tabaci (Hemiptera: Aleyrodidae) through nutritional factors and defense mechanisms. J. Econ. Entomol. 2024, 117, 817–824. [Google Scholar]
- Jiu, M.; Hu, J.; Wang, L.J.; Dong, J.F.; Song, Y.Q.; Sun, H.Z. Cryptic species identification and composition of Bemisia tabaci (Hemiptera: Aleyrodidae) complex in Henan province, China. J. Insect Sci. 2017, 17, 78. [Google Scholar]
- Elfekih, S.; Tay, W.T.; Gordon, K.; Court, L.N.; De Barro, P.J. Standardized molecular diagnostic tool for the identification of cryptic species within the Bemisia tabaci complex. Pest. Manag. Sci. 2018, 74, 170–173. [Google Scholar]
- Acharya, R.; Shrestha, Y.K.; Khatun, M.F.; Lee, K.-Y. Identification of begomoviruses from three cryptic species of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in Nepal. Agronomy 2021, 11, 2032. [Google Scholar] [CrossRef]
- Tang, J.; Qu, C.; Zhan, Q.; Zhang, D.; Wang, J.; Luo, C.; Wang, R. Baseline of susceptibility, risk assessment, biochemical mechanism, and fitness cost of resistance to dimpropyridaz, a novel pyridazine pyrazolecarboxamide insecticide, in Bemisia tabaci from China. Pestic. Biochem. Physiol. 2024, 203, 105987. [Google Scholar]
- Zhou, M.; Liu, Y.; Wang, Y.; Chang, Y.; Wu, Q.; Gong, W.; Du, Y. Effect of high temperature on abamectin and thiamethoxam tolerance in Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). Insects 2024, 15, 399. [Google Scholar] [CrossRef]
- Li, J.; Zhu, C.; Xu, Y.; He, H.; Zhao, C.; Yan, F. Molecular mechanism underlying ROS-mediated AKH resistance to imidacloprid in whitefly. Insects 2024, 15, 436. [Google Scholar] [CrossRef]
- Thao, M.L.L.; Baumann, P. Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr. Microbiol. 2004, 48, 140–144. [Google Scholar] [PubMed]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. Lond. B 2003, 270, 1857–1865. [Google Scholar]
- Zchori, F.E.; Brown, J.K. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2020, 95, 711–718. [Google Scholar]
- Gottlieb, Y.; Ghanim, M.; Chiel, E.; Gerling, D.; Portnoy, V.; Steinberg, S.; Tzuri, G.; Horowitz, A.R.; Belausov, E.; Mozes-Daube, N.; et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microb. 2006, 72, 3646–3652. [Google Scholar]
- Nirgianaki, A.; Banks, G.K.; Frohlich, D.R.; Veneti, Z.; Braig, H.R.; Miller, T.A.; Bedford, I.D.; Markham, P.G.; Savakis, C.; Bourtzis, K. Wolbachia infections of the whitefly Bemisia tabaci. Curr. Microbiol. 2003, 47, 93–101. [Google Scholar]
- Everett, K.D.E.; Thao, M.; Horn, M.; Dyszynski, G.E.; Baumann, P. Novel chlamydiae in whiteflies and scale insects: Endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int. J. Syst. Evol. Microbiol. 2005, 55, 1581–1587. [Google Scholar]
- Li, Y.H.; Ahmed, M.Z.; Li, S.J.; Lv, N.; Shi, P.Q.; Chen, X.S.; Qiu, B.L. Plant-mediated horizontal transmission of Rickettsia endosymbiont between different whitefly species. FEMS Microbiol. Ecol. 2017, 93, 138. [Google Scholar]
- Surapathrudu, K.; Murad, G. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar]
- Li, Q.; Fan, J.; Sun, J.R.; Wang, M.Q.; Francis, F.; Chen, J.L. Research progress in the interactions among the plants, insects and endosymbionts. J. Plant Prot. 2016, 43, 881–891. [Google Scholar]
- Hu, F.Y.; Chi-Wei, T. Nutritional Relationship between Bemisia tabaci and its primary endosymbiont, Portiera aleyrodidarum, during host plant acclimation. Insects 2020, 11, 498. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, C.; Yan, J.Y.; Wang, T.Y.; Yao, Y.L.; Ren, F.R.; Luan, J.B. Autophagy regulates whitefly-symbiont metabolic interactions. Appl. Environ. Microb. 2022, 88, e02089-21. [Google Scholar]
- Liu, Y.H.; Shah, M.; Song, Y.; Liu, T.X. Host plant affects symbiont abundance in Bemisia tabaci (Hemiptera: Aleyrodidae). Insects 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Bao, X.-Y.; Yan, J.Y.; Zhang, D.; Sun, X.; Li, C.Q.; Chen, Z.B.; Luan, J.B. Rickettsia symbionts spread via mixed mode transmission, increasing female fecundity and sex ratio shift by host hormone modulating, Proc. Natl. Acad. Sci. USA 2024, 121, e2406788121. [Google Scholar]
- Shi, P.Q.; Wang, L.; Chen, X.Y.; Wang, K.; Wu, Q.J.; Turlings Ted, C.J.; Zhang, P.J.; Qiu, B.L. Rickettsia transmission from whitefly to plants benefits herbivore insects but is detrimental to fungal and viral pathogens. Mbio 2024, 15, e02448-23. [Google Scholar]
- Qiu, B.L.; Chen, Y.P.; Liu, L.; Peng, W.L.; Li, X.X.; Mathur, M.Z.A.; Du, Y.Z.; Ren, S.X. Identification of three major Bemisia tabaci biotypes in China based on morphological and DNA polymorphisms. Prog. Nat. Sci. 2009, 6, 713–718. [Google Scholar]
- Chen, Q.; Liu, Y.S.; Zhu, G.R.; Xiao, L.F. Development, survivorship, reproduction and population increase of B-biotype Bemisia tabaci (Homoptera: Aleyrodidae) on five host plants. J. Shandong Agric. Univ. 2003, 34, 479–481. [Google Scholar]
- Yang, Y.Y.; Wang, Z.H.; Huang, J.; Peng, J.L.; Jiang, F.Y.; Liu, J.Y. The effects of four kinds of host plants on the development, survivorship and reproduction of Bemisia tabaci. Entomol. J. East. China 2006, 15, 276–280. [Google Scholar]
- Lv, N.; Peng, J.; Chen, X.Y.; Guo, C.F.; Sang, W.; Wang, X.M.; ZAhmed, M.; Xu, Y.Y.; Qiu, B.L. Antagonistic interaction between male-killing and cytoplasmic incompatibility induced by Cardinium and Wolbachia in the whitefly, Bemisia tabaci. Insect Sci. 2021, 28, 330–346. [Google Scholar]
- Chiel, E.; Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Katzir, N.; Inbar, M.; Ghanim, M. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull. Entomol. Res. 2007, 97, 407–413. [Google Scholar]
- Kliot, A.; Kontsedalov, S.; Lebedev, G.; Czosnek, H.; Ghanim, M. Combined infection with tomato yellow leaf curl virus and Rickettsia influences fecundity, attraction to infected plants and expression of immunity-related genes in the whitefly Bemisia tabaci. J. Gen. Virol. 2019, 100, 721–731. [Google Scholar]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. B-Biol. Sci. 1998, 265, 509–515. [Google Scholar]
- Shi, P.; Wang, L.; Liu, Y.; An, X.; Chen, X.; Ahmed, M.; Qiu, B.; Sang, W. Infection dynamics of endosymbionts reveal three novel localization patterns of Rickettsia during the development of whitefly Bemisia tabaci. FEMS Microbiol. Ecol. 2018, 94, 165. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Pan, H.P.; Chu, D.; Liu, B.M.; Xie, W.; Wang, S.L.; Wu, Q.J.; Xu, B.Y.; Zhang, Y.J. Relative amount of symbionts in insect hosts changes with host-plant adaptation and insecticide resistance. Environ. Entomol. 2013, 42, 74–78. [Google Scholar]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar]
- Ghanim, M.; Kontsedalov, S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest. Manag. Sci. 2009, 65, 939–942. [Google Scholar]
- Paschapur, A.U.; Singh, A.K.; Buski, R.; Guru, P.N.; Jeevan, B.; Mishra, K.K.; Kant, L. Unravelling geospatial distribution and genetic diversity of greenhouse whitefly, Trialeurodes vaporariorum (Westwood) from Himalayan Region. Sci. Rep. 2023, 13, 11946. [Google Scholar]
- Leonardo, T.E.; Muiru, G.T. Facultative symbionts are associated with host plant specialization in pea aphid populations. Proc. R. Soc. B-Biol. Sci. 2003, 270, 209–212. [Google Scholar]
- Simon, J.-C.; Carré, S.; Boutin, M.; Prunier-Leterme, N.; Sabater-Muñoz, B.; Latorre, A.; Bournoville, R. Host-based divergence in populations of the pea aphid: Insights from nuclear markers and the prevalence of facultative symbionts. Proc. R. Soc. B-Biol. Sci. 2003, 270, 1703–1712. [Google Scholar]
- Zhang, B.; Yang, W.; He, Q.; Chen, H.; Che, B.; Bai, X. Analysis of differential effects of host plants on the gut microbes of Rhoptroceros cyatheae. Front. Microbiol. 2024, 15, 1392586. [Google Scholar]
- Toju, H.; Fukatsu, T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011, 20, 853–868. [Google Scholar] [PubMed]
- Wilkinson, T.L.; Adams, D.; Minto, L.B.; Douglas, A.E. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J. Exp. Biol. 2001, 204, 3027–3038. [Google Scholar] [PubMed]
- Liang, Y.; Dikow, R.B.; Su, X.; Wen, J.; Ren, Z. Comparative genomics of the primary endosymbiont Buchnera aphidicola in aphid hosts and their coevolutionary relationships. BMC Biol. 2024, 22, 137. [Google Scholar] [PubMed]
- Łukasik, P.; Kolasa, M.R. With a little help from my friends: The roles of microbial symbionts in insect populations and communities. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230122. [Google Scholar]
- Cibichakravarthy, B.; Shaked, N.; Kapri, E.; Gottlieb, Y. Endosymbiont-derived metabolites are essential for tick host reproductive fitness. Msphere 2024, 9, e00693-23. [Google Scholar]
- Sloan, D.B.; Moran, N.A. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol. Lett. 2012, 8, 986–989. [Google Scholar]
- Santos-Garcia, D.; Farnier, P.-A.; Beitia, F.; Zchori-Fein, E.; Vavre, F.; Mouton, L.; Moya, A.; Latorre, A.; Silva, F.J. Complete genome sequence of “Candidatus Portiera aleyrodidarum” BT-QVLC, an obligate symbiont that supplies amino acids and carotenoids to Bemisia tabaci. J. Bacteriol. 2012, 194, 6654–6655. [Google Scholar]
- Costa, H.S.; Brown, J.K.; Byrne, D.N. Life history traits of the whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) on five virus-infected or healthy plant species. Environ. Entomol. 1991, 20, 1102–1107. [Google Scholar]
- Blackmer, J.L.; Byrne, D.N. Changes in amino acids in Cucumis melo in relation to life-history traits and flight propensity of Bemisia tabaci. Entomol. Exp. Appl. 2010, 93, 29–40. [Google Scholar]
- Yu, H.; Wang, K.; Yang, Z.; Li, X.; Liu, S.; Wang, L.; Zhang, H. A ferritin protein is involved in the development and reproduction of the whitefly, Bemisia tabaci. Environ. Entomol. 2023, 52, 750–758. [Google Scholar]
- Yang, Z.; Wang, K.; Liu, S.; Li, X.; Wang, H.; Wang, L.; Zhang, H.; Yu, H. Identification and functional analysis of isopentenyl pyrophosphate isomerase genes in the whiteflies Bemisia tabaci (Hemiptera: Aleyrodidae). J. Insect Sci. 2023, 23, 16. [Google Scholar]

| Symbiont | Primer Sequence (5′-3′) | Annealing Temperature | Product Size (bp) | Reference |
|---|---|---|---|---|
| Portiera | 28F: 5′-TGCAAGTCGAGCGGCATCAT-3′ 1098R: 5′-AAAGTTCCCGCCTTATGCGT-3′ | 60 °C | ~1000 | [23] |
| Hamiltonella | Ham-F: 5′-TGAGTAAAGTCTGGAATCTGG-3′ Ham-R: 5′-AGTTCAAGACCGCAACCTC-3′ | 58 °C | ~700 | [39] |
| Rickettsia | Rb-F: 5′-GCTCAGAACGAACGCTATC-3′ Rb-R: 5′-GAAGGAAAGCATCTCTGC-3′ | 58 °C | ~900 | [40] |
| Wolbachia | wsp-81F: 5′-TGGTCCAATAAGTGATGAAGAAAC-3′ wsp-691R: 5′-AAAAATTAAACGCTACTCCA-3′ | 55 °C | ~600 | [41] |
| Fritchea | U23F: 5′-GATGCCTTGGCATTGATAGGCGATGAAGGA-3′ 23SIGR5′-TGGCTCATCATGCAAAAGGCA-3′ | 55 °C | ~600 | [26] |
| Cardinium | CFB-F: 5′-GCGGTGTAAAATGAGCGTG-3′ CFB-R: 5′-ACCTMTTCTTAACTCAAGCCT-3′ | 58 °C | ~400 | [22] |
| Arsenophonus | Ars23S-15′-CGTTTGATGAATTCATAGTCAAA-3′ Ars23S-25′-GGTCCTCCAGTTAGTGTTACCCAAC-3′ | 52 °C | ~600 | [21] |
| Host Plant | Portiera | Hamiltonella | Rickettsia | Wolbachia | Fritchea | Cardinium | Arsenophonus |
|---|---|---|---|---|---|---|---|
| Poinsettia | 100% | 86.7% | 80.0% | - | - | - | - |
| Cabbage | 100% | 90.0% | 83.3% | - | - | - | - |
| Cotton | 100% | 83.3% | 86.7% | - | - | - | - |
| Tomato | 100% | 83.3% | 80.0% | - | - | - | - |
| Tobacco | 100% | 73.3% | 76.7% | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Yin, X.-J.; Fan, Z.-H.; Gu, X.-H.; Su, Z.-Q.; Luo, B.-R.; Qiu, B.-L.; Zhang, L.-H. Effects of Endosymbionts on the Nutritional Physiology and Biological Characteristics of Whitefly Bemisia tabaci. Insects 2025, 16, 703. https://doi.org/10.3390/insects16070703
Gao H, Yin X-J, Fan Z-H, Gu X-H, Su Z-Q, Luo B-R, Qiu B-L, Zhang L-H. Effects of Endosymbionts on the Nutritional Physiology and Biological Characteristics of Whitefly Bemisia tabaci. Insects. 2025; 16(7):703. https://doi.org/10.3390/insects16070703
Chicago/Turabian StyleGao, Han, Xiang-Jie Yin, Zhen-Huai Fan, Xiao-Hang Gu, Zheng-Qin Su, Bing-Rui Luo, Bao-Li Qiu, and Li-He Zhang. 2025. "Effects of Endosymbionts on the Nutritional Physiology and Biological Characteristics of Whitefly Bemisia tabaci" Insects 16, no. 7: 703. https://doi.org/10.3390/insects16070703
APA StyleGao, H., Yin, X.-J., Fan, Z.-H., Gu, X.-H., Su, Z.-Q., Luo, B.-R., Qiu, B.-L., & Zhang, L.-H. (2025). Effects of Endosymbionts on the Nutritional Physiology and Biological Characteristics of Whitefly Bemisia tabaci. Insects, 16(7), 703. https://doi.org/10.3390/insects16070703






