Cretaceous Connections Among Camel Cricket Lineages in the Himalaya Revealed Through Fossil-Calibrated Mitogenomic Phylogenetics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
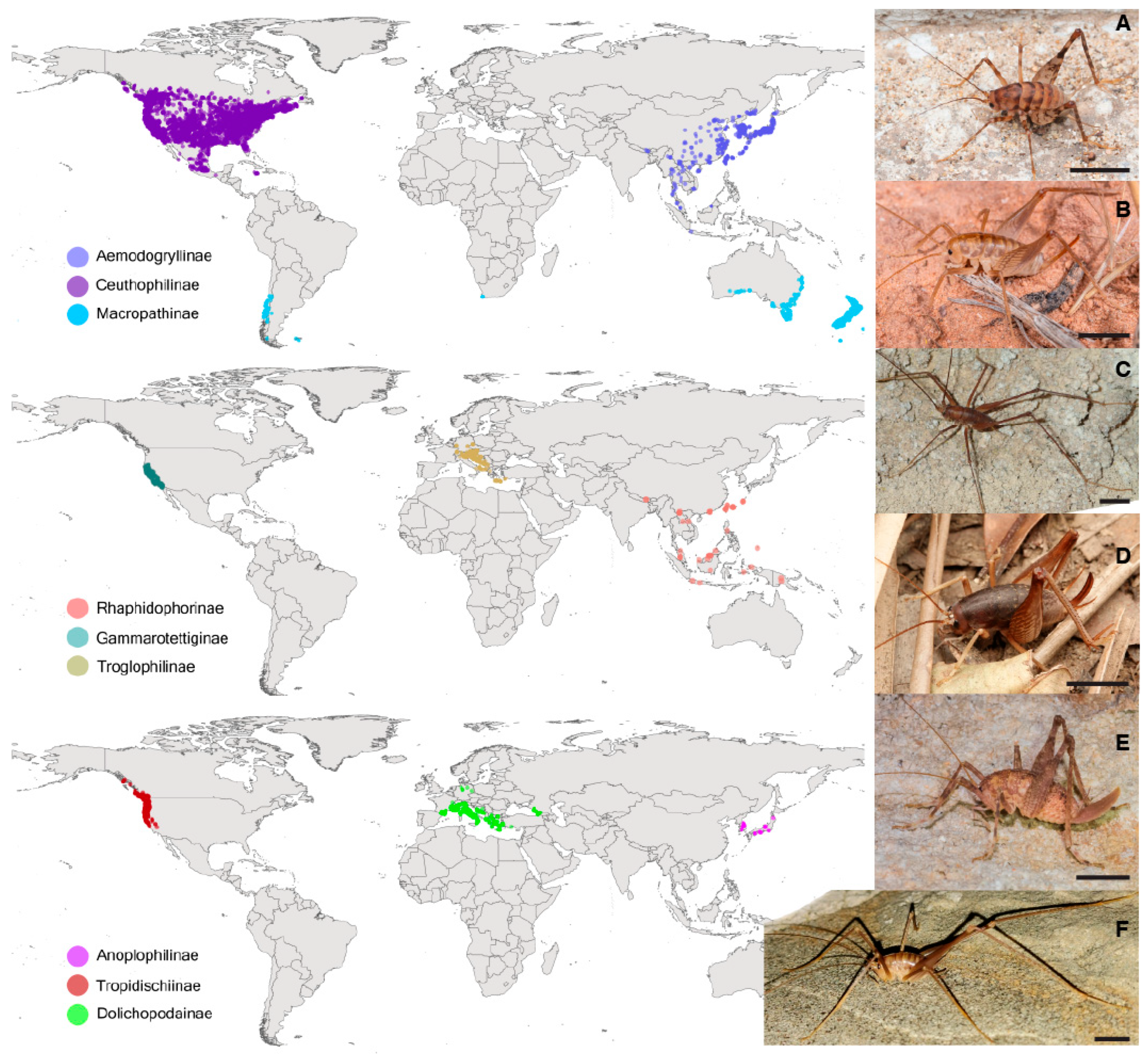
2.1. Taxon Sampling
2.2. DNA Sequencing
| Family | Subfamily | Taxa | Country | Specimen Code | Accession No. | Author |
|---|---|---|---|---|---|---|
| Rhaphidophoridae | Aemodogryllinae | Diestramima matermagna | Pema Gatshel, Bhutan | MPN_CW5536 | OR896621 | This study |
| Diestramima tsongkhapa | Trongsa, Bhutan | MPN_CW5525 | OR896622 | This study | ||
| Diestramima intermedia * | China | KX057718 | [50] | |||
| Diestramima tibetensis | China | KX057740 | [50] | |||
| Diestrammena sp. | China | MT849270 | [51] | |||
| Diestrammena japanica | Japan | MK347245 | [52] | |||
| Tachycines asynamorus * | China | KX057726 | [50] | |||
| Tachycines shuangcha | China | OM993275 | [53] | |||
| Tachycines zorzini | China | MW322826 | NC_057442 | [54] | ||
| Ceuthophilinae | Ceuthophilus sp. | Moab Desert, USA | MPN_CW4347 | OR880641 | This study | |
| Rhaphidophorinae | Stonychophora sp. | Vangunu, Solomon Is. | MPN_ORT15 | OR896624 | This study | |
| Rhaphidophora quadrispina | China | OL450400 | [55] | |||
| Rhaphidophora bicuspis | Thimphu, Bhutan | MPN_CW5529 | OR896623 | This study | ||
| Rhaphidophora bhutanensis | Pema Gatshel, Bhutan | MPN_CW5483 | OR896625 | This study | ||
| Rhaphidophora bilobata | Trongsa, Bhutan | MPN_CW5545 | OR896626 | This study | ||
| Troglophilinae | Troglophilus neglectus | Brje pri Kombu, Slovenia | EU938374 | [56] | ||
| Macropathinae | Macropathus sp. | Waitomo, NZ | MPN_CW109 | OR520204 | [6] | |
| Talitropsis sedilotti | Hawkes Bay, NZ | MPN_CW1830 | OR551721 | [6] | ||
| Parvotettix domesticus | Taronga, Tasmania | MPN_CW736 | OR551716 | [6] | ||
| Spelaeiacris monslamiensis | Hex River, South Africa | MPN_CW3801 | OR551731 | [6] | ||
| Prophalangopsidae | Cyphoderrinae | Cyphoderris monstrosa | KM657332 | [1] | ||
| Prophalangopsinae | Tarragoilus diuturnus | NC_021397 | [50] |
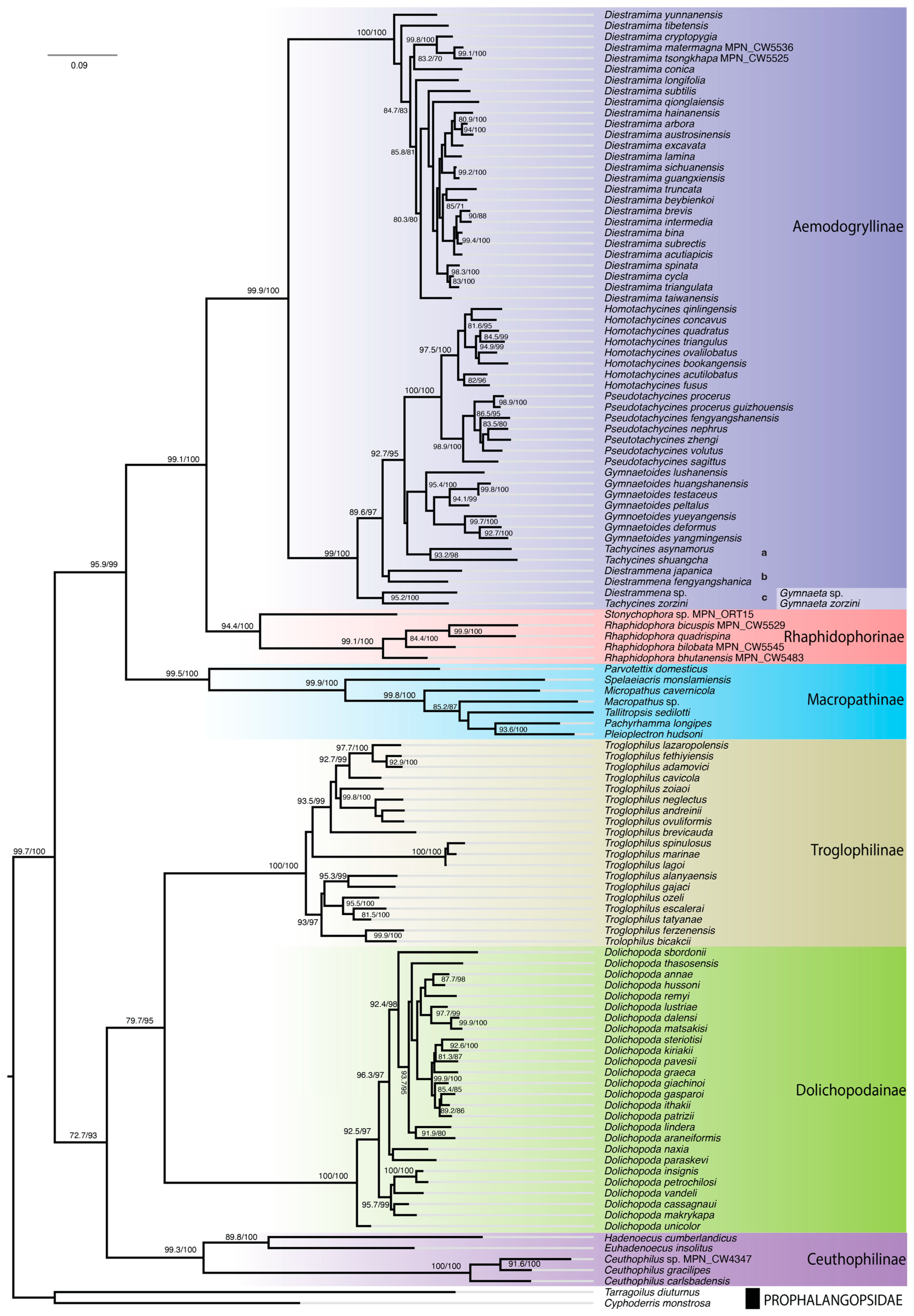
2.3. Phylogenetic Analysis
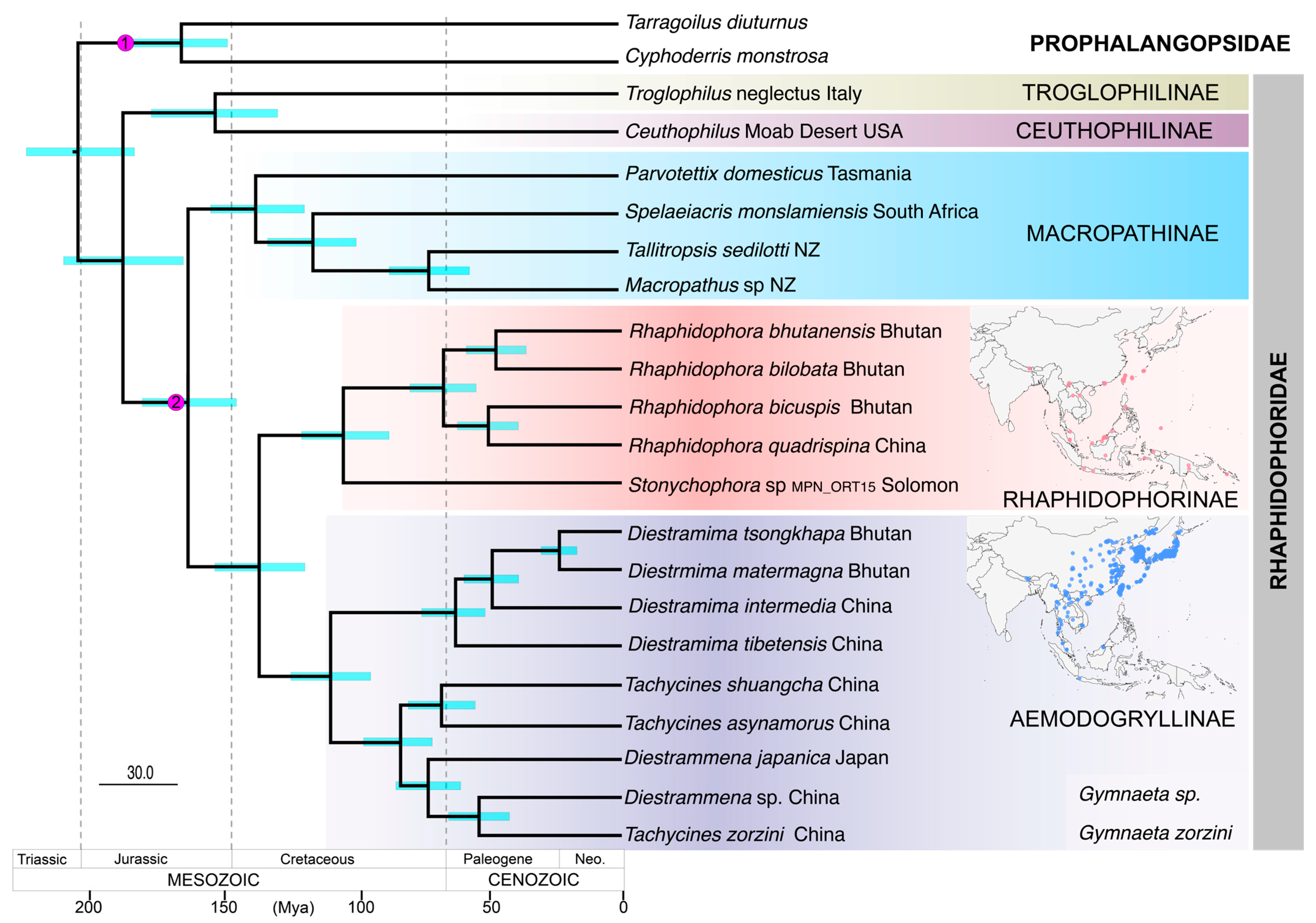
2.4. Divergence Time Estimate Analysis
- Fossils belonging to the genus Aboilus Martynov (201.3–157.3 million years ago [Mya]) are the oldest definitive Prophalangopsidae known [66,67]. These are well-recognized fossils previously used in divergence dating analysis of Orthoptera [1,50]. Aboilus consists of 20 extinct taxa [2], with fossils from the Jurassic and Cretaceous. We constrained two commonly used extant species of Prophalangopsidae in our molecular analyses (represented by Cyphoderris monstrosa Uhler and Tarragoilus diuturnus Gorochov) under a normal distribution prior with the minimum fossil age as a minimum soft bound (157.3 Mya) and 192 Mya as a maximum soft bound.
- As a secondary fossil calibration point, we constrained our analysis using a recent phylogenomic study of Southern Hemisphere rhaphidophorids calibrated with a Prophalangopsidae fossil (Aboilus) and a recent geological constraint [6]. We used 160 Mya as a mean age, with 95% Highest Posterior Density (HPD) values as soft minimum and maximum bounds at the node of Macropathinae and Aemodogryllinae + Rhaphidophorinae.
| BEAST RUN | Priors F/S | N. Gen. | Posterior | Tree Likelihood | Prior | Rhaphs. MRCA | 95% HPD | Ceu and Tro MRCA | 95% HPD | Rha and Aem MRCA | 95%HPD | Fossil Treatment: Aboilus (201–157 Mya) | Sec. Treatment: Mac/Aem + Rha Node Age (140–180 Mya) |
| 1 | LN/N | 10 | 411 | 327 | 368 | 188.7 | 166.6; 210.7 | 153.4 | 131; 178.7 | 137.6 | 121.5; 154.9 | Hard min = 157; mean =172; 97.5% max = 222 | 2.5% min = 140; mean = 160; 97.5 max = 180 |
| 2 | N/N | 10 | 1109 | 846 | 1819 | 188.8 | 165.7; 211.3 | 153.6 | 129.8; 178 | 138.1 | 120.7; 154.9 | 2.5% min = 152; mean = 172; 97.5% max = 192 | 2.5% min = 140; mean = 160; 97.5 max = 180 |
| 3 | E/E | 10 | 1019 | 775 | 1234 | 188.7 | 159.2; 226.8 | 153.7 | 124.7; 189.7 | 137.6 | 115.2; 166.1 | Hard min = 157; mean = 172; 97.5% max = 212 | Hard min = 140; mean = 160; 97.5% max = 214 |
| 4 | LN/E | 10 | 945 | 675 | 1173 | 188.6 | 156.4; 227.2 | 153.6 | 126.1; 189.7 | 135.3 | 113.2; 170.6 | Hard min = 157; mean = 172; 97.5% max = 222 | Hard min = 140; mean = 160; 97.5% max = 214 |
| 5 | N/N | 100 | 10278 | 7661 | 20,075 | 188.7 | 166.7; 211.9 | 153.6 | 130; 178.5 | 138.1 | 121.1;155 | 2.5% min = 152; mean = 172; 97.5% max = 192 | 2.5% min = 140; mean = 160 97.5 max = 180 |
| BEAST RUN | Prior S/S | N. Gen. | Posterior | Tree Likelihood | Prior | Rhaphs. MRCA | 95% HPD | Ceu and Tro MRCA | 95% HPD | Rha and Aem MRCA | 95% HPD | SEC. Node Age Treatment: Ceu and Tro (99.3–46.3 Mya) | Sec. Node Age Treatment: Mac/Aem + Rha Node Age (130–105 Mya) |
| 1 | N | 10 | 707 | 663 | 1245 | 109.5 | 98.0; 122.4 | 69.9 | 65.8; 73.6 | 88.5 | 77.6; 100.8 | 2.5% min = 48.9; mean = 68.5; 97.5% max = 88 | 2.5% min = 105; mean = 117; 97.5% max = 129 |
| 2 | E | 10 | 635 | 511.1 | 1266 | 113 | 105.3; 127.7 | 85.4 | 69.8; 102.2 | 90.4 | 81.4; 103.8 | Hard min = 56; mean = 68.5; 97.5% max = 102 | Hard min = 105; mean = 117; 97.5% max = 149 |
| 3 | U | 10 | 776 | 626.9 | 1388.3 | 121 | 106.6; 136.5 | 94.5 | 76.2; 115.9 | 95.5 | 84.1; 108.5 | Bounds: min = 46; mean = 72.5; max = 99 | Bounds: min = 105; mean = 118; max = 130 |
| 4 | N | 100 | 8860 | 8032 | 10,664 | 109.8 | 97.7; 122.1 | 69.8 | 66; 73.6 | 88.3 | 77.1; 100.4 | 2.5% min = 48.9; mean = 68.5; 97.5% max = 88 | 2.5% min = 105; mean = 117; 97.5% max = 129 |
3. Results
3.1. Systematics
3.2. Rhaphidophoridae Mitogenomic Data
3.3. Phylogenetic Analysis and Time Calibration
4. Discussion
4.1. Systematics
4.2. Lineage Age and Origin
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, H.; Amédégnato, C.; Cigliano, M.M.; Desutter-Grandcolas, L.; Heads, S.W.; Huang, Y.; Otte, D.; Whiting, M.F. 300 Million Years of Diversification: Elucidating the Patterns of Orthopteran Evolution Based on Comprehensive Taxon and Gene Sampling. Cladistics 2015, 31, 621–651. [Google Scholar] [CrossRef] [PubMed]
- Cigliano, M.; Braun, H.; Eades, D.; Otte, D. Orthoptera Species File. Available online: http://orthoptera.speciesfile.org/HomePage/Orthoptera/HomePage.aspx (accessed on 25 June 2025).
- Allegrucci, G.; Sbordoni, V. Insights into the Molecular Phylogeny of Rhaphidophoridae, an Ancient, Worldwide Lineage of Orthoptera. Mol. Phylogenet. Evol. 2019, 138, 126–138. [Google Scholar] [CrossRef]
- Trewick, S.A. A New Weta from the Chatham Islands (Orthoptera: Raphidophoridae). J. R. Soc. N. Z. 1999, 2, 165–173. [Google Scholar] [CrossRef]
- Trewick, S.A. Molecular Evidence for Dispersal Rather than Vicariance as the Origin of Flightless Insect Species on the Chatham Islands, New Zealand. J. Biogeogr. 2000, 27, 1189–1200. [Google Scholar] [CrossRef]
- Dowle, E.J.; Trewick, S.A.; Morgan-Richards, M. Fossil-Calibrated Phylogenies of Southern Cave Wētā Show Dispersal and Extinction Confound Biogeographic Signal. R. Soc. Open Sci. 2024, 11, 231118. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, S.; Song, H.; Shin, S. Phylogeny and Biogeography of the Wingless Orthopteran Family Rhaphidophoridae. Commun. Biol. 2024, 7, 401. [Google Scholar] [CrossRef]
- Grandcolas, P.; Trewick, S.A. What Is the Meaning of Extreme Phylogenetic Diversity? The Case of Phylogenetic Relict Species. In Biodiversity Conservation and Phylogenetic Systematics: Preserving Our Evolutionary Heritage in an Extinction Crisis; Pellens, R., Grandcolas, P., Eds.; Topics in Biodiversity and Conservation; Springer International Publishing: Cham, Swizterland, 2016; pp. 99–115. [Google Scholar] [CrossRef]
- Hubbell, T.H.; Norton, R.M. The Systematics and Biology of the Cave-Crickets of the North American Tribe Hadenoecini (Orthoptera Saltatoria: Ensifera: Rhaphidophoridae: Dolichopodinae); University of Michigan Museum of Zoology: Ann Arbor, MI, USA, 1978. [Google Scholar]
- Ragge, D.R. The Wing-Venation of the Orthoptera Saltatoria; The British Museum: London, UK, 1955; 159p. [Google Scholar]
- Sharov, A.G. Phylogeny of the Orthopteroidea; Rodendorf, B.B., Ed.; Trudy Paleontologicheskago Instituta Academia Nauk U.S.S.R.: Jerusalem, Israel, 1971; Volume 118, p. 251. [Google Scholar]
- Ander, K. Vergleichend-Anatomische Und Phylogenetische Studien Über Die Ensifera (Saltatoria). Opusc. Entomol. Suppl. 1939, 2, 1–306. [Google Scholar]
- Karny, H.H. Zur Kcnntnis Der Ostasiatischen Rhaphidophorinen (Orth., Salt., Gryllacrididae). Konowia 1934, 13, 214–230. [Google Scholar]
- Richards, A.M. The Effect of Weather on Rhaphidophoridae (Orthoptera) in New Zealand and Australia. Ann. Spéléologie 1965, 20, 391–400. [Google Scholar]
- Allegrucci, G.; Trewick, S.A.; Fortunato, A.; Carchini, G.; Sbordoni, V. Cave Crickets and Cave Weta (Orthoptera, Rhaphidophoridae) from the Southern End of the World: A Molecular Phylogeny Test of Biogeographical Hypotheses. J. Orthoptera Res. 2010, 19, 121–130. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, Z.; Zheng, X.; Wang, T.; Ma, L.; Shi, F. Phylogeny and Phylogeography of Diestramima Cave Crickets (Orthoptera: Rhaphidophoridae): Speciation Driven by Multiple Dispersal and Vicariance Events. Syst. Entomol. 2022, 47, 179–201. [Google Scholar] [CrossRef]
- Allegrucci, G.; Trucchi, E.; Sbordoni, V. Tempo and Mode of Species Diversification in Dolichopoda Cave Crickets (Orthoptera, Rhaphidophoridae). Mol. Phylogenet. Evol. 2011, 60, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Allegrucci, G.; Ketmaier, V.; Russo, C.D.; Rampini, M.; Sbordoni, V.; Cobolli, M. Molecular Phylogeography of Troglophilus Cave Crickets (Orthoptera, Rhaphidophoridae): A Combination of Vicariance and Dispersal Drove Diversification in the East Mediterranean Region. J. Zool. Syst. Evol. Res. 2017, 55, 310–325. [Google Scholar] [CrossRef]
- Waters, J.M.; Craw, D. Goodbye Gondwana? New Zealand Biogeography, Geology, and the Problem of Circularity. Syst. Biol. 2006, 55, 351–356. [Google Scholar] [CrossRef]
- Bargelloni, L.; Marcato, S.; Zane, L.; Patarnello, T. Mitochondrial Phylogeny of Notothenioids: A Molecular Approach to Antarctic Fish Evolution and Biogeography. Syst. Biol. 2000, 49, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H.; Holland, B.S. Dispersal Is Fundamental to Biogeography and the Evolution of Biodiversity on Oceanic Islands. J. Biogeogr. 2006, 33, 193–198. [Google Scholar] [CrossRef]
- Trewick, S.A.; Morgan-Richards, M. After the Deluge: Mitochondrial DNA Indicates Miocene Radiation and Pliocene Adaptation of Tree and Giant Weta (Orthoptera: Anostostomatidae). J. Biogeogr. 2005, 32, 295–309. [Google Scholar] [CrossRef]
- Borissov, S.B.; Heller, K.-G.; Çıplak, B.; Chobanov, D.P. Origin, Evolution and Systematics of the Genus Poecilimon (Orthoptera: Tettigoniidae)—An Outburst of Diversification in the Aegean Area. Syst. Entomol. 2023, 48, 198–220. [Google Scholar] [CrossRef]
- Trichas, A.; Smirli, M.; Papadopoulou, A.; Anastasiou, I.; Keskin, B.; Poulakakis, N. Dispersal versus Vicariance in the Aegean: Combining Molecular and Morphological Phylogenies of Eastern Mediterranean Dendarus (Coleoptera: Tenebrionidae) Sheds New Light on the Phylogeography of the Aegean Area. Zool. J. Linn. Soc. 2020, 190, 824–843. [Google Scholar] [CrossRef]
- Wallace, A.R. The Geographical Distribution of Animals; with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface; Harper & Brothers: New York, NY, USA, 1876. [Google Scholar]
- Crisp, M.D.; Trewick, S.A.; Cook, L.G. Hypothesis Testing in Biogeography. Trends Ecol. Evol. 2011, 26, 66–72. [Google Scholar] [CrossRef]
- Trewick, S.A. Vicars and Vagrants: Assembly of the New Zealand Avifauna. Australas. Sci. 2011, 32, 24–27. [Google Scholar]
- Ho, S.Y.W.; Phillips, M.J. Accounting for Calibration Uncertainty in Phylogenetic Estimation of Evolutionary Divergence Times. Syst. Biol. 2009, 58, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Chopard, L. Biospeleologica N. LXIII. Orthopteres et Dermaptéres (Premiere Serie). Arch. Zool. Exp. Gen. Paris 1936, 78, 195–214. [Google Scholar]
- Gorochov, A.V. A New Representative of the Family Rhaphidophoridae (Orthoptera) from Baltic Amber [in Russian]. Paleontol. Zhurnal 1989, 3, 108–110. [Google Scholar]
- Azar, D.; Maalouf, R.; Nel, A. An Enigmatic Tettigoniidea from the Lower Cretaceous Amber of Bqaatouta, Lebanon (Orthoptera, Ensifera). Palaeoentomology 2022, 5, 233–239. [Google Scholar] [CrossRef]
- Beasley-Hall, P.G.; Tierney, S.M.; Weinstein, P.; Austin, A.D. A Revised Phylogeny of Macropathine Cave Crickets (Orthoptera: Rhaphidophoridae) Uncovers a Paraphyletic Australian Fauna. Mol. Phylogenet. Evol. 2018, 126, 153–161. [Google Scholar] [CrossRef]
- Adelung, N. Beitrag Zur Kenntnis Der Palaarktischen Stenopelmatiden (Orthoptera, Locustodea). Extrait L’Annuaire Musee Zoologique L’ Academie Imperiale Science St. Petersbourg 1902, 7, 55–75. [Google Scholar]
- Furukawa, H. On Two Cave-Dwelling Orthopterans, Diestrammena, from Japan. J. Fac. Sci. Imp. Univ. Tokyo 1933, 3, 205–216. [Google Scholar]
- Gorochov, A.V.; Storozhenko, S.Y. On the Fauna of the Subfamily Aemodogryllinae (Orthoptera, Rhaphidophoridae) in Vietnam. Proc. Zool. Inst. Russ. Acad. Sci. 1992, 245, 17–34. [Google Scholar]
- Gorochov, A.V. Material on the Fauna and Systematics of Stenopelmatoidea (Orthoptera) from Indochina and Some Other Territories. I. [in Russian]. Entomol. Obozr. 1998, 77, 73–105. [Google Scholar]
- Zhu, Q.; Wang, H.; Zhou, Z.; Shi, F. Phylogeny and Integrative Taxonomy of the Genera Gymnaetoides and Pseudotachycines (Orthoptera: Rhaphidophoridae). Insects 2022, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Dawwrueng, P.; Gorochov, A.V.; Tanomtong, A.; Suwannapoom, C. Contribution to the Knowledge of Rhaphidophorinae (Orthoptera: Ensifera: Rhaphidophoridae) from Thailand: Three Genera Neorhaphidophora, Eurhaphidophora and Minirhaphidophora. Zootaxa 2020, 4853, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Gorochov, A.V.; Storozhenko, S.Y. New and Little-Known Taxa of the Tribe Diestramimini (Orthoptera: Rhaphidophoridae: Aemodogryllinae) from Southeast Asia. Part 1. Zoosystematica Ross. 2015, 24, 48–84. [Google Scholar] [CrossRef]
- Gorochov, A.V.; Storozhenko, S.Y. New and Little-Known Taxa of the Tribe Diestramimini (Orthoptera: Rhaphidophoridae: Aemodogryllinae) from Southeast Asia. Part 2. Zoosystematica Ross. 2019, 28, 132–154. [Google Scholar] [CrossRef]
- Dorji, C.; Morgan-Richards, M.; Trewick, S.A. Little-Known Wingless Crickets of Bhutan (Rhaphidophoridae): Discovery and Description of Nine New Species. Zootaxa 2025, 5653, 1–32. [Google Scholar] [CrossRef]
- Willemse, C. On a Collection of Indo-Australian, Melanesian and Micronesian Tettigoniidae. Natuurhistorisch Maandbl. 1942, 31, 94–100. [Google Scholar]
- Scudder, S. Hubbard. 1861. On the Genus Raphidophora, Serville; with Descriptions of Four Species from the Caves of Kentucky, and from the Pacific Coast. Proc. Boston Soc. Nat. Hist. 1861, 8, 6–14. [Google Scholar] [CrossRef]
- Sunnucks, P.; Hales, D.F. Numerous Transposed Sequences of Mitochondrial Cytochrome Oxidase I-II in Aphids of the Genus Sitobion (Hemiptera: Aphididae). Mol. Biol. Evol. 1996, 13, 510–524. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Koot, E.M.; Morgan-Richards, M.; Trewick, S.A. Climate Change and Alpine-Adapted Insects: Modelling Environmental Envelopes of a Grasshopper Radiation. R. Soc. Open Sci. 2022, 9, 211596. [Google Scholar] [CrossRef]
- Vaux, F.; Hills, S.F.K.; Marshall, B.A.; Trewick, S.A.; Morgan-Richards, M. A Phylogeny of Southern Hemisphere Whelks (Gastropoda: Buccinulidae) and Concordance with the Fossil Record. Mol. Phylogenet. Evol. 2017, 114, 367–381. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de Novo Metazoan Mitochondrial Genome Annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Jiang, H.; Liu, X.; Li, K. A New Genus of Rhaphidophorinae (Orthoptera, Rhaphidophoridae) from China. Zootaxa 2018, 4500, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, L.; Liu, N.; Guo, H.; Guan, B.; Di, J.; Shi, F. Towards a Higher-Level Ensifera Phylogeny Inferred from Mitogenome Sequences. Mol. Phylogenet. Evol. 2017, 108, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Huang, Y.; Mao, Y.; Zhang, N.; Nie, Y.; Zhang, X.; Zhou, Y.; Mao, S. The Evolutionary Patterns of Genome Size in Ensifera (Insecta: Orthoptera). Front. Genet. 2021, 12, 693541. [Google Scholar] [CrossRef]
- Guan, D.-L.; Xu, S.-Q. The Complete Mitochondrial Genome of an Orthoptera Insect Diestrammena japonica (Rhaphidophoroidea; Rhaphidophoridae; Aemodogryllinae); National Center for Biotechnology Information: Bethesda, MD, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MK347245 (accessed on 6 May 2025).
- Hong, B. The Complete Mitochondrial Genome of Tachycines shuangcha; National Center for Biotechnology Information: Bethesda, MD, USA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OM993275 (accessed on 6 May 2025).
- Wang, Y.; Zhan, H.; Lv, X.; Li, B.; Yang, X. The Complete Mitochondrial Genome of Tachycines (Gymnaeta) zorzini (Orthoptera: Rhaphidophoridae). Mitochondrial DNA Part B 2021, 6, 1173–1174. [Google Scholar] [CrossRef]
- Lu, X. Rhaphidophora quadrispina, Direct Submission; National Center for Biotechnology Information: Bethesda, MD, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OL450400 (accessed on 6 May 2025).
- Fenn, J.D.; Song, H.; Cameron, S.L.; Whiting, M.F. A Preliminary Mitochondrial Genome Phylogeny of Orthoptera (Insecta) and Approaches to Maximizing Phylogenetic Signal Found within Mitochondrial Genome Data. Mol. Phylogenet. Evol. 2008, 49, 59–68. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Huang, Y.; Lin, L.-L.; Wang, X.-Y.; Zheng, Z.-M. The Phylogeny of the Orthoptera (Insecta) as Deduced from Mitogenomic Gene Sequences. Zool. Stud. 2013, 52, 37. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.; Xiao, B.; Jiang, G. The Comparative Mitogenomics and Phylogenetics of the Two Grouse-Grasshoppers (Insecta, Orthoptera, Tetrigoidea). Biol. Res. 2017, 50, 34. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Seo, T.-K.; Kishino, H.; Thorne, J.L. Incorporating Gene-Specific Variation When Inferring and Evaluating Optimal Evolutionary Tree Topologies from Multilocus Sequence Data. Proc. Natl. Acad. Sci. USA 2005, 102, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Béthoux, O.; Shin, S.; Donath, A.; Letsch, H.; Liu, S.; McKenna, D.D.; Meng, G.; Misof, B.; Podsiadlowski, L.; et al. Phylogenomic Analysis Sheds Light on the Evolutionary Pathways towards Acoustic Communication in Orthoptera. Nat. Commun. 2020, 11, 4939. [Google Scholar] [CrossRef]
- Wang, H.; Sha, L.; Zhang, Q.; Fang, Y.; Wang, B.; Zhang, H. Alcheringa Australas. J. Palaeontol. 2015, 39, 250–258. [Google Scholar] [CrossRef]
- Lin, Q.-B.; Huang, D.-Y. Revision of “Parahagla Lamina” Lin, 1982 and Two New Species of Aboilus (Orthoptera: Prophalangopsidae) from the Early-Middle Jurassic of Northwest China. Prog. Nat. Sci. 2006, 16, 303–307. [Google Scholar]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Koot, E.M.; Morgan-Richards, M.; Trewick, S.A. An Alpine Grasshopper Radiation Older than the Mountains, on Kā Tiritiri o Te Moana (Southern Alps) of Aotearoa (New Zealand). Mol. Phylogenet. Evol. 2020, 147, 106783. [Google Scholar] [CrossRef]
- Rambaut, A. Figtree Ver 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Barba-Montoya, J.; Tao, Q.; Kumar, S. Molecular and Morphological Clocks for Estimating Evolutionary Divergence Times. BMC Ecol. Evol. 2021, 21, 83. [Google Scholar] [CrossRef]
- Saladin, B.; Leslie, A.B.; Wüest, R.O.; Litsios, G.; Conti, E.; Salamin, N.; Zimmermann, N.E. Fossils Matter: Improved Estimates of Divergence Times in Pinus Reveal Older Diversification. BMC Evol. Biol. 2017, 17, 95. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.-W. A Review of the Subgenus Diestrammena (Gymnaeta) from China (Orthoptera: Rhaphidophoridae Aemodogryllinae). Zootaxa 2009, 2272, 21–36. [Google Scholar] [CrossRef]
- Inoue, J.; Donoghue, P.C.J.; Yang, Z. The Impact of the Representation of Fossil Calibrations on Bayesian Estimation of Species Divergence Times. Syst. Biol. 2010, 59, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Grandcolas, P.; Nattier, R.; Trewick, S. Relict Species: A Relict Concept? Trends Ecol. Evol. 2014, 29, 655–663. [Google Scholar] [CrossRef]
- Clapham, M.E.; Karr, J.A.; Nicholson, D.B.; Ross, A.J.; Mayhew, P.J. Ancient Origin of High Taxonomic Richness among Insects. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152476. [Google Scholar] [CrossRef]
- Schachat, S.R.; Labandeira, C.C.; Clapham, M.E.; Payne, J.L. A Cretaceous Peak in Family-Level Insect Diversity Estimated with Mark–Recapture Methodology. Proc. R. Soc. B Biol. Sci. 2019, 286, 20192054. [Google Scholar] [CrossRef]
- Jud, N.A. Fossil Evidence for a Herbaceous Diversification of Early Eudicot Angiosperms during the Early Cretaceous. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151045. [Google Scholar] [CrossRef]
- Silvestro, D.; Bacon, C.D.; Ding, W.; Zhang, Q.; Donoghue, P.C.J.; Antonelli, A.; Xing, Y. Fossil Data Support a Pre-Cretaceous Origin of Flowering Plants. Nat. Ecol. Evol. 2021, 5, 449–457. [Google Scholar] [CrossRef]
- Brikiatis, L. The De Geer, Thulean and Beringia Routes: Key Concepts for Understanding Early Cenozoic Biogeography. J. Biogeogr. 2014, 41, 1036–1054. [Google Scholar] [CrossRef]
- Wen, J.; Nie, Z.-L.; Ickert-Bond, S.M. Intercontinental Disjunctions between Eastern Asia and Western North America in Vascular Plants Highlight the Biogeographic Importance of the Bering Land Bridge from Late Cretaceous to Neogene. J. Syst. Evol. 2016, 54, 469–490. [Google Scholar] [CrossRef]
- Trewick, S.A.; Morgan-Richards, M. Phylogenetics and Conservation in New Zealand: The Long and the Short of It. In Biodiversity Conservation and Phylogenetic Systematics; Pellens, R., Grandcolas, P., Eds.; Topics in Biodiversity and Conservation; Springer International Publishing: Cham, Swizterland, 2016; Volume 14, pp. 81–97. [Google Scholar] [CrossRef]
- An, W.; Hu, X.; Garzanti, E.; Wang, J.-G.; Liu, Q. New Precise Dating of the India-Asia Collision in the Tibetan Himalaya at 61 Ma. Geophys. Res. Lett. 2021, 48, e2020GL090641. [Google Scholar] [CrossRef]
- Pusok, A.E.; Stegman, D.R. The Convergence History of India-Eurasia Records Multiple Subduction Dynamics Processes. Sci. Adv. 2020, 6, eaaz8681. [Google Scholar] [CrossRef] [PubMed]
- White, L.T.; Lister, G.S. The Collision of India with Asia. J. Geodyn. 2012, 56–57, 7–17. [Google Scholar] [CrossRef]
- Hall, R. Cenozoic Geological and Plate Tectonic Evolution of SE Asia and the SW Pacific: Computer-Based Reconstructions, Model and Animations. J. Asian Earth Sci. 2002, 20, 353–431. [Google Scholar] [CrossRef]
- Mann, P.; Taylor, F.W.; Lagoe, M.B.; Quarles, A.; Burr, G. Accelerating Late Quaternary Uplift of the New Georgia Island Group (Solomon Island Arc) in Response to Subduction of the Recently Active Woodlark Spreading Center and Coleman Seamount. Tectonophysics 1998, 295, 259–306. [Google Scholar] [CrossRef]
- Simpson, G.G. Too Many Lines; The Limits of the Oriental and Australian Zoogeographic Regions. Proc. Am. Philos. Soc. 1977, 121, 107–120. [Google Scholar]
- Keppel, G.; Lowe, A.J.; Possingham, H.P. Changing Perspectives on the Biogeography of the Tropical South Pacific: Influences of Dispersal, Vicariance and Extinction. J. Biogeogr. 2009, 36, 1035–1054. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorji, C.; Morgan-Richards, M.; Trewick, S.A. Cretaceous Connections Among Camel Cricket Lineages in the Himalaya Revealed Through Fossil-Calibrated Mitogenomic Phylogenetics. Insects 2025, 16, 670. https://doi.org/10.3390/insects16070670
Dorji C, Morgan-Richards M, Trewick SA. Cretaceous Connections Among Camel Cricket Lineages in the Himalaya Revealed Through Fossil-Calibrated Mitogenomic Phylogenetics. Insects. 2025; 16(7):670. https://doi.org/10.3390/insects16070670
Chicago/Turabian StyleDorji, Cheten, Mary Morgan-Richards, and Steven A. Trewick. 2025. "Cretaceous Connections Among Camel Cricket Lineages in the Himalaya Revealed Through Fossil-Calibrated Mitogenomic Phylogenetics" Insects 16, no. 7: 670. https://doi.org/10.3390/insects16070670
APA StyleDorji, C., Morgan-Richards, M., & Trewick, S. A. (2025). Cretaceous Connections Among Camel Cricket Lineages in the Himalaya Revealed Through Fossil-Calibrated Mitogenomic Phylogenetics. Insects, 16(7), 670. https://doi.org/10.3390/insects16070670







