Determining the Effectiveness of Saccharomyces cerevisiae as a Postbiotic in Mass-Reared Acheta domesticus (House Cricket)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Set Up and Feed Preparation
2.2. Cricket Set Up
2.3. Total Biomass, Average Cricket Weight, and Survivability
2.4. Nutritional Analyses
2.5. Cricket Dissections and DNA Extraction
2.6. Viral qPCR
2.7. Viral qPCR
2.8. Bioinformatics Analysis Pipeline
2.9. Statistical Analysis
3. Results
3.1. Total Biomass, Average Cricket Weight, and Survivability
3.2. Nutritional Analysis
3.3. Viral qPCR
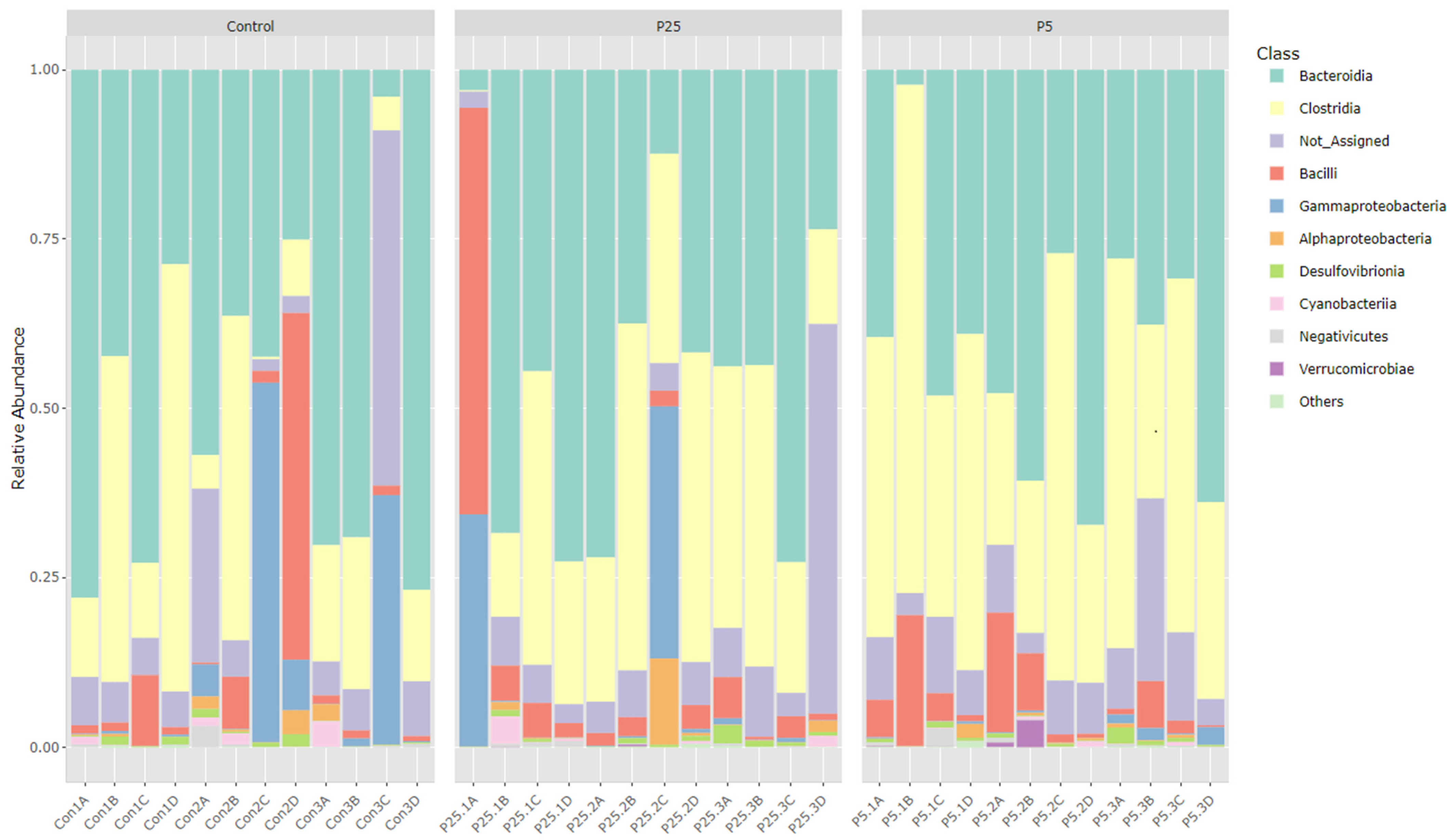
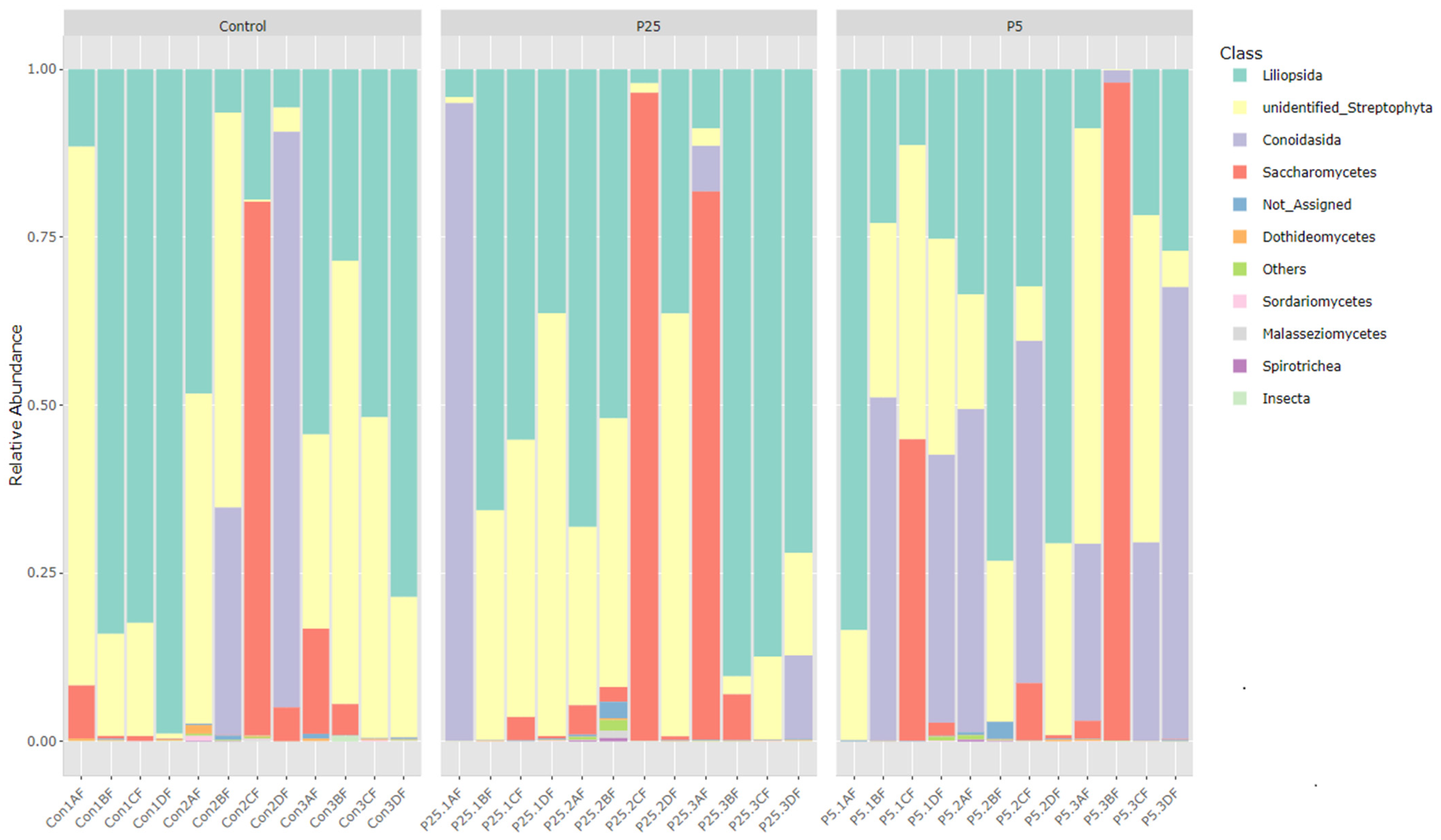
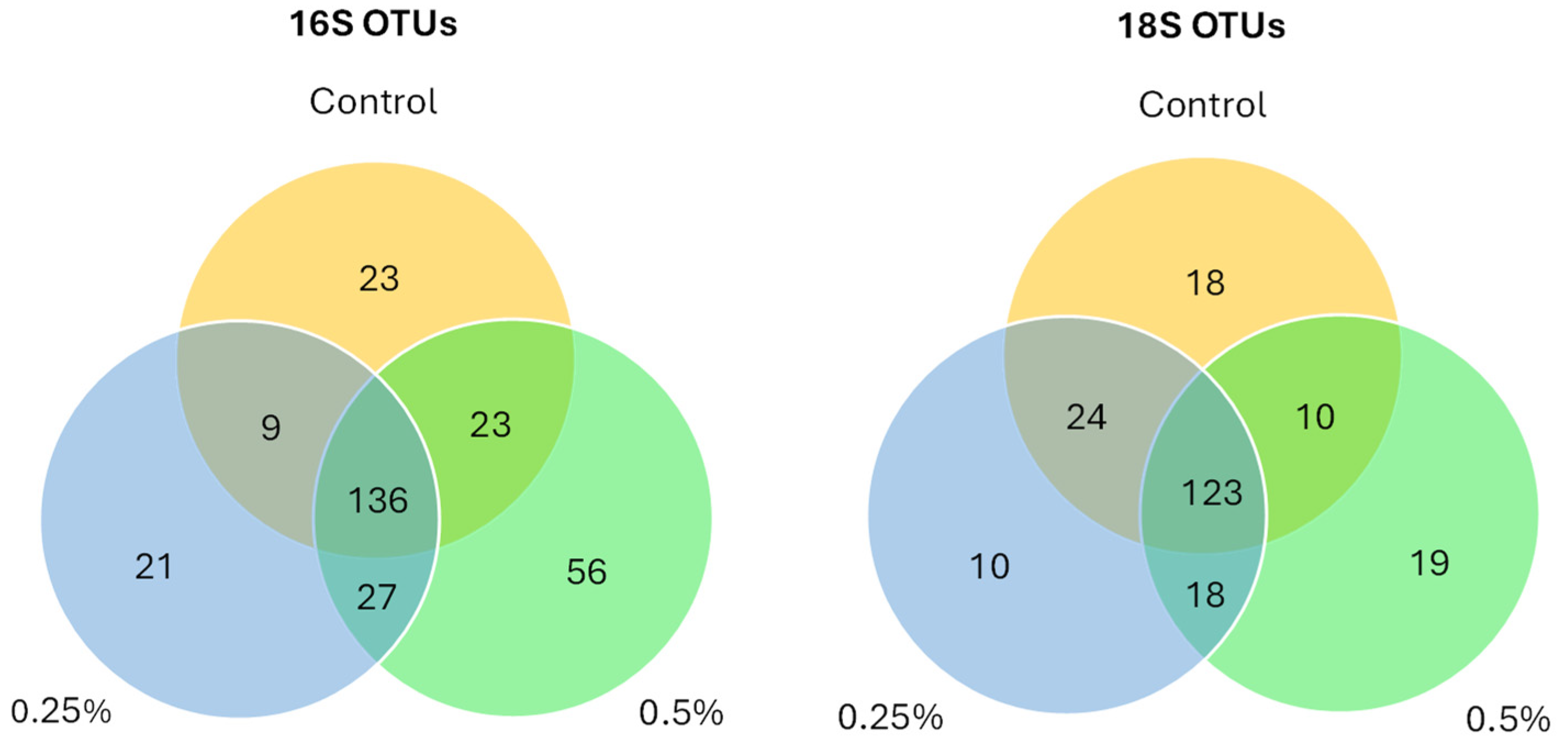
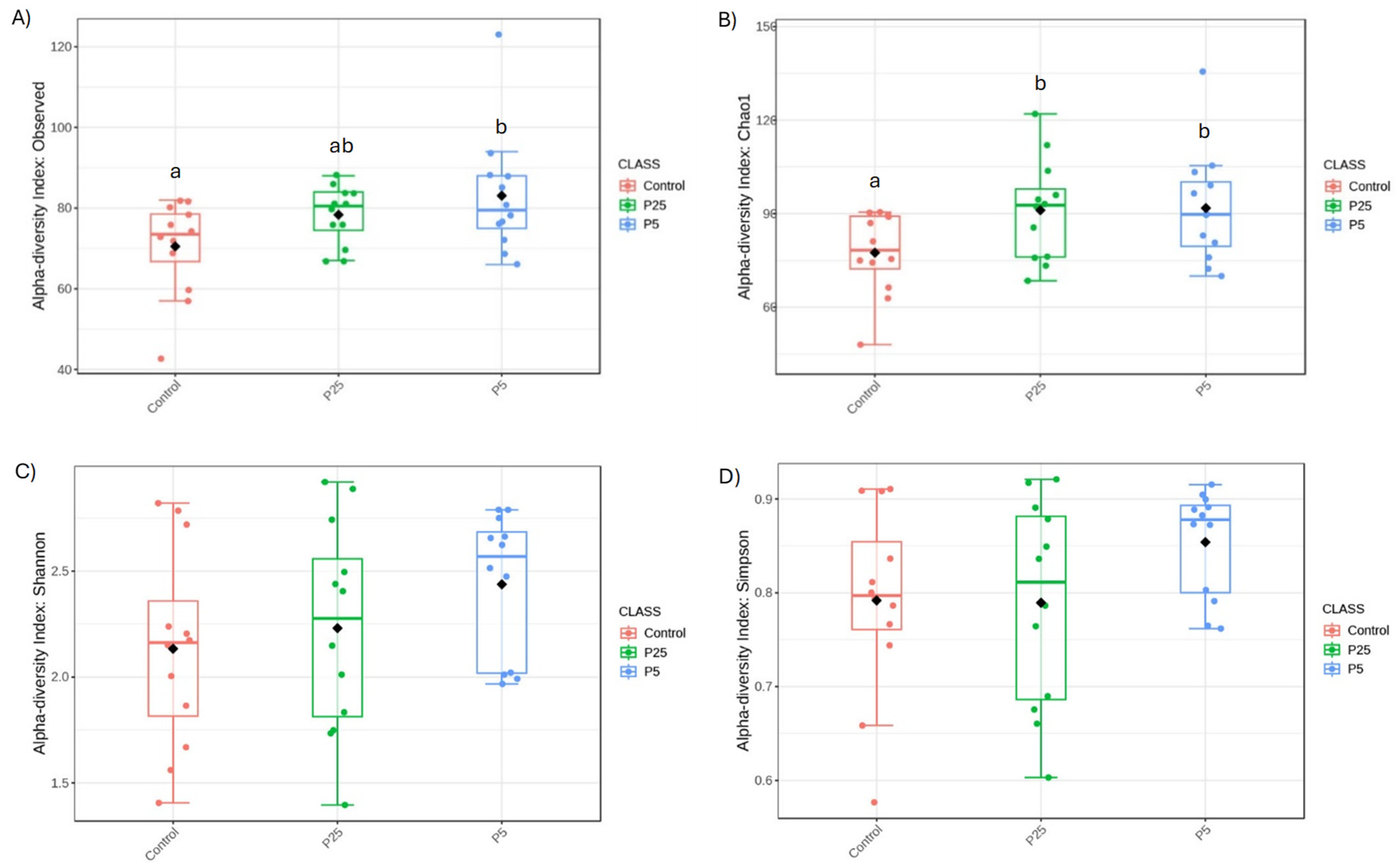
3.4. 16S/18S Microbiome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AdDV | Acheta domesticus densovirus |
| AdVVV | Acheta domesticus volvovirus |
| ANOVA | Analysis of variance |
| BSF | Black soldier fly |
| C | Celsius |
| CI | Confidence interval |
| CrIV | Cricket iridovirus or invertebrate iridovirus type 6 |
| CT | Cycle threshold |
| DM | Dry matter |
| DNA | Deoxyribonucleic acid |
| F | Fahrenheit |
| FDR | False discovery rate |
| GI | Gastrointestinal |
| LEfSe | Linear discriminate analysis effect size |
| LDA | Linear discriminate analysis |
| LSD | Least square differences |
| NFE | Nitrogen-free extract |
| µL | Microliter |
| nM | Nanomolar |
| NMDS | Non-metric multi-dimensional scaling |
| OTU | Operational taxonomic unit |
| PERMANOVA | Permutational multivariate analysis of variance |
| qPCR | Quantitative polymerase chain reaction |
| RH | Relative humidity |
| rRNA | Ribosomal ribonucleic acid |
| SD | Standard deviation |
| TBM | Total biomass |
References
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B. Insects as Food and Feed: Nutrient Composition and Environmental Impact. Master’s Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Maciel-Vegara, G.; Ros, V.I.D. Viruses of insects reared for food and feed. J. Inverteb Pathol. 2017, 147, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Meynadier, G.; Matz, G.; Veyrunes, J.C.; Bres, N. Virose de type densonucleose chez les orthoptères. Annls Soc. Ent. Fr. 1977, 13, 487–493. [Google Scholar] [CrossRef]
- Styer, E.L.; Hamm, J.J. Report of a densovirus in a commercial cricket operation in the southeastern United States. J. Invertebr. Pathol. 1991, 58, 283–285. [Google Scholar] [CrossRef]
- Weissman, D.B.; Gray, D.A.; Pham, H.T.; Tijssen, P. Billions and billions sold: Pet-feeder crickets (Orthoptera: Gryllidae), commercial cricket farms, an epizootic densovirus, and government regulations make for a potential disaster. Zootaxa 2012, 3504, 67–88. [Google Scholar] [CrossRef]
- Boykin, K.L.; Bitter, A.; Lex, Z.N.; Tuminello, J.; Mitchell, M.A. Characterizing the Roles of Life Stage and Season on the Prevalence of Select Viral Pathogens in Acheta domesticus Crickets on a Commercial Cricket Farm in the United States. Vet. Sci. 2025, 12, 191. [Google Scholar] [CrossRef]
- Duffield, K.R.; Hunt, J.; Sadd, B.M.; Sakaluk, S.K.; Oppert, B.; Rosario, K.; Behle, R.W.; Ramirez, J.L. Active and Covert Infections of Cricket Iridovirus and Acheta domesticus Densovirus in Reared Gryllodes sigillatus Crickets. Front. Microbiol. 2021, 12, 780796. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Alberoni, D. Probiotics for Honeybees’ Health. In Probiotics and Prebiotics in Animal Health and Food Safety; Di Gioia, D., Biavati, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 219–245. ISBN 978-3-319-71948-1. [Google Scholar]
- Hamdi, C.; Balloi, A.; Essanaa, J.; Crotti, E.; Gonella, E.; Raddadi, N.; Ricci, I.; Boudabous, A.; Borin, S.; Manino, A.; et al. Gut microbiome dysbiosis and honeybee health: Gut microbiome dysbiosis and honeybee health. J. Appl. Entomol. 2011, 135, 524–533. [Google Scholar] [CrossRef]
- Kyritsis, G.A.; Augustinos, A.A.; Cáceres, C.; Bourtzis, K. Medfly Gut Microbiota and Enhancement of the Sterile Insect Technique: Similarities and Differences of Klebsiella oxytoca and Enterobacter sp. AA26 Probiotics during the Larval and Adult Stages of the VIENNA 8D53+ Genetic Sexing Strain. Front. Microbiol. 2017, 8, 2064. [Google Scholar] [CrossRef]
- Richard, N.; Rabot, R.; Beutin, C.; Loon, J.J.A.V. Live Yeast Probiotic Can Boost Growth Performances of Yellow Meal Worm and Black Soldier Fly Larvae; Wageningen University: Wageningen, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Savio, C.; Mugo-Kamiri, L.; Upfold, J.K. Bugs in Bugs: The Role of Probiotics and Prebiotics in Maintenance of Health in Mass-Reared Insects. Insects 2022, 13, 376. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- AVMA Guidelines for the Euthanasia of Animals: 2020 Edition, Leary, S.L., Ed.; 2020th ed.; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; ISBN 978-1-882691-09-8. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 22nd ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2023. [Google Scholar]
- Papp, T.; Spann, D.; Marschang, R.E. Development and use of a real-time polymerase chain reaction for the detection of group ii invertebrate iridoviruses in pet lizards and prey insects. J. Zoo. Wildl. Med. 2014, 45, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons: Practical guidance for choosing the best multiple comparisons test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef] [PubMed]
- Thiese, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J. Thorac. Dis. 2016, 8, E928–E931. [Google Scholar] [CrossRef]
- White, C.N. The perilous P value. J. Am. Vet. Med. Assoc. 2025, 263, 267–270. [Google Scholar] [CrossRef]
- Yang, P.; Wang, F.; Gan, J.; He, Z.; Sun, L.; Feng, Y.; Wang, C.; Zhao, M. Effects of Saccharomyces cerevisiae on Growth Performance and Intestinal Bacterium of Gryllus bimaculatus. J. Yunnan Agric. Univ. 2023, 38, 1–11. [Google Scholar] [CrossRef]
- Vaga, M.; Berggren, Å.; Jansson, A. Growth, survival and development of house crickets (Acheta domesticus) fed flowering plants. JIFF 2021, 7, 151–161. [Google Scholar] [CrossRef]
- Nowosielski, J.W.; Patton, R.L. Life-tables for the house cricket, Acheta domesticus L., and the effect of intra-specific factors on longevity. J. Insect Physiol. 1965, 11, 201–209. [Google Scholar] [CrossRef]
- Tennis, P.S.; Koonce, J.F.; Teraguchi, M. The effects of population density and food surface area on body weight of Acheta domesticus (L.) (Orthoptera: Gryllidae). Can. J. Zool. 1977, 55, 2004–2010. [Google Scholar] [CrossRef]
- Van Peer, M.; Berrens, S.; Coudron, C.; Noyens, I.; Verheyen, G.R.; Van Miert, S. Towards good practices for research on Acheta domesticus, the house cricket. J. Insects Food Feed. 2024, 10, 1235–1251. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of Diet on the Growth Performance, Feed Conversion, and Nutrient Content of the House Cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Mahavidanage, S.; Fuciarelli, T.M.; Li, X.; Rollo, C.D. The effects of rearing density on growth, survival, and starvation resistance of the house cricket Acheta domesticus. JOR 2023, 32, 25–31. [Google Scholar] [CrossRef]
- Bertola, M.; Mutinelli, F. A Systematic Review on Viruses in Mass-Reared Edible Insect Species. Viruses 2021, 13, 2280. [Google Scholar] [CrossRef]
- Kleespies, R.G.; Tidona, C.A.; Darai, G. Characterization of a New Iridovirus Isolated from Crickets and Investigations on the Host Range. J. Invertebr. Pathol. 1999, 73, 84–90. [Google Scholar] [CrossRef]
- Pham, H.T.; Bergoin, M.; Tijssen, P. Acheta domesticus Volvovirus, a Novel Single-Stranded Circular DNA Virus of the House Cricket. Genome Announc. 2013, 1, e00079-13. [Google Scholar] [CrossRef]
- Orinda, M.A.; Mosi, R.O.; Ayieko, M.A.; Amimo, A. Growth performance of Common house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus) crickets fed on agro-byproducts. J. Entomol. Zool. Stud. 2017, 5, 1664–1668. [Google Scholar]
- El Deen, S.N.; Lamaj, F.; Verrastro, V.; Al Bitar, L.; Baldacchino, F. Effects of two diets on adults’ survival and productivity in mass-rearing ofTenebrio molitor (Coleoptera: Tenebrionidae). JIFF 2021, 7, 1149–1158. [Google Scholar] [CrossRef]
- Esaivani, C.; Vasanthi, K.; Bharathi, R.; Chairman, K. Impact of probiotic saccharomyces cerevisiae on the enzymatic profile and the economic parameters of silkworm bombyx mori L. Adv. Biol. 2014, 1, 1–8. [Google Scholar]
- Kannan, M.; Vitenberg, T.; Ben-Mordechai, L.; Khatib, S.; Opatovsky, I. Effect of yeast supplementation on growth parameters and metabolomics of black soldier fly larvae, Hermetia illucens (L.) (Diptera: Stratiomyidae). JIFF 2023, 9, 1353–1364. [Google Scholar] [CrossRef]
- Kim, S.; Park, I.; Park, H.; Lee, H.S.; Song, J.-H. Growth performance of the edible mealworm species, Tenebrio molitor (Coleoptera: Tenebrionidae) on diets composed of brewer’s yeast. Int. J. Ind. Entomol. 2019, 39, 54–59. [Google Scholar] [CrossRef]
- Opatovsky, I.; Vitenberg, T.; Jonas-Levi, A.; Gutman, R. Does Consumption of Baker’s Yeast ( Saccharomyces cerevisiae ) by Black Soldier Fly (Diptera: Stratiomyidae) Larvae Affect Their Fatty Acid Composition? J. Insect Sci. 2021, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Riaz, K.; Iqbal, T.; Khan, S.; Usman, A.; Al-Ghamdi, M.S.; Shami, A.; El Hadi Mohamed, R.A.; Almadiy, A.A.; Al Galil, F.M.A.; Alfuhaid, N.A.; et al. Growth Optimization and Rearing of Mealworm (Tenebrio molitor L.) as a Sustainable Food Source. Foods 2023, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Spitaler, U.; Bianchi, F.; Eisenstecken, D.; Castellan, I.; Angeli, S.; Dordevic, N.; Robatscher, P.; Vogel, R.F.; Koschier, E.H.; Schmidt, S. Yeast species affects feeding and fitness of Drosophila suzukii adults. J. Pest. Sci. 2020, 93, 1295–1309. [Google Scholar] [CrossRef]
- Taha, R.H.; Kamel, H.M. Micro-Organisms Supplementation to Mulberry Silkworm, Bombyx mori L. Egypt. Acad. J. Biol. Sci. A Entomol. 2017, 10, 57–64. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; Van Huis, A.; Van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Duysburgh, C.; Miclotte, L.; Green, J.B.; Watts, K.T.; Sardi, M.I.; Chakrabarti, A.; Khafipour, E.; Marzorati, M. Saccharomyces cerevisiae derived postbiotic alters gut microbiome metabolism in the human distal colon resulting in immunomodulatory potential in vitro. Front. Microbiol. 2024, 15, 1358456. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Granberg, F.; Low, M.; Onorati, P.; Semberg, E.; Jansson, A.; Berggren, Å. Virus Diversity and Loads in Crickets Reared for Feed: Implications for Husbandry. Front. Vet. Sci. 2021, 8, 642085. [Google Scholar] [CrossRef]
- Boykin, K.L. Characterizing the roles of bacterial and viral agents in house crickets (Acheta domesticus) at commercial rearing facilities in the United States. Dissertation, Louisiana State University, Baton Rouge, LA, USA, 2024. [Google Scholar]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.J.; Van Der Velden, S.; Ríos-Morales, M.; Van Faassen, M.J.R.; Loreti, M.G.; De Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Feng, P.; Li, Q.; Sun, H.; Gao, J.; Ye, X.; Tao, Y.; Tian, Y.; Wang, P. Effects of fulvic acid on growth performance, serum index, gut microbiota, and metabolites of Xianju yellow chicken. Front. Nutr. 2022, 9, 963271. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Maiques, E.; Angelova, A.; Carrasco, P.; Moya, A.; Latorre, A. Diet shapes the gut microbiota of the omnivorous cockroach Blattella germanica. FEMS Microbiol. Ecol. 2015, 91, fiv022. [Google Scholar] [CrossRef]
- Crawley, H. The polycistid gregarines of the United States: Third contribution. Proc. Natl. Acad. Sci. USA 1907, 59, 220–228. [Google Scholar]
- Hoshide, K. Notes on the gregarines in Japan 6: Two cephaline gregarines from Gryllus yemma Ohamchi et Matsuura. Bull. Fac. Educ. Yamaguchi Univ. 1973, 23, 77–85. [Google Scholar]
- Rueckert, S.; Betts, E.L.; Tsaousis, A.D. The Symbiotic Spectrum: Where Do the Gregarines Fit? Trends Parasitol. 2019, 35, 687–694. [Google Scholar] [CrossRef]
- Bessette, E.; Williams, B. Protists in the Insect Rearing Industry: Benign Passengers or Potential Risk? Insects 2022, 13, 482. [Google Scholar] [CrossRef]
- Sumner, R. Influence of gregarines on the growtwh in the mealworm. Science 1933, 78, 125. [Google Scholar] [CrossRef]
- Valigurová, A. Sophisticated Adaptations of Gregarina cuneata (Apicomplexa) Feeding Stages for Epicellular Parasitism. PLoS ONE 2012, 7, e42606. [Google Scholar] [CrossRef]
- Lipa, J.J. Studies on gregarines (Gregarinomorpha) of arthropods in Poland. Acta Protozool. 1967, 5, 97–179. [Google Scholar]
- Rodriguez, Y.; Omoto, C.K.; Gomulkiewicz, R. Individual and Population Effects of Eugregarine, Gregarina niphandrodes (Eugregarinida: Gregarinidae), on Tenebrio molitor (Coleoptera: Tenebrionidae). Environ. Entomol. 2007, 36, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Slowik, A.R.; Herren, P.; Bessette, E.; Lim, F.S.; Hernández-Pelegrín, L.; Savio, C. Harmful and beneficial symbionts of Tenebrio molitor and their implications for disease management. JIFF 2023, 9, 1381–1395. [Google Scholar] [CrossRef]
- Zuk, M. The effects of gregarine parasites on longevity, weight loss, fecundity and developmental time in the field crickets Gryllus veletis and G.pennsylvanicus. Ecol. Entomol. 1987, 12, 349–354. [Google Scholar] [CrossRef]
- Gielen, R.; Põldmaa, K.; Tammaru, T. In search of ecological determinants of fungal infections: A semi-field experiment with folivorous moths. Ecol. Evol. 2022, 12, e8926. [Google Scholar] [CrossRef]
- Seye, F.; Faye, O.; Ndiaye, M.; Njie, E.; Marie Afoutou, J. Pathogenicity of the Fungus, Aspergillus clavatus, Isolated from the Locust, Oedaleus senegalensis, Against Larvae of the Mosquitoes Aedes aegypti, Anopheles gambiae and Culex quinquefasciatus. J. Insect Sci. 2009, 9, 1–7. [Google Scholar] [CrossRef][Green Version]
- Becchimanzi, A.; Nicoletti, R. Aspergillus-bees: A dynamic symbiotic association. Front. Microbiol. 2022, 13, 968963. [Google Scholar] [CrossRef]
- Moubasher, A. Yeasts and filamentous fungi inhabiting guts of three insect species in Assiut, Egypt. Mycosphere 2017, 8, 1297–1316. [Google Scholar] [CrossRef]
- Xu, X.; Shao, M.; Yin, C.; Mao, Z.; Shi, J.; Yu, X.; Wang, Y.; Sun, F.; Zhang, Y. Diversity, Bacterial Symbionts, and Antimicrobial Potential of Termite-Associated Fungi. Front. Microbiol. 2020, 11, 300. [Google Scholar] [CrossRef]
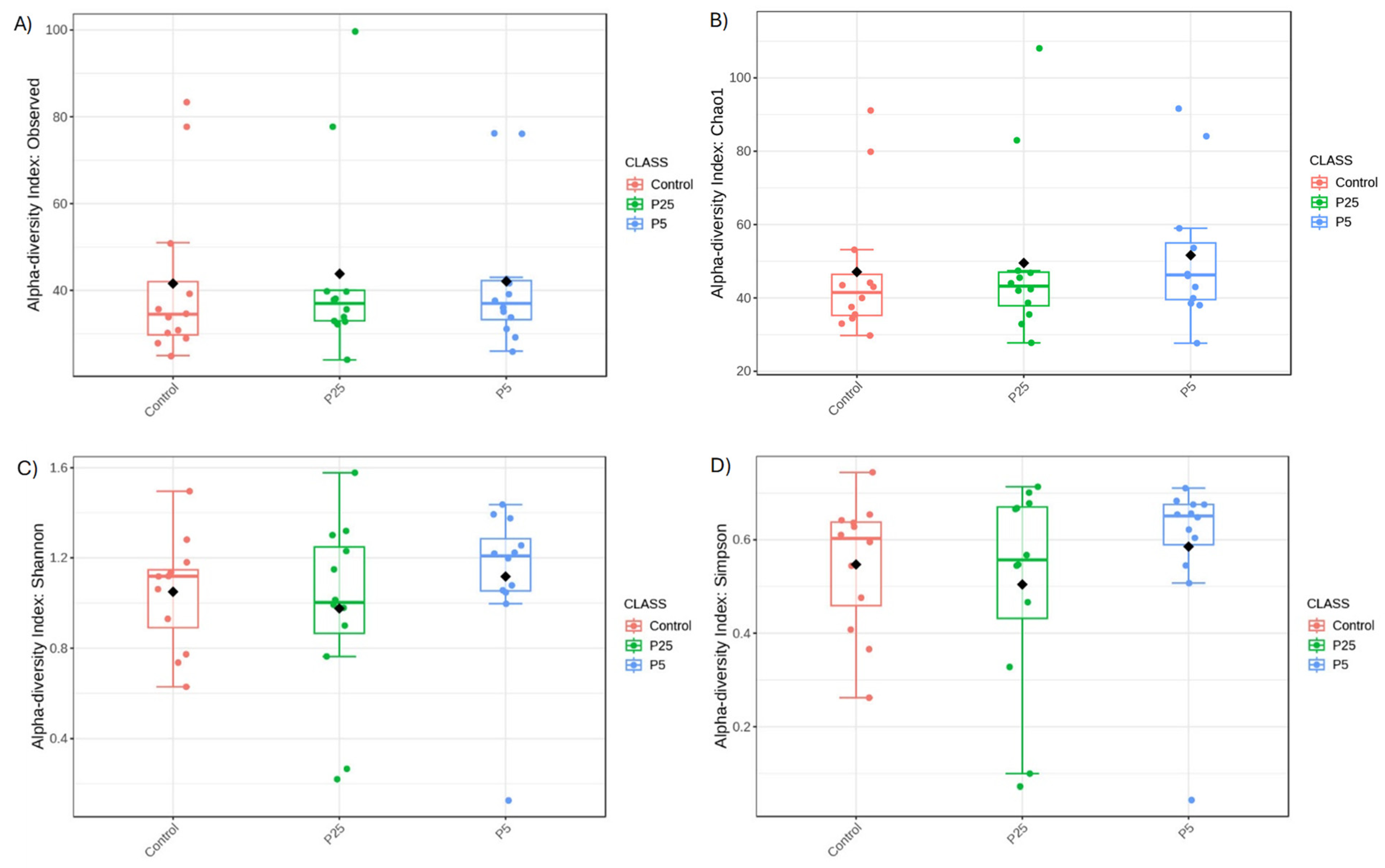
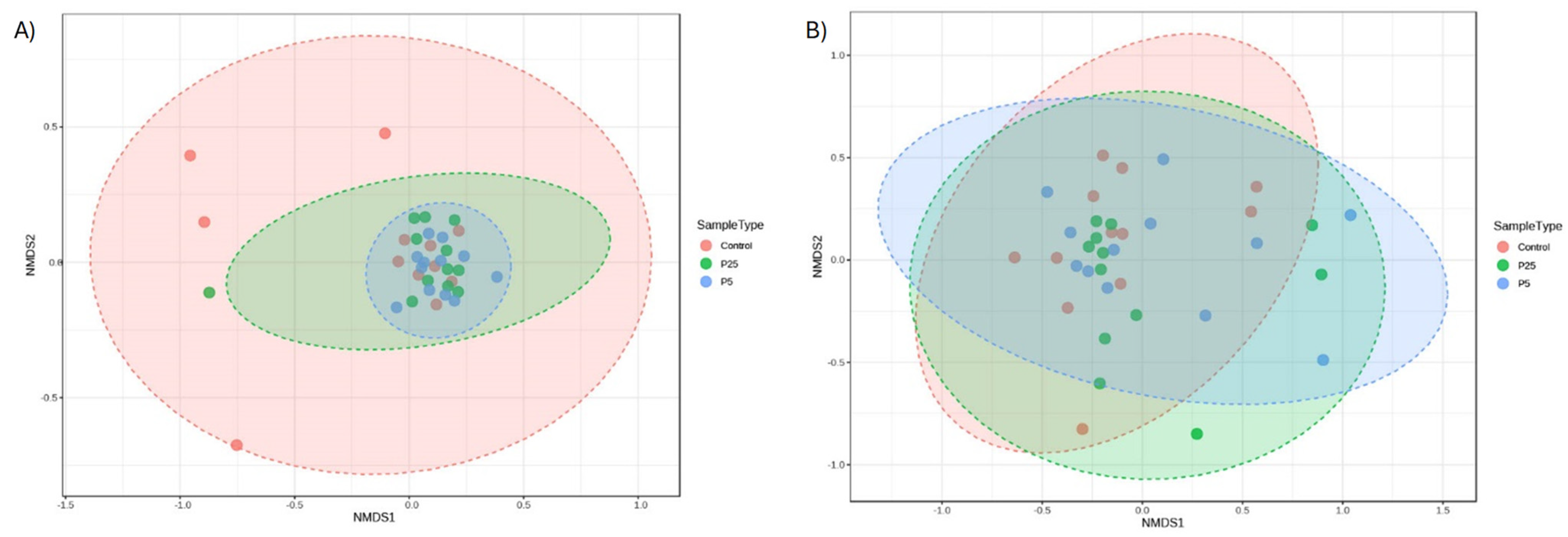
| Treatment | Total Biomass (g) 2 | Average Body Weight per Cricket (mg) | % Survival 2 |
|---|---|---|---|
| Control (n = 5) | 982 ± 165 a | 310 ± 27 | 25.33 ± 3.39 a |
| 0.25% (n = 5) | 1005 ± 192 a | 319 ± 43 | 25.23 ± 3.75 a |
| 0.5% (n = 5) | 1278 ± 206 b | 336 ± 43 | 30.91 ± 4.08 b |
| Substrate Analyzed | Dry Matter (%) | Crude Protein (% DM) | Fiber (% DM) | NFE 1 (% DM) | Crude Fat 2 (% DM) | Ash 3 (% DM) |
|---|---|---|---|---|---|---|
| Feed | ||||||
| Control diet (n = 2) | 89.1 ± 0.1 | 21.5 ± 0.4 | 6.9 ± 0.1 | 64.6 ± 0.4 | 5.6 ± 0.1 | 7.1 ± 0.1 |
| 0.25% diet (n = 2) | 89.0 ± 0.1 | 21.5 ± 0.9 | 6.7 ± 0.5 | 65.0 ± 1.1 | 5.5 ± 0.4 | 6.9 ± 0.2 |
| 0.5% diet (n = 2) | 89.3 ± 0.3 | 21.6 ± 0.0 | 6.7 ± 0.0 | 64.8 ± 0.2 | 5.6 ± 0.1 | 7.0 ± 0.1 |
| Crickets | ||||||
| Control (n = 6) | 27.1 ± 0.4 | 65.5 ± 0.4 | 6.1 ± 0.2 | N/A | 22.2 (21.8–23.0) | 6.4 ± 1.0 a |
| 0.25% treatment (n = 6) | 27.4 ± 0.6 | 66.6 ± 1.0 | 6.6 ± 0.6 | N/A | 22.4 (19.9–22.7) | 7.4 ± 0.4 b |
| 0.5% treatment (n = 6) | 27.8 ± 0.5 | 65.7 ± 2.1 | 6.0 ± 0.6 | N/A | 22.3 (17.6–23.3) | 6.8 ± 0.3 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boykin, K.L.; Neff, E.; Mitchell, M.A. Determining the Effectiveness of Saccharomyces cerevisiae as a Postbiotic in Mass-Reared Acheta domesticus (House Cricket). Insects 2025, 16, 702. https://doi.org/10.3390/insects16070702
Boykin KL, Neff E, Mitchell MA. Determining the Effectiveness of Saccharomyces cerevisiae as a Postbiotic in Mass-Reared Acheta domesticus (House Cricket). Insects. 2025; 16(7):702. https://doi.org/10.3390/insects16070702
Chicago/Turabian StyleBoykin, Kimberly L., Erik Neff, and Mark A. Mitchell. 2025. "Determining the Effectiveness of Saccharomyces cerevisiae as a Postbiotic in Mass-Reared Acheta domesticus (House Cricket)" Insects 16, no. 7: 702. https://doi.org/10.3390/insects16070702
APA StyleBoykin, K. L., Neff, E., & Mitchell, M. A. (2025). Determining the Effectiveness of Saccharomyces cerevisiae as a Postbiotic in Mass-Reared Acheta domesticus (House Cricket). Insects, 16(7), 702. https://doi.org/10.3390/insects16070702






