Comparative Analysis of Diel and Circadian Eclosion Rhythms and Clock Gene Expression Between Sexes in the Migratory Moth Spodoptera frugiperda
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Stock
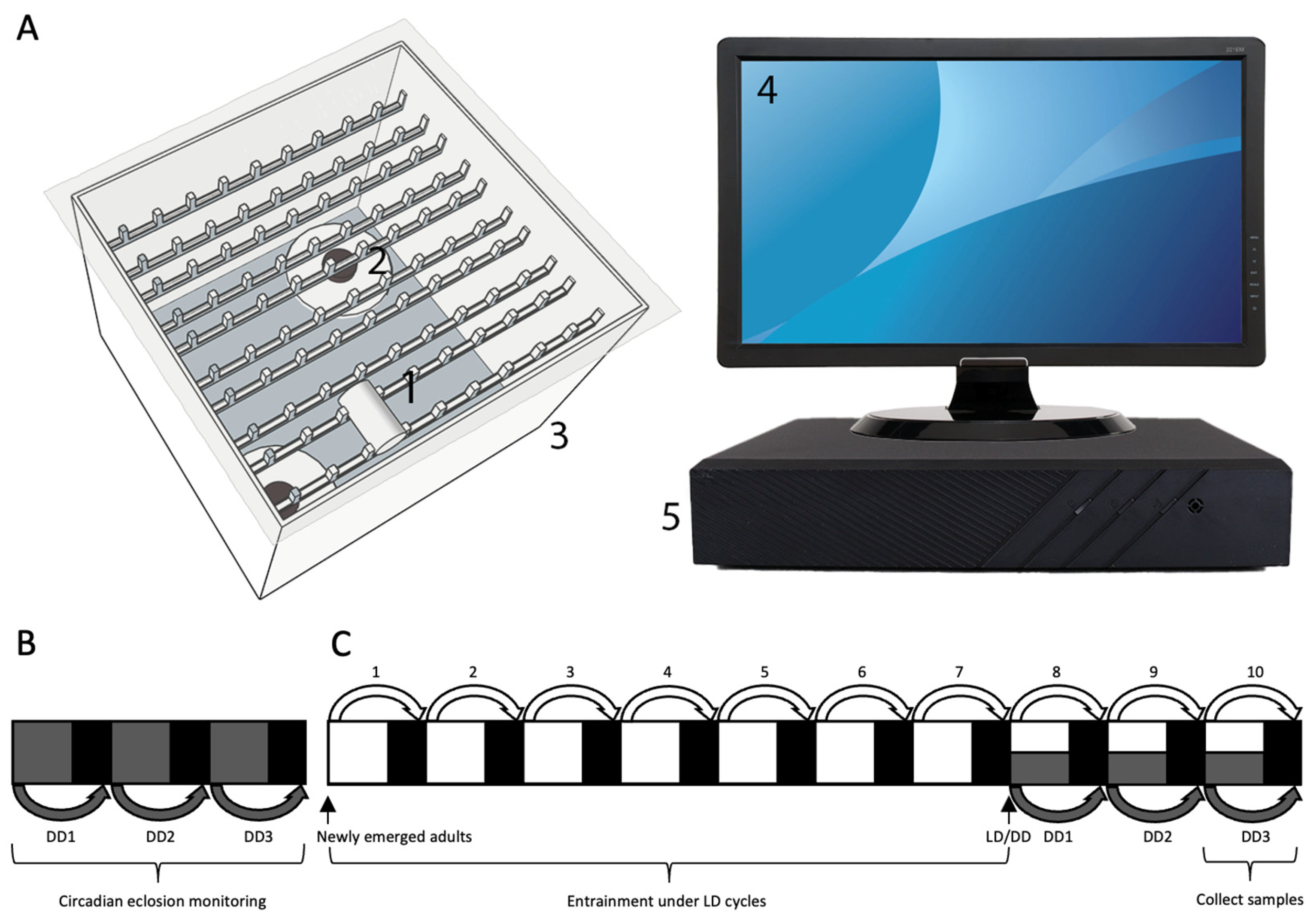
2.2. Diel Eclosion Behavior Assay Under the Summer-like L14: D10 Cycle
2.3. Circadian Eclosion Behavior Assay Under Constant Darkness
2.4. Tissue Collection
2.5. RNA Isolation and cDNA Synthesis
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
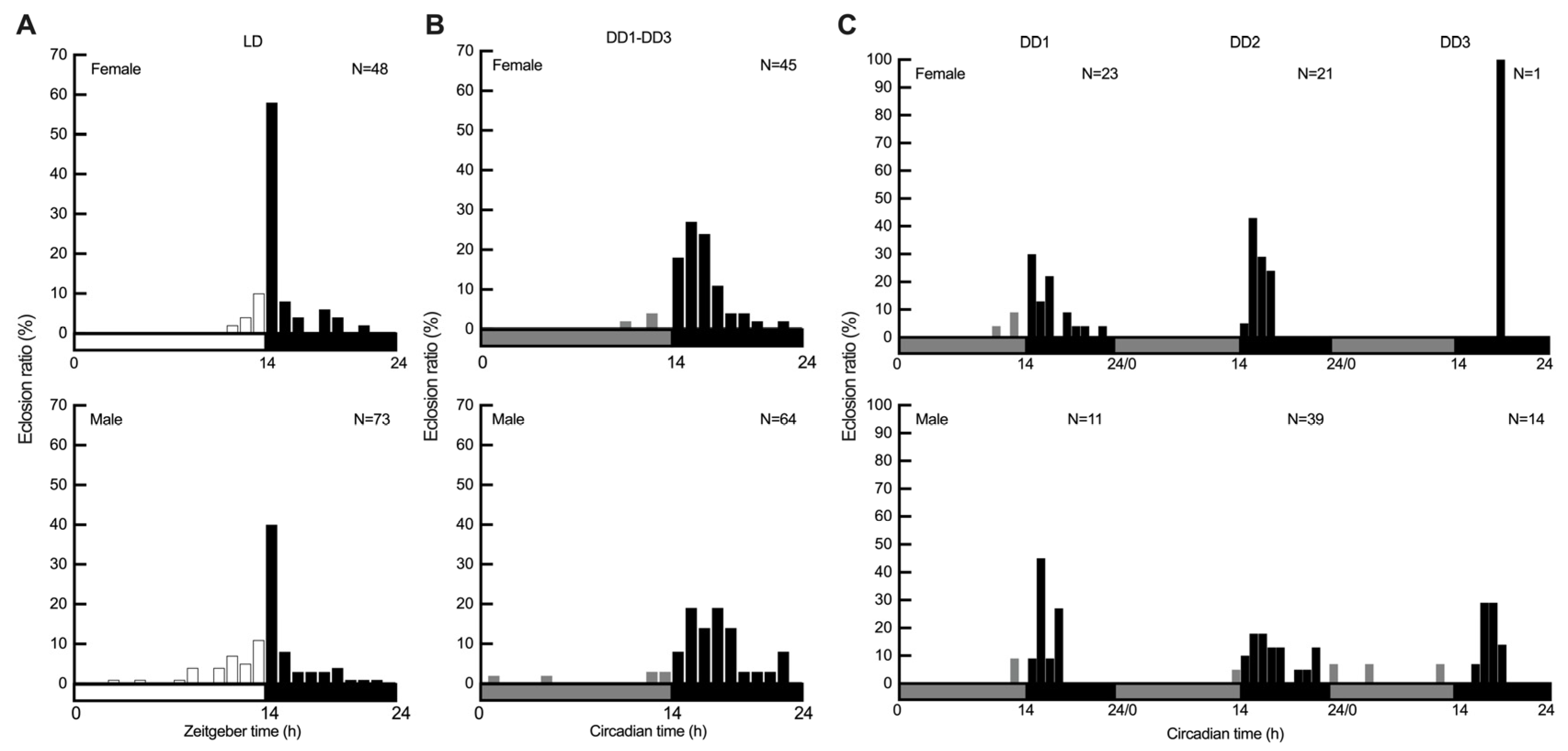
3.1. Diel and Circadian Eclosion Rhythms of S. frugiperda Entrained to Summer-like Photoperiod
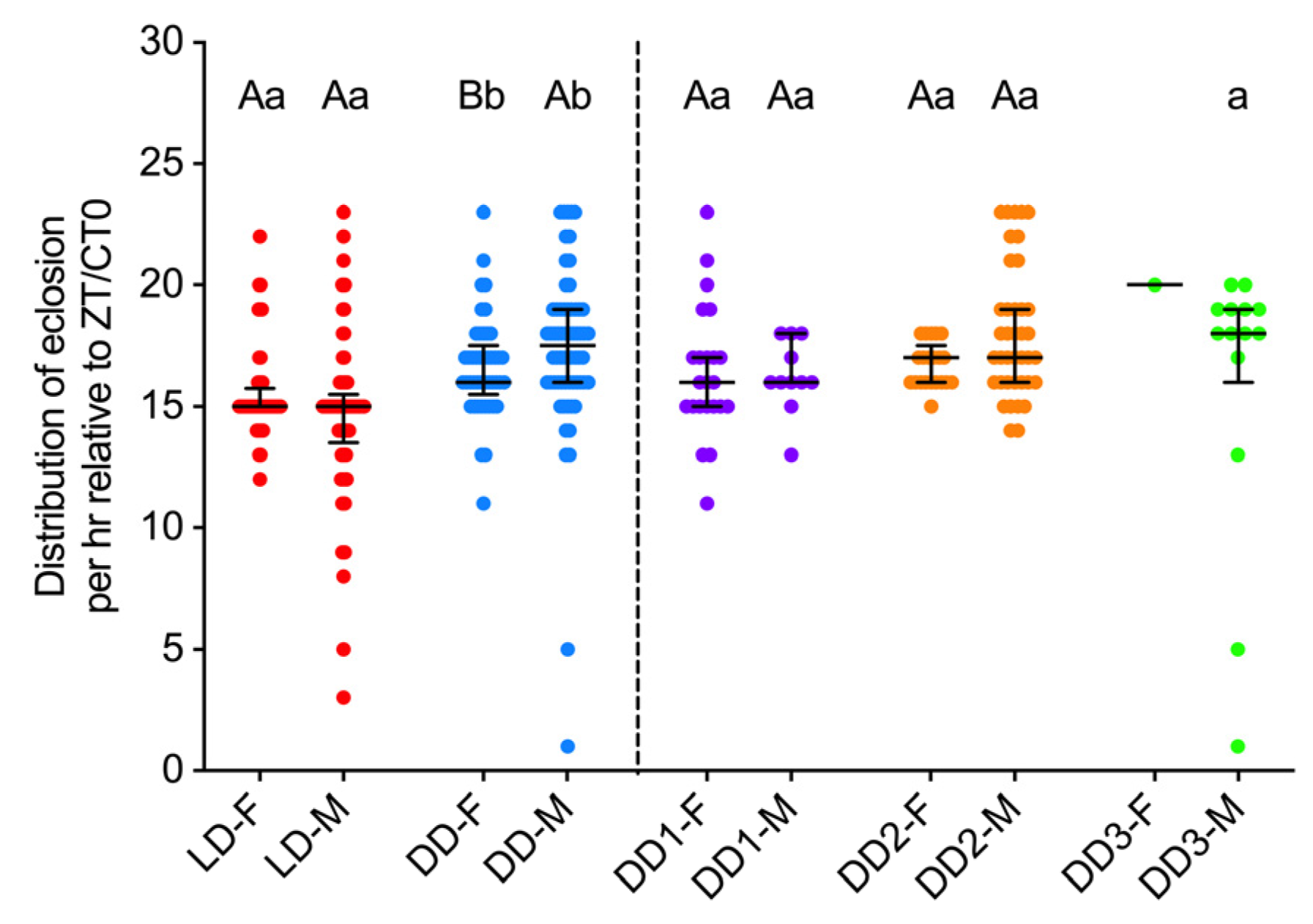
3.2. Distribution of Pupa Eclosion Between Sexes and Light Regimes
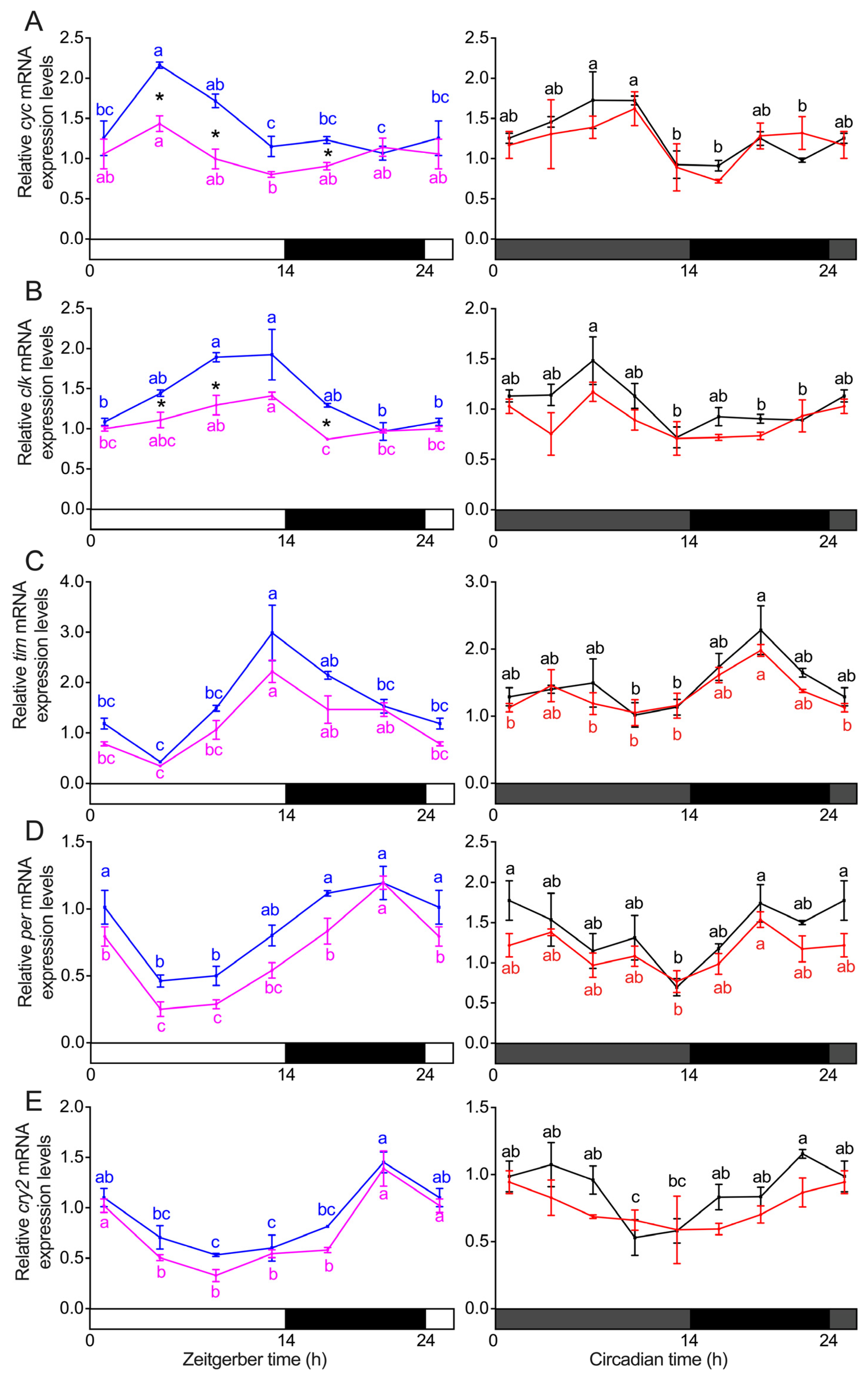
3.3. Transcript Expression of Clock Genes in the Heads of S. frugiperda
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L14: D10 | 14 h light: 10 h dark |
| DD | Constant darkness |
| FAW | Fall armyworm |
| TTFL | Transcriptional–translational feedback loop |
| LD | Light–dark |
| ZT | Zeitgeber time |
| DD1 | The first day of constant darkness |
| DD2 | The second day of constant darkness |
| DD3 | The third day of constant darkness |
| CT | Circadian time |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| K-S | Kolmogorov–Smirnov |
| M-W | Mann–Whitney |
References
- Hardin, P.E. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 2011, 74, 141–173. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 2014, 35, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Cain, S.W.; Chang, A.M.; Phillips, A.J.; Munch, M.Y.; Gronfier, C.; Wyatt, J.K.; Dijk, D.J.; Wright, K.P., Jr.; Czeisler, C.A. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108, 15602–15608. [Google Scholar] [CrossRef] [PubMed]
- Force, E.; Sokolowski, M.B.C.; Suray, C.; Debernard, S.; Chatterjee, A.; Dacher, M. Regulation of feeding dynamics by the circadian clock, light and sex in an adult nocturnal insect. Front. Physiol. 2024, 14, 1304626. [Google Scholar] [CrossRef]
- Helfrich-Förster, C. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster sex-specific differences suggest a different quality of activity. J. Biol. Rhythms 2000, 15, 135–154. [Google Scholar] [CrossRef]
- Joye, D.A.M.; Evans, J.A. Sex differences in daily timekeeping and circadian clock circuits. Semin. Cell Dev. Biol. 2022, 126, 45–55. [Google Scholar] [CrossRef]
- Krizo, J.A.; Mintz, E.M. Sex differences in behavioral circadian rhythms in laboratory rodents. Front. Endocrinol. 2015, 5, 234. [Google Scholar] [CrossRef]
- Rund, S.S.; Lee, S.J.; Bush, B.R.; Duffield, G.E. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles Gambiae mosquitoes. J. Insect Physiol. 2012, 58, 1609–1619. [Google Scholar] [CrossRef]
- Walton, J.C.; Bumgarner, J.R.; Nelson, R.J. Sex differences in circadian rhythms. Cold Spring Harbor Perspect. Biol. 2022, 14, a039107. [Google Scholar] [CrossRef]
- Bünning, E. Zur Kenntnis Der Endonomen Tagesrhythmik Bei Insekten Und Bei Pflanzen. Ber. Dtsch. Bot. Ges. 1935, 53, 594–623. [Google Scholar] [CrossRef]
- Kalmus, H. Periodizität und autochronie (ideochronie) als zeitregelnde eigenschaffen der organismen. Biol. Gen. 1935, 11, 93–114. [Google Scholar]
- Saunders, D.S. Insect Clocks; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Numata, H.; Tomioka, K. Insect Chronobiology; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Pittendrigh, C.S. On temperature independence in the clock system controlling emergence time in Drosophila. Proc. Natl. Acad. Sci. USA 1954, 40, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C.S.; Skopik, S.D. Circadian systems. V. The driving oscillation and the temporal sequence of development. Proc. Natl. Acad. Sci. USA 1970, 65, 500–507. [Google Scholar] [CrossRef]
- Merlin, C.; Reppert, S.M. The evolution of circadian clocks in insects. Integr. Comp. Biol. 2009, 49, E114. [Google Scholar]
- Mark, B.; Bustos-González, L.; Cascallares, G.; Conejera, F.; Ewer, J. The circadian clock gates Drosophila adult emergence by controlling the timecourse of metamorphosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023249118. [Google Scholar] [CrossRef]
- Wegener, C.; Amini, E.; Cavieres-Lepe, J.; Ewer, J. Neuronal and endocrine mechanisms underlying the circadian gating of eclosion: Insights from Drosophila. Curr. Opin. Insect Sci. 2024, 66, 101286. [Google Scholar] [CrossRef]
- Pilorz, V.; Helfrich-Förster, C.; Oster, H. The role of the circadian clock system in physiology. Pflügers Arch. Eur. J. Physiol. 2018, 470, 227–239. [Google Scholar] [CrossRef]
- Tomioka, K.; Matsumoto, A. Circadian molecular clockworks in non-model insects. Curr. Opin. Insect Sci. 2015, 7, 58–64. [Google Scholar] [CrossRef]
- Dushay, M.S.; Konopka, R.J.; Orr, D.; Greenacre, M.L.; Kyriacou, C.P.; Rosbash, M.; Hall, J.C. Phenotypic and genetic analysis of clock, a new circadian rhythm mutant in Drosophila melanogaster. Genetics 1990, 125, 557–578. [Google Scholar] [CrossRef]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Price, J.L.; Man, B.; Young, M.W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science 1994, 263, 1603–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Markert, M.J.; Groves, S.C.; Hardin, P.E.; Merlin, C. Vertebrate-like CRYPTOCHROME 2 from monarch regulates circadian transcription via independent repression of CLOCK and BMAL1 activity. Proc. Natl. Acad. Sci. USA 2017, 114, E7516–E7525. [Google Scholar] [CrossRef] [PubMed]
- Iiams, S.E.; Lugena, A.B.; Zhang, Y.; Hayden, A.N.; Merlin, C. Photoperiodic and clock regulation of the vitamin A pathway in the brain mediates seasonal responsiveness in the monarch butterfly. Proc. Natl. Acad. Sci. USA 2019, 116, 25214–25221. [Google Scholar] [CrossRef]
- Iiams, S.E.; Wan, G.J.; Zhang, J.W.; Lugena, A.B.; Zhang, Y.; Hayden, A.N.; Merlin, C. Loss of functional cryptochrome 1 reduces robustness of 24-hour behavioral rhythms in monarch butterflies. iScience 2024, 27, 108980. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Watari, Y.; Tanaka, K.; Goto, S.G. Temperature cycle amplitude alters the adult eclosion time and expression pattern of the circadian clock gene period in the onion fly. J. Insect Physiol. 2016, 86, 54–59. [Google Scholar] [CrossRef]
- Myers, E.M. The circadian control of eclosion. Chronobiol. Int. 2003, 20, 775–794. [Google Scholar] [CrossRef]
- Massy, R.; Hawkes, W.L.S.; Doyle, T.; Troscianko, J.; Menz, M.H.M.; Roberts, N.W.; Chapman, J.W.; Wotton, K.R. Hoverflies use a time-compensated sun compass to orientate during autumn migration. Proc. Biol. Sci. 2021, 288, 20211805. [Google Scholar] [CrossRef]
- Ji, J.Y.; Liu, Y.Q.; Zhang, L.; Cheng, Y.X.; Stanley, D.; Jiang, X.F. The clock gene, period, influences migratory flight and reproduction of the oriental armyworm, Mythimna separata (Walker). Insect Sci. 2023, 30, 650–6604. [Google Scholar] [CrossRef]
- Merlin, C.; Iiams, S.E.; Lugena, A.B. Monarch butterfly migration moving into the genetic era. Trends Genet. 2020, 36, 689–701. [Google Scholar] [CrossRef]
- Merlin, C.; Liedvogel, M. The genetics and epigenetics of animal migration and orientation: Birds, butterflies and beyond. J. Exp. Biol. 2019, 222, jeb191890. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2022, 43, 102198. [Google Scholar] [CrossRef]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, evolution, and management options of an invasive species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef]
- Chen, H.; Wu, M.F.; Liu, J.; Shen, A.D.; Jiang, Y.Y.; Hu, G. Migratory routes and occurrence divisions of the fall armyworm Spodoptera frugiperda in China. J. Plant Prot. 2020, 47, 747–757. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jiang, Y.Y.; Zhai, B.P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2019, 76, 454–463. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Lv, H.; Dong, Y.Y.; Huang, W.J.; Hu, G.; Liu, Y.; Chen, H.; Geng, Y.; Bai, J.; Guo, P.; et al. Mapping the spatio-temporal distribution of fall armyworm in China by coupling multi-factors. Remote Sens. 2022, 14, 4415. [Google Scholar] [CrossRef]
- Jiang, C.X.; Zhang, X.Y.; Wu, J.; Feng, C.H.; Ma, L.; Hu, G.; Li, Q. The source areas and migratory pathways of the fall armyworm Spodoptera frugiperda (Smith) in Sichuan province, China. Insects 2022, 13, 935. [Google Scholar] [CrossRef]
- Wu, Q.L.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the north China plain. Insect Sci. 2021, 28, 649–661. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Huang, L.; Liu, J.; Zhang, H.B.; Qiu, K.; Lu, F.; Hu, G. Migration dynamics of fall armyworm Spodoptera frugiperda (Smith) in the Yangtze river delta. Insects 2023, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wan, G.J.; Li, J.C.; Ma, Y.B.; Reynolds, D.R.; Dreyer, D.; Warrant, E.J.; Chapman, J.W.; Hu, G. Adaptive migratory orientation of an invasive pest on a new continent. iScience 2023, 26, 108281. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Hahn, D.A.; Merlin, C.; Holzapfel, C.M.; Bradshaw, W.E. Keeping time without a Spine: What can the insect clock teach us about seasonal adaptation? Phil. Trans. R. Soc. B 2017, 372, 20160257. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Liu, B.; Zheng, W.G.; Liu, C.H.; Liu, Z.X.; He, Y.; Li, X.; Wu, C.; Wang, P.; Liu, K.Y.; et al. Chromosome-level genome of black cutworm provides novel insights into polyphagy and seasonal migration in insects. BMC Biol. 2023, 21, 2. [Google Scholar] [CrossRef]
- Lv, C.N.; Huang, X.; Wang, W.T.; He, Y.K.; Hu, G.; Pan, W.D.; Chen, F.J.; Wan, G.J. The circadian eclosion rhythm of Spodoptera frugiperda in response to photoperiod cues. Chin. J. Appl. Entomol. 2023, 60, 1722–1732. [Google Scholar]
- Hänniger, S.; Dumas, P.; Schöfl, G.; Gebauer-Jung, S.; Vogel, H.; Unbehend, M.; Heckel, D.G.; Groot, A.T. Genetic basis of allochronic differentiation in the fall armyworm. BMC Evol. Biol. 2017, 17, 68. [Google Scholar] [CrossRef]
- Tessnow, A.E.; Nagoshi, R.N.; Meagher, R.L.; Gilligan, T.M.; Sadd, B.M.; Carriere, Y.; Davis, H.N.; Fleischer, S.J.; Richers, K.; Palumbo, J.C.; et al. Genomic patterns of strain-specific genetic structure, linkage, and selection across fall armyworm populations. BMC Genom. 2025, 26, 116. [Google Scholar] [CrossRef]
- van Doorn, G.S.; Schepers, J.; Hut, R.A.; Groot, A.T. Sex-specific expression of circadian rhythms enables allochronic speciation. Evol. Lett. 2024, 9, 65–76. [Google Scholar] [CrossRef]
- Wang, X.; Du, Z.Y.; Duan, Y.G.; Liu, S.L.; Liu, J.; Li, B.Y.; Ma, L.; Wu, Y.F.; Tian, L.; Song, F.; et al. Population genomics analyses reveal the role of hybridization in the rapid invasion of fall armyworm. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Wan, G.; Sword, G.A.; Du, J.; Huang, Q.; Chen, W.; Warrant, E. Editorial: Magnetobiology and chronobiology: New opportunities for smart phytoprotection. Front. Plant. Sci. 2025, 16, 1594646. [Google Scholar] [CrossRef]
- Li, Z.Y.; Dai, Q.X.; Kuang, Z.L.; Liang, M.R.; Wang, L.; Lu, Y.Y.; Chen, K. Effects of three artificial diets on development and reproduction of the fall armyworm Spodoptera frugiperda (J.E. Smith). J. Environ. Entomol. 2019, 41, 1147–1154. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, L.; Wei, Y.J.; Zhang, M.; Pan, W.D.; Sword, G.A.; Yang, F.; Chen, F.J.; Wan, G.J. Reliable reference genes for gene expression analyses under the hypomagnetic field in a migratory insect. Front. Physiol. 2022, 13, 954228. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.; Parsons, R.; Garner, N.; Oster, H.; Rawashdeh, O. CircaCompare: A method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Bioinformatics 2019, 36, 1208–1212. [Google Scholar] [CrossRef]
- Stanewsky, R. Clock mechanisms in Drosophila. Cell Tissue Res. 2002, 309, 11–26. [Google Scholar] [CrossRef]
- Merlin, C. Lepidopteran Circadian Clocks: From Molecules to Behavior. In Molecular Biology and Genetics of the Lepidoptera, 1st ed.; Taylor & Francis: London, UK, 2009; pp. 153–168. [Google Scholar] [CrossRef]
- Bertossa, R.C.; van Dijk, J.; Beersma, D.G.; Beukeboom, L.W. Circadian rhythms of adult emergence and activity but not eclosion in males of the parasitic wasp Nasonia vitripennis. J. Insect Physiol. 2010, 56, 805–812. [Google Scholar] [CrossRef][Green Version]
- Nartey, M.A.; Sun, X.; Qin, S.; Hou, C.X.; Li, M.W. CRISPR/Cas9-based knockout reveals that the clock gene timeless is indispensable for regulating circadian behavioral rhythms in Bombyx mori. Insect Sci. 2021, 28, 1414–1425. [Google Scholar] [CrossRef]
- Wang, D.F.; Chen, J.; Yuan, Y.; Yu, L.Q.; Yang, G.; Chen, W.F. CRISPR/Cas9-mediated knockout of Period reveals its function in the circadian rhythms of the diamondback moth Plutella xylostella. Insect Sci. 2023, 30, 637–649. [Google Scholar] [CrossRef]
- Beer, K.; Helfrich-Förster, C. Model and non-model insects in chronobiology. Front. Behav. Neurosci. 2020, 14, 601676. [Google Scholar] [CrossRef]
- Bidell, D.; Feige, N.D.; Triphan, T.; Muller, C.; Pauls, D.; Helfrich-Förster, C.; Selcho, M. Photoreceptors for immediate effects of light on circadian behavior. iScience 2024, 27, 109819. [Google Scholar] [CrossRef]
- Malekera, M.J.; Acharya, R.; Mostafiz, M.M.; Hwang, H.S.; Bhusal, N.; Lee, K.Y. Temperature-dependent development models describing the effects of temperature on the development of the fall armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Insects 2022, 13, 1084. [Google Scholar] [CrossRef]
- Wei, Q.; He, J.C.; Wang, W.X.; Lai, F.X.; Wan, P.J.; Fu, Q. Role of the clock gene Period in regulating circadian rhythm of courtship vibrations in Nilaparvata lugens. Insect Biochem. Mol. Biol. 2025, 177, 104250. [Google Scholar] [CrossRef]
- Li, G.Y.; Cui, Q.; Zheng, S.R.; Zhang, K.X.; Wang, Y.H.; Zhan, S.; Fang, G.Q. Molecular basis of circadian rhythm divergence between diurnal and nocturnal Lepidoperans. iScience 2025, 28, 112206. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, Y.; Peng, H.; Deng, Z. Sex chromosome dosage compensation in insects. Insects 2025, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Dayton, J.N.; Dopman, E.B. The period gene alters daily and seasonal timing in Ostrinia nubilalis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Brady, D.; Saviane, A.; Cappellozza, S.; Sandrelli, F. The circadian clock in Lepidoptera. Front. Physiol. 2021, 12, 776826. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.Y.; Peng, Y.; Liang, X.Y.; Wilson, K.; Chipabika, G.; Karangwa, P.; Uzayisenga, B.; Mensah, B.A.; Kachigamba, D.L.; et al. Global genomic signature reveals the evolution of fall armyworm in the eastern hemisphere. Mol. Ecol. 2023, 32, 5463–5478. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, F.; Wan, X.S.; Xu, J.; Ye, H. Reproductive behavior and circadian rhythms of Spodoptera frugiperda. J. Environ. Entomol. 2022, 44, 509–522. [Google Scholar] [CrossRef]
- He, L.M.; Ge, S.S.; Zhang, H.W.; He, W.; Yan, R.; Wu, K.M. Photoregime affects development, reproduction, and flight performance of the invasive fall armyworm (Lepidoptera: Noctuidae) in China. Environ. Entomol. 2021, 50, 367–381. [Google Scholar] [CrossRef]
| Gene | GenBank No. | Sequence (5′ to 3′; F, Forward; R, Reverse) | Purpose |
|---|---|---|---|
| Qcyc | XM_035583465.2 | F: CAGCAGTCGTCGCATCAACA R: TCGCAGCCAGACATCTCGT | qRT-PCR for cycle |
| Qclk | XM_050704408.1 | F: GGCACCTCAGGCTACGATTAC R: ATCCACTGCTGCCCTTTTGTT | qRT-PCR for clock |
| Qtim | XM_050697432.1 | F: GTCTTCATTTGGTTGTTACGGCTA R: CAAGACCAGAAGCGACCTCA | qRT-PCR for timeless |
| Qper | XM_035573031.2 | F: ATTGTCAGGTTTCAGCGACTTT R: CGATTCTAAGGCACCTGTTACGA | qRT-PCR for period |
| Qcry2 | XM_050697787.1 | F: GGTCGGCATCACTGTCACATC R: CGCTTTGCCACCATTTCGTTCT | qRT-PCR for cryptochrome 2 |
| QEF1-α | U20139.1 | F: CGAAACCGCTATACTATGTCACC R: ATACCAGCCTCGAACTCACC | Reference gene elongation Factor 1-α |
| Qrpl32 | AF400195.1 | F: GGTTCACAGCCGTGTTTCAAT R: ATGGCGGATAAACCTTTTCGTC | Reference gene ribosomal protein L32 |
| cyc | clk | tim | per | cry2 | ||
|---|---|---|---|---|---|---|
| Female | LD | 0.0071 ** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| DD3 | ns | 0.044 * | 0.0058 ** | 0.010 * | ns | |
| Male | LD | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| DD3 | <0.001 *** | 0.0010 ** | 0.0059 ** | 0.0021 ** | <0.001 *** |
| Gene | Comparison Groups | Mesor Difference (p-Value) | Amplitude Difference (p-Value) | Phase Difference (p-Value) |
|---|---|---|---|---|
| cyc | LD-F vs. LD-M | −0.38 (<0.001 ***) | −0.24 (0.057) | −3.37 (0.044 *) |
| LD-F vs. DD3-F | na | na | na | |
| DD3-F vs. DD3-M | na | na | na | |
| LD-M vs. DD3-M | na | na | −0.16 (0.90) | |
| clk | LD-F vs. LD-M | −0.32 (<0.001 ***) | −0.29 (0.0038 **) | −0.64 (0.60) |
| LD-F vs. DD3-F | na | na | 5.59 (0.0069 **) | |
| DD3-F vs. DD3-M | −0.17 (0.012 *) | −0.10 (0.27) | −1.12 (0.58) | |
| LD-M vs. DD3-M | na | na | 5.11 (<0.001 ***) | |
| tim | LD-F vs. LD-M | −0.40 (0.014 *) | −0.27 (0.22) | 0.42 (0.68) |
| LD-F vs. DD3-F | na | na | −3.86 (0.015 *) | |
| DD3-F vs. DD3-M | −0.13 (0.24) | −0.094 (0.55) | −0.65 (0.72) | |
| LD-M vs. DD3-M | na | na | −4.93 (0.0021 **) | |
| per | LD-F vs. LD-M | −0.20 (<0.001 ***) | 0.055 (0.42) | 0.45 (0.46) |
| LD-F vs. DD3-F | na | na | −3.21 (0.017 *) | |
| DD3-F vs. DD3-M | −0.22 (0.024 *) | −0.17 (0.22) | −0.41 (0.83) | |
| LD-M vs. DD3-M | na | na | −4.07 (0.0038 **) | |
| cry2 | LD-F vs. LD-M | −0.14 (0.048 *) | 0.037 (0.70) | −0.050 (0.95) |
| LD-F vs. DD3-F | na | na | na | |
| DD3-F vs. DD3-M | na | na | na | |
| LD-M vs. DD3-M | na | na | −2.42 (0.021 *) |
| LD-F (Hours) | LD-M (Hours) | DD3-F (Hours) | DD3-M (Hours) | |
|---|---|---|---|---|
| cyc | 2.96 | 6.33 | na | 6.49 |
| clk | 9.87 | 10.52 | 4.29 | 5.40 |
| tim | 15.42 | 15.00 | 19.28 | 19.93 |
| per | 20.19 | 19.74 | 23.40 | 23.81 |
| cry2 | 21.98 | 22.03 | na | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Ren, Y.; Krylov, V.V.; Wang, Y.; Li, Y.; Pan, W.; Hu, G.; Chen, F.; Wan, G. Comparative Analysis of Diel and Circadian Eclosion Rhythms and Clock Gene Expression Between Sexes in the Migratory Moth Spodoptera frugiperda. Insects 2025, 16, 705. https://doi.org/10.3390/insects16070705
Lv C, Ren Y, Krylov VV, Wang Y, Li Y, Pan W, Hu G, Chen F, Wan G. Comparative Analysis of Diel and Circadian Eclosion Rhythms and Clock Gene Expression Between Sexes in the Migratory Moth Spodoptera frugiperda. Insects. 2025; 16(7):705. https://doi.org/10.3390/insects16070705
Chicago/Turabian StyleLv, Changning, Yibo Ren, Viacheslav V. Krylov, Yumeng Wang, Yuanyuan Li, Weidong Pan, Gao Hu, Fajun Chen, and Guijun Wan. 2025. "Comparative Analysis of Diel and Circadian Eclosion Rhythms and Clock Gene Expression Between Sexes in the Migratory Moth Spodoptera frugiperda" Insects 16, no. 7: 705. https://doi.org/10.3390/insects16070705
APA StyleLv, C., Ren, Y., Krylov, V. V., Wang, Y., Li, Y., Pan, W., Hu, G., Chen, F., & Wan, G. (2025). Comparative Analysis of Diel and Circadian Eclosion Rhythms and Clock Gene Expression Between Sexes in the Migratory Moth Spodoptera frugiperda. Insects, 16(7), 705. https://doi.org/10.3390/insects16070705









