Industrial-Scale Bioconversion of Three-Phase Residue by Musca domestica Larvae: Dynamics of Gut Microbiota and Their Ecological Driver
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
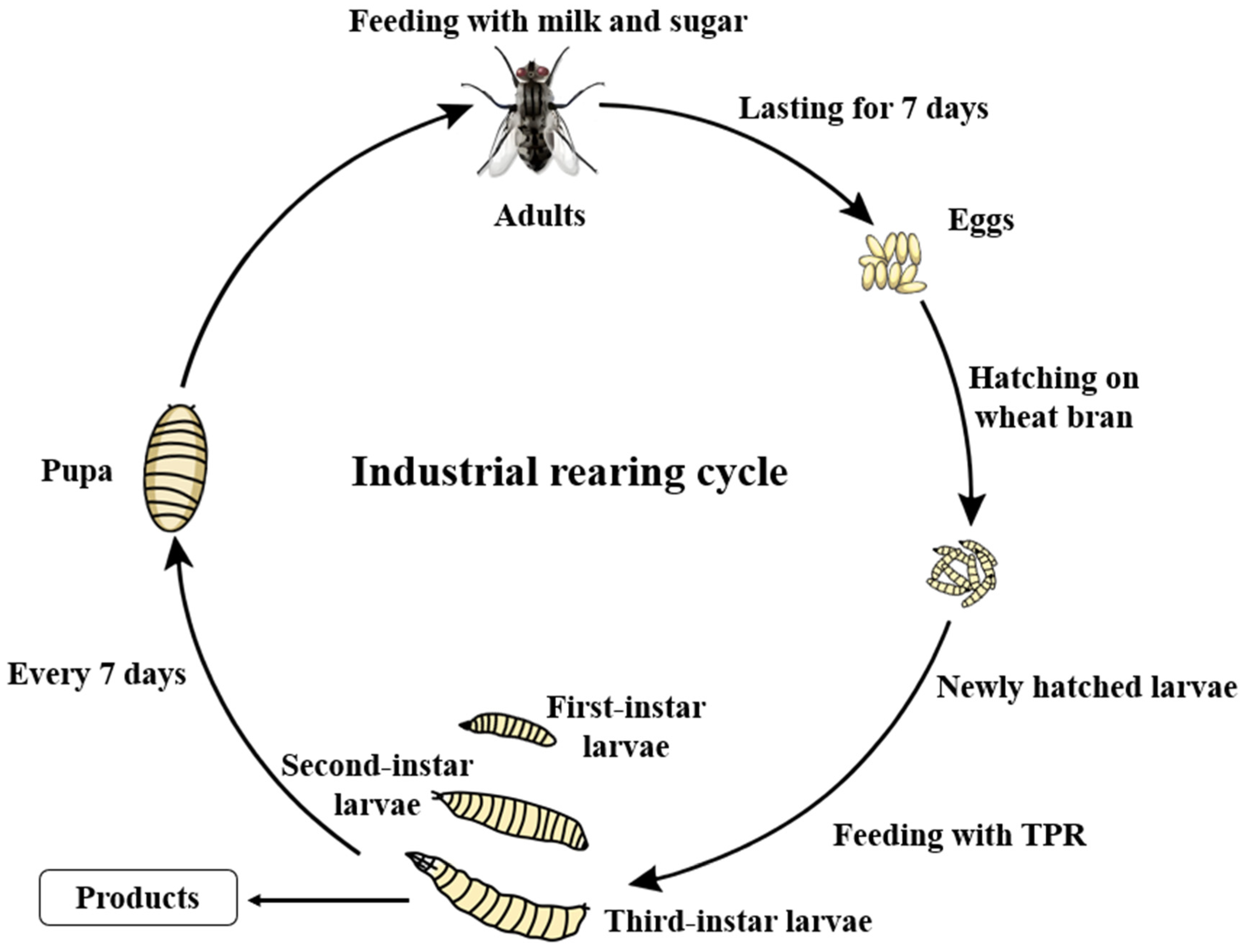
2.1. Industrial-Scale Production of M. domestica Larvae
2.2. TPR
2.3. Sampling
2.4. Processing of Larval Samples
2.5. DNA Extraction and Analysis
2.6. Determination of Physical and Chemical Parameters
2.7. Statistical Analysis
3. Results
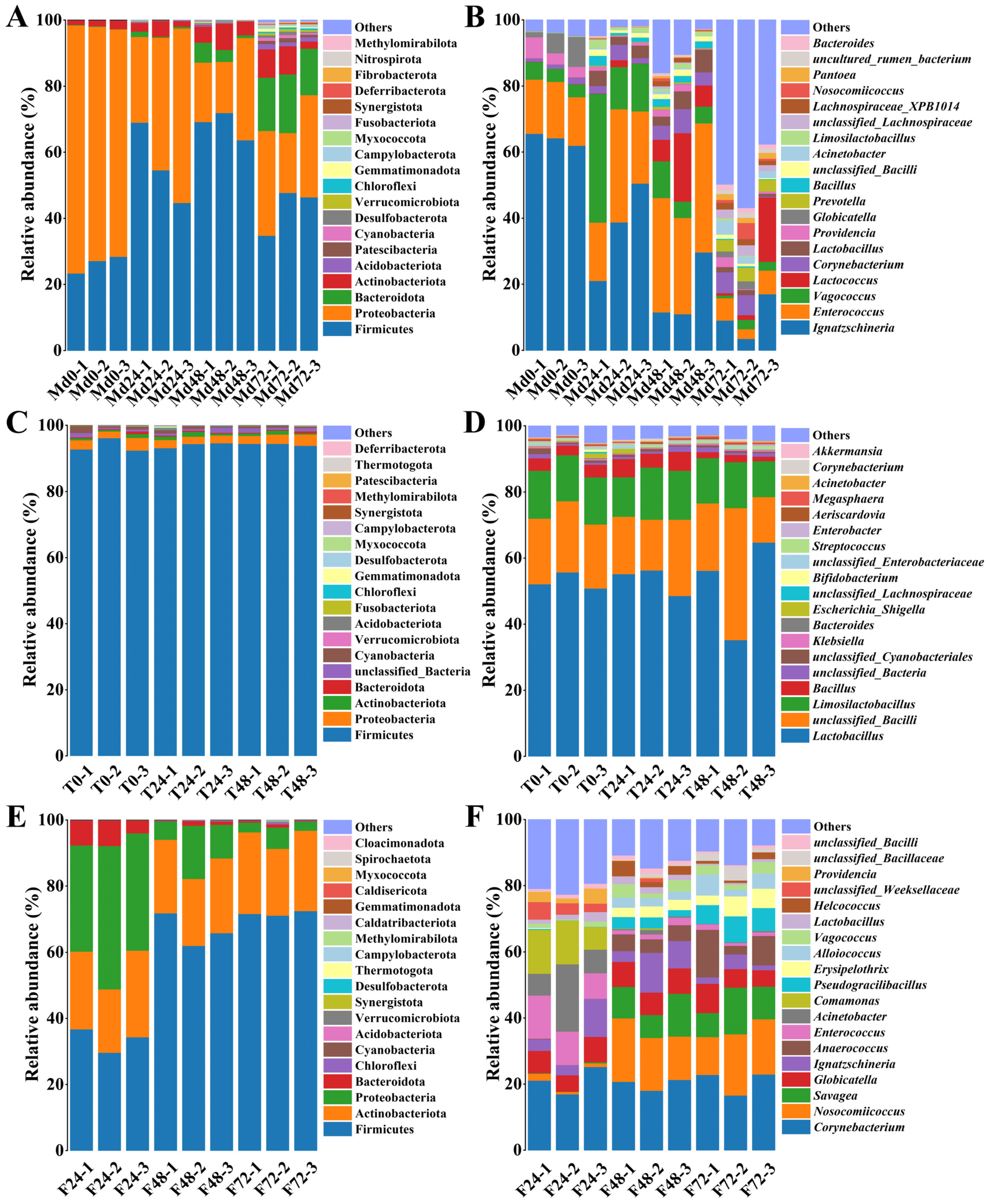
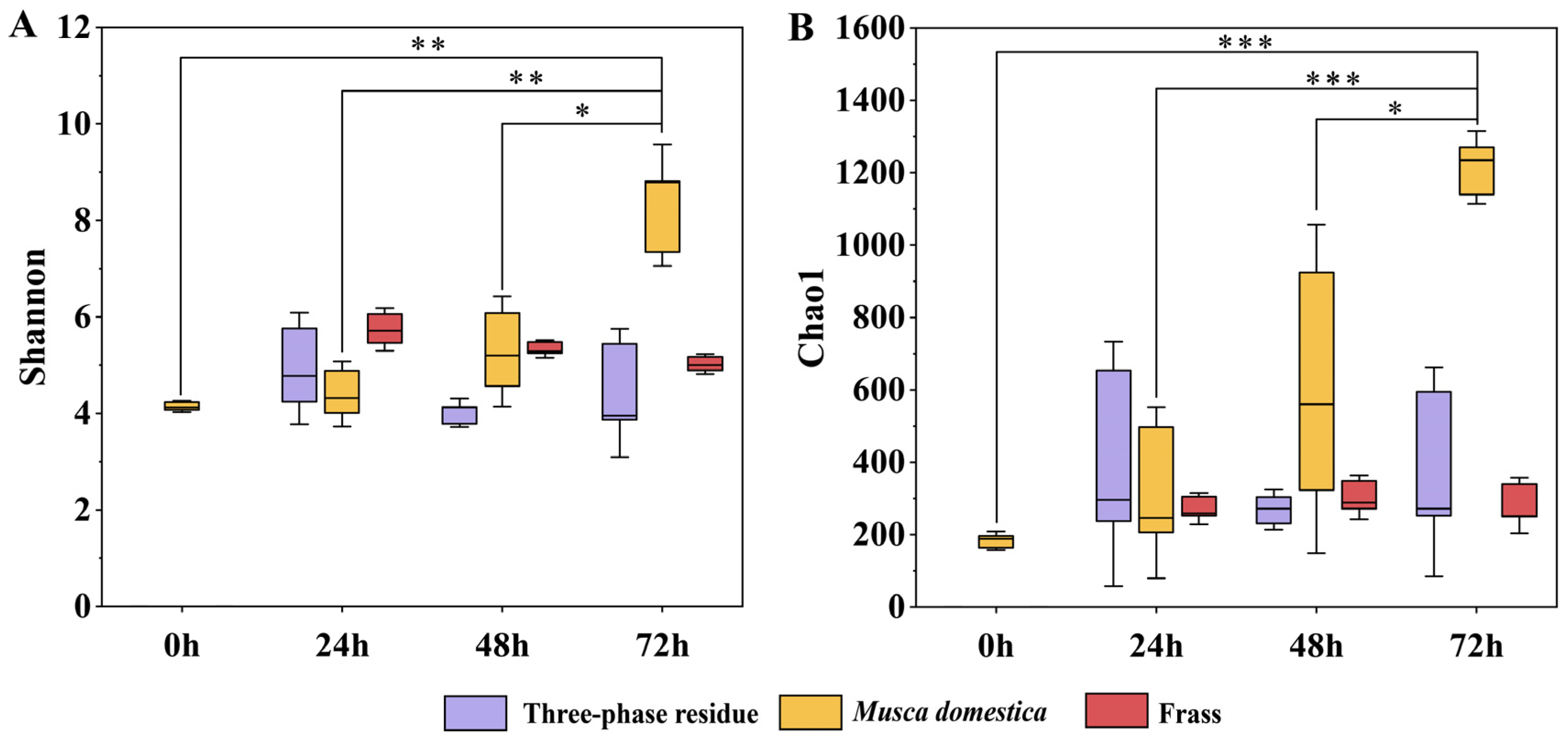
3.1. Diversity of Bacterial Communities
3.2. Taxonomic Composition of Bacterial Communities
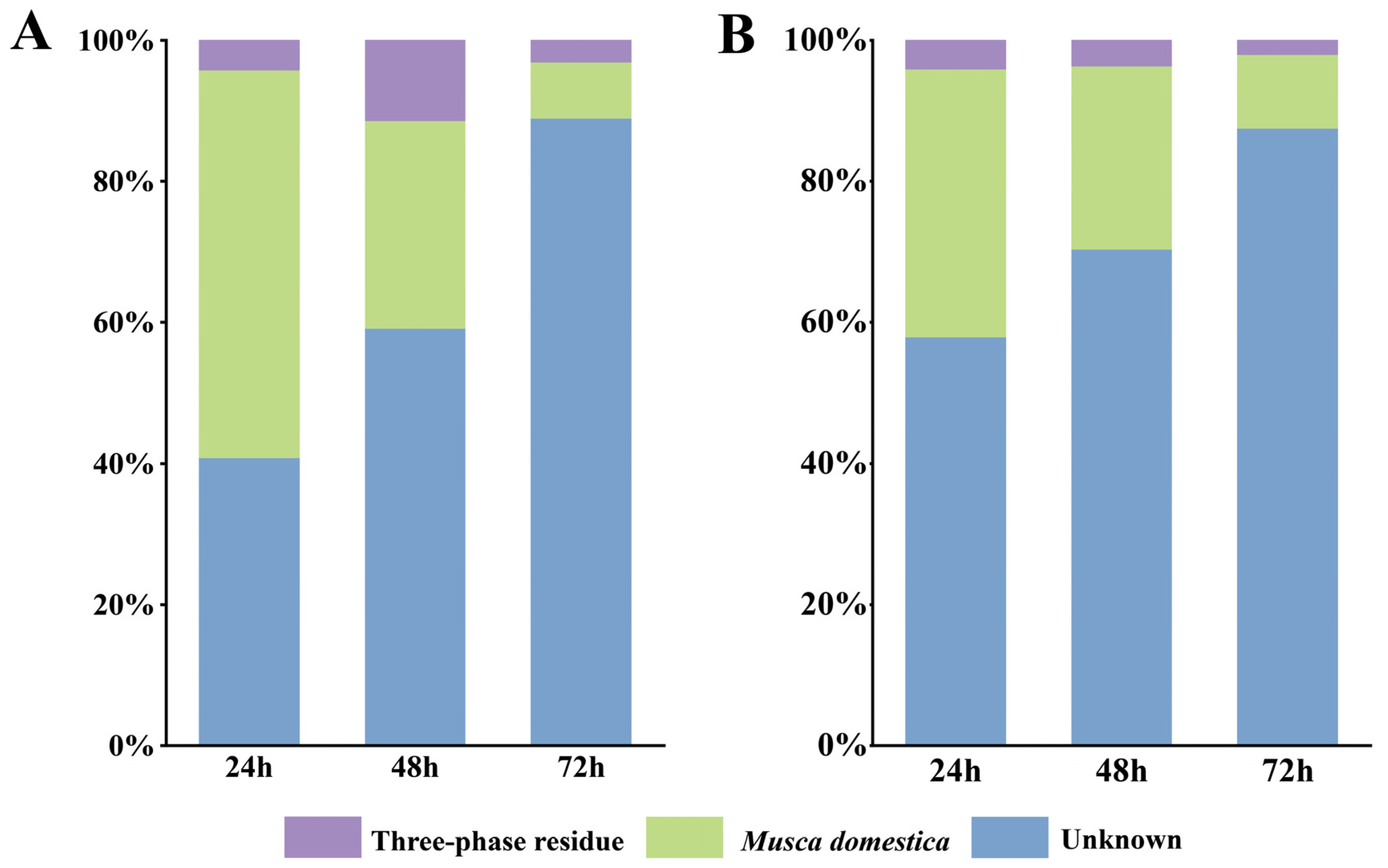
3.3. Horizontal Transfer of Bacteria
3.4. Physicochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. BioMed. Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, Inefficiencies and Waste in the Global Food System. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The Use of Fly Larvae for Organic Waste Treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental Impact of Food Waste Bioconversion by Insects: Application of Life Cycle Assessment to Process Using Hermetia Illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Van Huis, A.; Dicke, M.; Van Loon, J.J.A. Insects to Feed the World. JIFF 2015, 1, 3–5. [Google Scholar] [CrossRef]
- Sanchez Matos, J.; Barberino, A.T.M.S.; De Araujo, L.P.; Lôbo, I.P.; De Almeida Neto, J.A. Potentials and Limitations of the Bioconversion of Animal Manure Using Fly Larvae. Waste Biomass Valor. 2021, 12, 3497–3520. [Google Scholar] [CrossRef]
- Cui, G.; Lü, F.; Lu, T.; Zhang, H.; He, P. Feasibility of Housefly Larvae-Mediated Vermicomposting for Recycling Food Waste Added Digestate as Additive. J. Environ. Sci. 2023, 128, 150–160. [Google Scholar] [CrossRef]
- Niu, Y.; Zheng, D.; Yao, B.; Cai, Z.; Zhao, Z.; Wu, S.; Cong, P.; Yang, D. A Novel Bioconversion for Value-Added Products from Food Waste Using Musca Domestica. Waste Manag. 2017, 61, 455–460. [Google Scholar] [CrossRef]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of Fruit Byproducts through Bioconversion by Hermetia Illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 991–1010. [Google Scholar] [CrossRef]
- Lim, J.-W.; Mohd-Noor, S.-N.; Wong, C.-Y.; Lam, M.-K.; Goh, P.-S.; Beniers, J.J.A.; Oh, W.-D.; Jumbri, K.; Ghani, N.A. Palatability of Black Soldier Fly Larvae in Valorizing Mixed Waste Coconut Endosperm and Soybean Curd Residue into Larval Lipid and Protein Sources. J. Environ. Manag. 2019, 231, 129–136. [Google Scholar] [CrossRef]
- Li, X.; Rahimnejad, S.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Substituting Fish Meal with Housefly (Musca Domestica) Maggot Meal in Diets for Bullfrog Rana (Lithobates) Catesbeiana: Effects on Growth, Digestive Enzymes Activity, Antioxidant Capacity and Gut Health. Aquaculture 2019, 499, 295–305. [Google Scholar] [CrossRef]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable Use of Hermetia Illucens Insect Biomass for Feed and Food: Attributional and Consequential Life Cycle Assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Xiang, F.; Sheng, J.; Li, G.; Ma, J.; Wang, X.; Jiang, C.; Zhang, Z. Black Soldier Fly Larvae Vermicompost Alters Soil Biochemistry and Bacterial Community Composition. Appl. Microbiol. Biotechnol. 2022, 106, 4315–4328. [Google Scholar] [CrossRef]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass Derived from Black Soldier Fly Larvae Treatment of Biodegradable Wastes. A Critical Review and Future Perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects—Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- De Smet, J.; Wynants, E.; Cos, P.; Van Campenhout, L. Microbial Community Dynamics during Rearing of Black Soldier Fly Larvae (Hermetia Illucens) and Impact on Exploitation Potential. Appl. Env. Microbiol. 2018, 84, e02722-17. [Google Scholar] [CrossRef]
- Zhang, W.; Stelinski, L.L.; Mohamed, A.; Wang, G.; Tettamanti, G.; Chen, M.; Hong, M.; Daly, E.Z.; Bruin, J.; Renault, D.; et al. Unlocking agro-ecosystem sustainability: Exploring the bottom-up effects of microbes, plants, and insect herbivores. Integr. Zool. 2024, 20, 465–484. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Shang, R.; Wang, G.; Zhang, J. Improvement in Bioconversion Efficiency and Reduction of Ammonia Emission by Introduction of Fruit Fermentation Broth in a Black Soldier Fly Larvae and Kitchen Waste Conversion System. Insect Sci. 2023, 30, 975–990. [Google Scholar] [CrossRef]
- Li, X.; Mei, C.; Luo, X.; Wulamu, D.; Zhan, S.; Huang, Y.; Yang, H. Dynamics of the Intestinal Bacterial Community in Black Soldier Fly Larval Guts and Its Influence on Insect Growth and Development. Insect Sci. 2023, 30, 947–963. [Google Scholar] [CrossRef]
- Mazza, L.; Xiao, X.; Ur Rehman, K.; Cai, M.; Zhang, D.; Fasulo, S.; Tomberlin, J.K.; Zheng, L.; Soomro, A.A.; Yu, Z.; et al. Management of Chicken Manure Using Black Soldier Fly (Diptera: Stratiomyidae) Larvae Assisted by Companion Bacteria. Waste Manag. 2020, 102, 312–318. [Google Scholar] [CrossRef]
- Pei, Y.; Zhao, S.; Chen, X.; Zhang, J.; Ni, H.; Sun, M.; Lin, H.; Liu, X.; Chen, H.; Yang, S. Bacillus Velezensis EEAM 10B Strengthens Nutrient Metabolic Process in Black Soldier Fly Larvae (Hermetia Illucens) via Changing Gut Microbiome and Metabolic Pathways. Front. Nutr. 2022, 9, 880488. [Google Scholar] [CrossRef]
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Yu, J.; Van Huis, A.; Yu, Z.; Fasulo, S.; et al. Efficient Co-Conversion Process of Chicken Manure into Protein Feed and Organic Fertilizer by Hermetia Illucens L. (Diptera: Stratiomyidae) Larvae and Functional Bacteria. J. Environ. Manag. 2018, 217, 668–676. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Yao, D.; Zhang, X.; Zhang, Q.; Liu, W.; Li, Y.; Yin, Y.; An, S.; Zhang, R.; et al. Aerobic and Facultative Anaerobic Klebsiella Pneumoniae Strains Establish Mutual Competition and Jointly Promote Musca Domestica Development. Front. Immunol. 2023, 14, 1102065. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhang, X.; Zhang, K.; Liu, W.; Zhang, R.; Zhang, Z. Enterobacter Hormaechei in the Intestines of Housefly Larvae Promotes Host Growth by Inhibiting Harmful Intestinal Bacteria. Parasites Vectors 2021, 14, 598. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhang, X.; Zhang, K.; Li, Y.; Yin, Y.; Zhang, R.; Zhang, Z. Beneficial Bacteria in the Intestines of Housefly Larvae Promote Larval Development and Humoral Phenoloxidase Activity, While Harmful Bacteria Do the Opposite. Front. Immunol. 2022, 13, 938972. [Google Scholar] [CrossRef]
- Kooienga, E.M.; Baugher, C.; Currin, M.; Tomberlin, J.K.; Jordan, H.R. Effects of Bacterial Supplementation on Black Soldier Fly Growth and Development at Benchtop and Industrial Scale. Front. Microbiol. 2020, 11, 587979. [Google Scholar] [CrossRef]
- Somroo, A.A.; Ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus Buchneri on Soybean Curd Residue Co-Conversion by Black Soldier Fly Larvae (Hermetia Illucens) for Food and Feedstock Production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef]
- Yu, G.; Cheng, P.; Chen, Y.; Li, Y.; Yang, Z.; Chen, Y.; Tomberlin, J.K. Inoculating Poultry Manure With Companion Bacteria Influences Growth and Development of Black Soldier Fly (Diptera: Stratiomyidae) Larvae. Env. Entomol. 2011, 40, 30–35. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, T.; Chen, X.; Yu, Z. Life-Cycle Assessment of Energy Consumption and Environmental Impact of an Integrated Food Waste-Based Biogas Plant. Appl. Energy 2015, 151, 227–236. [Google Scholar] [CrossRef]
- Wu, M.; Hu, J.; Shen, F.; Huang, M.; Zhao, L.; Tian, D.; Zhang, Y.; Liu, Y.; Zeng, Y.; Deng, S. Conceptually Integrating a Multi-Product Strategy for the Valorization of Kitchen Waste towards a More Sustainable Management. J. Clean. Prod. 2021, 306, 127292. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, J.; Zhang, R.; Huang, Z.; Wan, Q.; Zhang, Z. Comparative Analysis of Gut Bacterial Communities in Housefly Larvae Fed Different Diets Using a High-Throughput Sequencing Approach. FEMS Microbiol. Lett. 2019, 366, fnz126. [Google Scholar] [CrossRef]
- Gold, M.; Von Allmen, F.; Zurbrügg, C.; Zhang, J.; Mathys, A. Identification of Bacteria in Two Food Waste Black Soldier Fly Larvae Rearing Residues. Front. Microbiol. 2020, 11, 582867. [Google Scholar] [CrossRef]
- Querejeta, M.; Hervé, V.; Perdereau, E.; Marchal, L.; Herniou, E.A.; Boyer, S.; Giron, D. Changes in Bacterial Community Structure Across the Different Life Stages of Black Soldier Fly (Hermetia Illucens). Microb. Ecol. 2023, 86, 1254–1267. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Zhu, F.; Wang, X.; Wang, X.; Lei, C. The Gut Microbiota in Larvae of the Housefly Musca Domestica and Their Horizontal Transfer through Feeding. AMB Expr. 2017, 7, 147. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-N.; Zhang, Y.; Cao, Z.-H.; Wang, X.-Y.; Ma, B.; Wu, W.-B.; Hu, N.; Huo, Z.-Y.; Yuan, Q.-B. Occurrence of super antibiotic resistance genes in the downstream of the Yangtze River in China: Prevalence and antibiotic resistance profiles. Sci. Total Environ. 2019, 651, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yuan, Q.; Li, Z.; Du, Z.; Wu, G.; Yu, J.; Hu, J. Translocation and dissemination of gut bacteria after severe traumatic brain injury. Microorganisms 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, N.; Michaelsen, T.Y.; Ejbye-Ernst, R.; Jensen, A.; Nielsen, M.E.; Bahrndorff, S.; Nielsen, J.L. Housefly (Musca Domestica L.) Associated Microbiota across Different Life Stages. Sci. Rep. 2020, 10, 7842. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, H.; Zhang, X.; Liu, Y.; Chen, H.; Zheng, L.; Zhai, Y.; Zheng, H. The honeybee gut resistome and its role in antibiotic resistance dissemination. Integr. Zool. 2023, 18, 1014–1026. [Google Scholar] [CrossRef]
- Hu, N.; Fan, X.; Long, W.; Fan, Y.; Gao, H. Improvement in Efficiency of Kitchen Waste Bioconversion by Housefly Larvae Using New Net-Bottom Method. Environ. Technol. Innov. 2024, 33, 103525. [Google Scholar] [CrossRef]
- Li, X.; Zhou, S.; Zhang, J.; Zhou, Z.; Xiong, Q. Directional Changes in the Intestinal Bacterial Community in Black Soldier Fly (Hermetia Illucens) Larvae. Animals 2021, 11, 3475. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Chapman, S.J.; Su, J.; Wang, C. Changes in Gut Bacterial Communities and the Incidence of Antibiotic Resistance Genes during Degradation of Antibiotics by Black Soldier Fly Larvae. Environ. Int. 2020, 142, 105834. [Google Scholar] [CrossRef]
- Xian, Z.-N.; Yin, C.-F.; Zheng, L.; Zhou, N.-Y.; Xu, Y. Biodegradation of Additive-Free Polypropylene by Bacterial Consortia Enriched from the Ocean and from the Gut of Tenebrio Molitor Larvae. Sci. Total Environ. 2023, 892, 164721. [Google Scholar] [CrossRef]
- Miller, C.; Bates, S.T.; Gielda, L.M.; Creighton, J.C. The Role of Parental Care in the Establishment of the Offspring Digestive Tract Microbiome in Nicrophorus Defodiens. Anim. Behav. 2021, 172, 35–44. [Google Scholar] [CrossRef]
- Kumar, D.; Sun, Z.; Cao, G.; Xue, R.; Hu, X.; Gong, C. Study of Gut Bacterial Diversity of Bombyx Mandarina and Bombyx Mori through 16S rRNA Gene Sequencing. J. Asia-Pac. Entomol. 2019, 22, 522–530. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhang, X.; Zhang, R.; Zhang, Z. Negative Impact of Pseudomonas Aeruginosa Y12 on Its Host Musca Domestica. Front. Microbiol. 2021, 12, 691158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Zhang, Q.; Zhang, K.; Liu, W.; Zhang, R.; Zhang, Z. The Expansion of a Single Bacteriophage Leads to Bacterial Disturbance in Gut and Reduction of Larval Growth in Musca Domestica. Front. Immunol. 2022, 13, 885722. [Google Scholar] [CrossRef] [PubMed]
- Carina Audisio, M.; Torres, M.J.; Sabaté, D.C.; Ibarguren, C.; Apella, M.C. Properties of Different Lactic Acid Bacteria Isolated from Apis Mellifera L. Bee-Gut. Microbiol. Res. 2011, 166, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, J.; Cheng, X.; Li, C.; Yuan, Z. Relationship of Black Soldier Fly Larvae (BSFL) Gut Microbiota and Bioconversion Efficiency with Properties of Substrates. Waste Manag. 2024, 180, 106–114. [Google Scholar] [CrossRef]
- Choi, J.W.; Jeon, E.J.; Jeong, K.J. Recent Advances in Engineering Corynebacterium Glutamicum for Utilization of Hemicellulosic Biomass. Curr. Opin. Biotechnol. 2019, 57, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, Q.; Li, D. Mechanism Underlying Effects of Cellulose-Degrading Microbial Inoculation on Amino Acid Degradation and Biosynthesis during Composting. Bioresour. Technol. 2024, 403, 130899. [Google Scholar] [CrossRef]
- Gálvez, E.J.C.; Iljazovic, A.; Amend, L.; Lesker, T.R.; Renault, T.; Thiemann, S.; Hao, L.; Roy, U.; Gronow, A.; Charpentier, E.; et al. Distinct Polysaccharide Utilization Determines Interspecies Competition between Intestinal Prevotella spp. Cell Host Microbe. 2020, 28, 838–852.e6. [Google Scholar] [CrossRef]
- Xiang, F.; Zhang, Q.; Xu, X.; Zhang, Z. Black Soldier Fly Larvae Recruit Functional Microbiota into the Intestines and Residues to Promote Lignocellulosic Degradation in Domestic Biodegradable Waste. Environ. Pollut. 2024, 340, 122676. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, L.; Li, M.; Zhu, Y.; Liang, D.; Ma, Y.; Sun, L.; Zhao, G.; Tu, Q. Probiotic Bacillus as Fermentation Agents: Status, Potential Insights, and Future Perspectives. Food Chem. X 2024, 22, 101465. [Google Scholar] [CrossRef]
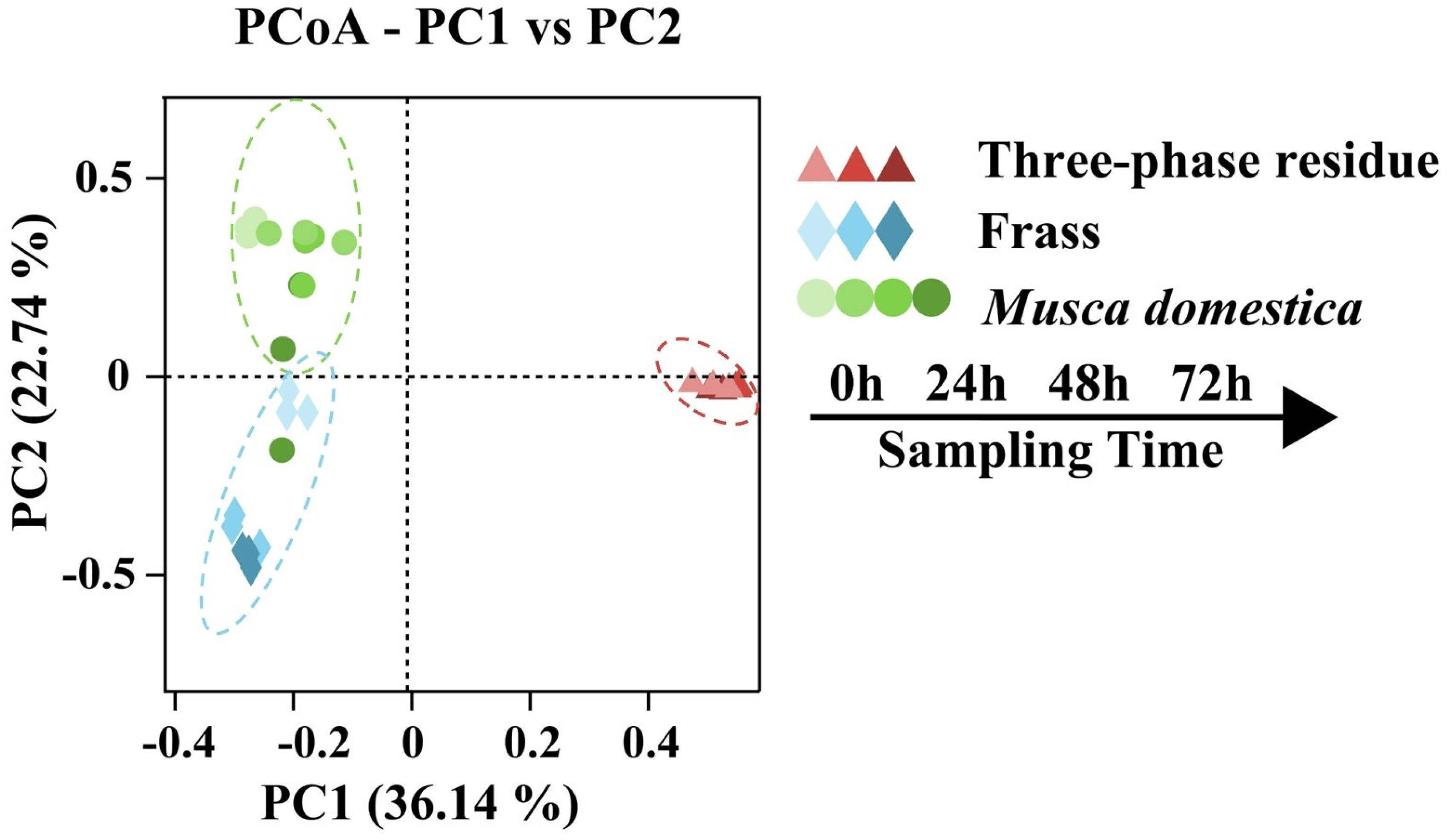
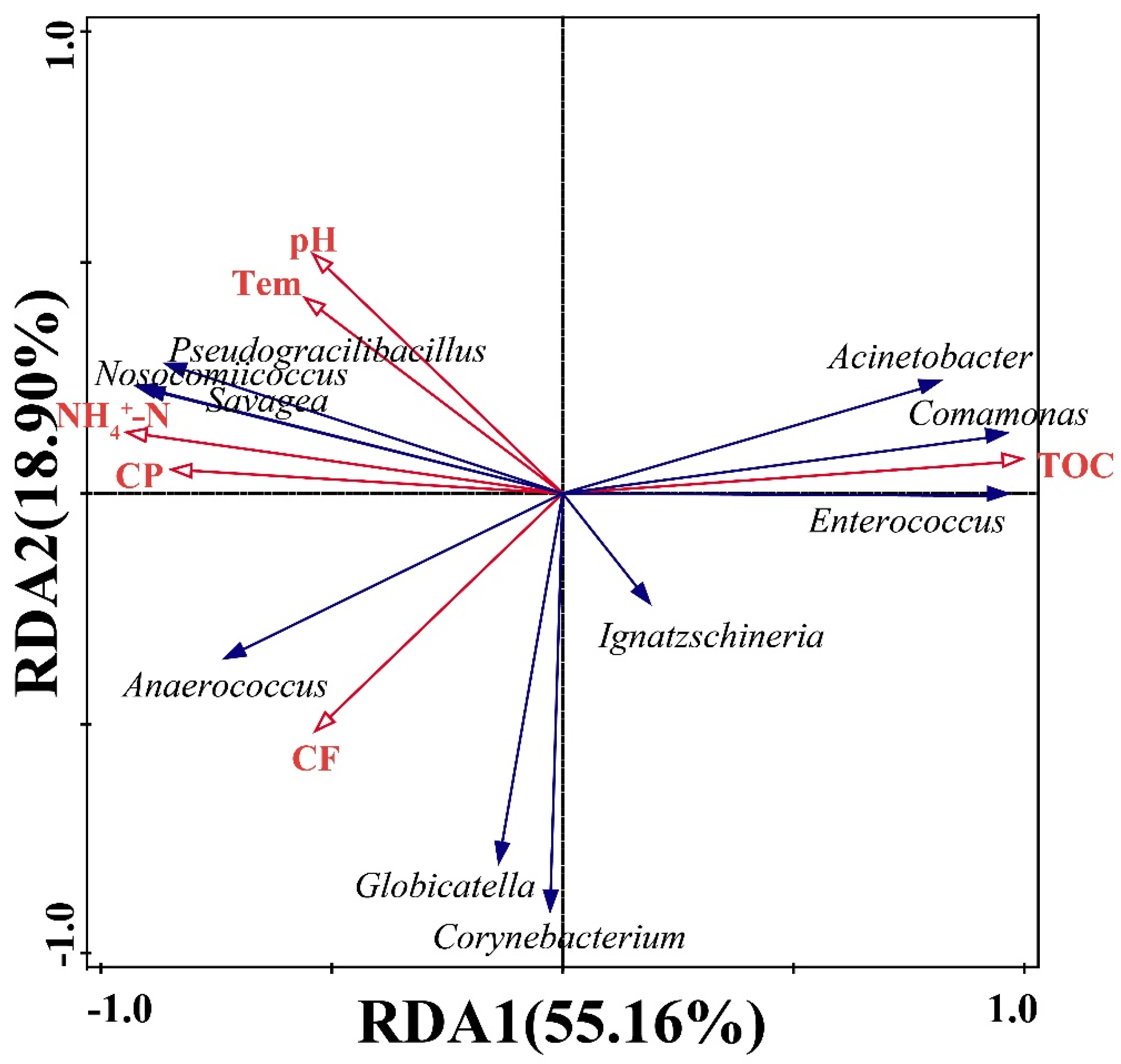
| Sample | Clean Reads | Number of ASV | Chao1 Estimate | Shannon Index |
|---|---|---|---|---|
| T0 | 73,016 ± 316 | 381 ± 80 | 395 ± 84 | 4.92 ± 0.62 |
| T24 | 72,789 ± 186 | 255 ± 28 | 269 ± 29 | 4.01 ± 0.16 |
| T48 | 72,457 ± 308 | 360 ± 53 | 373 ± 57 | 4.42 ± 0.72 |
| Md0 | 72,596 ± 457 | 159 ± 10 | 183 ± 13 | 4.14 ± 0.06 |
| Md24 | 72,380 ± 296 | 306 ± 27 | 316 ± 28 | 4.41 ± 0.37 |
| Md48 | 72,245 ± 63 | 592 ± 42 | 602 ± 47 | 5.28 ± 0.62 |
| Md72 | 73,530 ± 2226 | 1199 ± 46 | 1214 ± 54 | 8.32 ± 0.68 |
| F24 | 732,623 ± 99 | 257 ± 22 | 272 ± 23 | 5.74 ± 0.24 |
| F48 | 61,129 ± 1165 | 286 ± 37 | 303 ± 33 | 5.34 ± 0.10 |
| F72 | 76,563 ± 147 | 271 ± 39 | 281 ± 42 | 5.02 ± 0.11 |
| Sample | MC (%) | pH | TOC (%) | CP (%) | CF (%) | NH4+ (g/kg) |
|---|---|---|---|---|---|---|
| T0 | 76.46 ± 0.12 ab | 4.06 ± 0.06 a | 37.28 ± 0.52 a | 39.23 ± 0.83 a | 11.53 ± 0.54 a | 0.21 ± 0.01 a |
| T24 | 76.14 ± 0.37 a | 4.08 ± 0.05 a | 36.97 ± 0.71 ab | 39.62 ± 0.16 a | 11.67 ± 0.33 a | 0.23 ± 0.03 a |
| T48 | 75.77 ± 0.63 a | 4.03 ± 0.06 a | 36.09 ± 0.32 b | 39.49 ± 0.40 a | 11.92 ± 0.79 a | 0.26 ± 0.04 a |
| Md24 | 79.56 ± 0.02 c | ND | ND | 39.85 ± 0.67 a | 30.33 ± 2.17 b | ND |
| Md48 | 77.46 ± 0.11 b | ND | ND | 43.48 ± 0.64 b | 32.80 ± 1.99 c | ND |
| Md72 | 74.52 ± 0.38 d | ND | ND | 48.07 ± 0.35 c | 28.95 ± 1.21 b | ND |
| F24 | 61.35 ± 0.86 e | 6.45 ± 0.38 b | 36.46 ± 1.00 ab | 30.68 ± 1.31 e | 6.69 ± 0.91 d | 3.27 ± 0.34 b |
| F48 | 60.34 ± 0.42 e | 6.54 ± 0.26 b | 32.24 ± 0.63 c | 35.55 ± 1.24 d | 8.35 ± 1.50 d | 5.79 ± 0.23 c |
| F72 | 58.27 ± 0.52 f | 7.21 ± 0.32 c | 31.31 ± 0.18 c | 34.55 ± 0.52 d | 7.40 ± 0.54 d | 7.29 ± 0.39 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.; Pang, J.; Yan, W.; Hu, N. Industrial-Scale Bioconversion of Three-Phase Residue by Musca domestica Larvae: Dynamics of Gut Microbiota and Their Ecological Driver. Insects 2025, 16, 686. https://doi.org/10.3390/insects16070686
Long W, Pang J, Yan W, Hu N. Industrial-Scale Bioconversion of Three-Phase Residue by Musca domestica Larvae: Dynamics of Gut Microbiota and Their Ecological Driver. Insects. 2025; 16(7):686. https://doi.org/10.3390/insects16070686
Chicago/Turabian StyleLong, Wenna, Junran Pang, Wantao Yan, and Nan Hu. 2025. "Industrial-Scale Bioconversion of Three-Phase Residue by Musca domestica Larvae: Dynamics of Gut Microbiota and Their Ecological Driver" Insects 16, no. 7: 686. https://doi.org/10.3390/insects16070686
APA StyleLong, W., Pang, J., Yan, W., & Hu, N. (2025). Industrial-Scale Bioconversion of Three-Phase Residue by Musca domestica Larvae: Dynamics of Gut Microbiota and Their Ecological Driver. Insects, 16(7), 686. https://doi.org/10.3390/insects16070686





