Availability, Accessibility, and Suitability of Native Flowers from Central Chile to Mastrus ridens, a Parasitoid of Codling Moth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasitoid Populations Rearing
2.2. Plant Species Selection
2.3. Nectar Availability
2.4. Nectar Accessibility for M. ridens
2.5. Nectar Suitability
2.5.1. Nectar Solutions
2.5.2. Cut Flowers
2.5.3. Statistical Analysis
3. Results
3.1. Nectar Availability
3.2. Nectar Accessibility for M. ridens
3.3. Nectar Suitability: Parasitoid Longevity
3.3.1. Nectar Solutions
3.3.2. Cut Flowers
3.3.3. Nectar-Providing Technique
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimpel, G.E.; Mills, N.J. Biological Control: Ecology and Applications; Cambridge University Press: New York, NY, USA, 2017; ISBN 110810746X/9781108107464. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing Ecosystem Services from Conservation Biological Control: The Role of Habitat Management. Biol. Control 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A Functional Overview of Conservation Biological Control. Crop. Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing Arthropod-Mediated Ecosystem Services in Agricultural Landscapes: The Role of Native Plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Zaviezo, T.; Muñoz, A.E. Conservation Biological Control of Arthropod Pests Using Native Plants. Curr. Opin. Insect Sci. 2023, 56, 101022. [Google Scholar] [CrossRef]
- Iuliano, B.; Gratton, C. Temporal Resource (Dis)Continuity for Conservation Biological Control: From Field to Landscape Scales. Front. Sustain. Food Syst. 2020, 4, 547848. [Google Scholar] [CrossRef]
- Agustí, N.; Shayler, S.P.; Harwood, J.D.; Vaughan, I.P.; Sunderland, K.D.; Symondson, W.O.C. Collembola as Alternative Prey Sustaining Spiders in Arable Ecosystems: Prey Detection within Predators Using Molecular Markers. Mol. Ecol. 2003, 12, 3467–3475. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E.; Leibee, G.L. Comparing Floral Nectar and Aphid Honeydew Diets on the Longevity and Nutrient Levels of a Parasitoid Wasp. Entomol. Exp. Appl. 2004, 111, 189–199. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Wratten, S.D. Improved Fitness of Aphid Parasitoids Receiving Resource Subsidies. Ecology 2004, 85, 658–666. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.; Bukovinszkine-Kiss, G.; Van Lenteren, J. Sugar Resources Are Vital for Diadegma Semiclausum Fecundity under Field Conditions. Basic Appl. Ecol. 2006, 7, 133–140. [Google Scholar] [CrossRef]
- Lundgren, J.G. Relationships of Natural Enemies and Non-Prey Foods; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Tena, A.; Pekas, A.; Cano, D.; Wäckers, F.L.; Urbaneja, A. Sugar Provisioning Maximizes the Biocontrol Service of Parasitoids. J. Appl. Ecol. 2015, 52, 795–804. [Google Scholar] [CrossRef]
- Gonzalez, D.; Nave, A.; Gonçalves, F.; Nunes, F.M.; Campos, M.; Torres, L. Higher Longevity and Fecundity of Chrysoperla Carnea, a Predator of Olive Pests, on Some Native Flowering Mediterranean Plants. Agron. Sustain. Dev. 2016, 36, 30. [Google Scholar] [CrossRef]
- Irvin, N.A.; Hoddle, M.S. The Effects of Floral Nectar, Extrafloral Nectar and Hemipteran Honeydew on the Fitness of Tamarixia Radiata (Hymenoptera: Eulophidae), a Parasitoid of Diaphorina Citri. Biol. Control 2021, 163, 104753. [Google Scholar] [CrossRef]
- Taylor, R.M.; Pfannenstiel, R.S. Nectar Feeding by Wandering Spiders on Cotton Plants. Environ. Entomol. 2008, 37, 996–1002. [Google Scholar] [CrossRef]
- Wolf, S.; Romeis, J.; Collatz, J. Utilization of Plant-Derived Food Sources from Annual Flower Strips by the Invasive Harlequin Ladybird Harmonia Axyridis. Biol. Control 2018, 122, 118–126. [Google Scholar] [CrossRef]
- Alcalá Herrera, R.; Fernández Sierra, M.L.; Ruano, F. The Suitability of Native Flowers as Pollen Sources for Chrysoperla Lucasina (Neuroptera: Chrysopidae). PLoS ONE 2020, 15, e0239847. [Google Scholar] [CrossRef]
- Pan, H.; Xiu, C.; Liu, B.; Wyckhuys, K.A.G.; Lu, Y. Whorl-Stage Maize Provides a Microclimate Refuge for Predatory Ladybeetles. Biol. Control 2020, 142, 104162. [Google Scholar] [CrossRef]
- JERVIS, M.A.; KIDD, N.A.C. Host-Feeding Strategies in Hymenopteran Parasitoids. Biol. Rev. 1986, 61, 395–434. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource Acquisition, Allocation, and Utilization in Parasitoid Reproductive Strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Harvey, J.A.; Mills, N.J. Influence of Adult Nutrition on the Relationship between Body Size and Reproductive Parameters in a Parasitoid Wasp. Ecol. Entomol. 2005, 30, 571–580. [Google Scholar] [CrossRef]
- Russell, M. A Meta-Analysis of Physiological and Behavioral Responses of Parasitoid Wasps to Flowers of Individual Plant Species. Biol. Control 2015, 82, 96–103. [Google Scholar] [CrossRef]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The Impact of Adult Diet on Parasitoid Reproductive Performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Zaviezo, T.; Romero, A.; Calleja, F.; Calvo, C.; Osorio, R.; Casanoves, F.; Irles, P. Intraspecific Variation in Biocontrol Traits in Mastrus Ridens (Hymenoptera: Ichneumonidae) Laboratory Populations. BioControl 2021, 66, 475–485. [Google Scholar] [CrossRef]
- Mátray, S.; Herz, A. Flowering Plants Serve Nutritional Needs of Ascogaster Quadridentata (Hymenoptera: Braconidae), a Key Parasitoid of Codling Moth. Biol. Control 2022, 171, 104950. [Google Scholar] [CrossRef]
- Wäckers, F.L. A Comparison of Nectar- and Honeydew Sugars with Respect to Their Utilization by the Hymenopteran Parasitoid Cotesia Glomerata. J. Insect Physiol. 2001, 47, 1077–1084. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C.; Fitton, M.G.; Huddleston, T.; Dawah, H.A. Flower-Visiting by Hymenopteran Parasitoids. J. Nat. Hist. 1993, 27, 67–105. [Google Scholar] [CrossRef]
- Steppuhn, A.; Wäckers, F.L. HPLC Sugar Analysis Reveals the Nutritional State and the Feeding History of Parasitoids. Funct. Ecol. 2004, 18, 812–819. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E. Floral Resources Impact Longevity and Oviposition Rate of a Parasitoid in the Field. J. Anim. Ecol. 2008, 77, 565–572. [Google Scholar] [CrossRef]
- Tena, A.; Wäckers, F.L.; Heimpel, G.E.; Urbaneja, A.; Pekas, A. Parasitoid Nutritional Ecology in a Community Context: The Importance of Honeydew and Implications for Biological Control. Curr. Opin. Insect Sci. 2016, 14, 100–104. [Google Scholar] [CrossRef]
- Fernández de Bobadilla, M.; Ramírez, N.M.; Calvo-Agudo, M.; Dicke, M.; Tena, A. Honeydew Management to Promote Biological Control. Curr. Opin. Insect Sci. 2024, 61, 101151. [Google Scholar] [CrossRef] [PubMed]
- Chhagan, A.; Davis, V.A.; Hunt, S.; MacDonald, F.; Santos, K.; Richards, K.; Bell, V.A. Effect of Floral Resources and Mealybug Honeydew on the Longevity of the Parasitoid, Anagyrus Fusciventris. Biocontrol Sci. Technol. 2024, 34, 316–322. [Google Scholar] [CrossRef]
- Wäckers, F.L.; van Rijn, P.C.J.; Heimpel, G.E. Honeydew as a Food Source for Natural Enemies: Making the Best of a Bad Meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Strange-George, J.E.; Kulhanek, C.A.; Wäckers, F.L.; Heimpel, G.E. Sugar Feeding by the Aphid Parasitoid Binodoxys Communis: How Does Honeydew Compare with Other Sugar Sources? J. Insect Physiol. 2008, 54, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Tena, A.; Llácer, E.; Urbaneja, A. Biological Control of a Non-Honeydew Producer Mediated by a Distinct Hierarchy of Honeydew Quality. Biol. Control 2013, 67, 117–122. [Google Scholar] [CrossRef]
- Damien, M.; Llopis, S.; Desneux, N.; Van Baaren, J.; Le Lann, C. How Does Floral Nectar Quality Affect Life History Strategies in Parasitic Wasps? Entomol. Gen. 2020, 40, 147–156. [Google Scholar] [CrossRef]
- Lee, J.C.; Andow, D.A.; Heimpel, G.E. Influence of Floral Resources on Sugar Feeding and Nutrient Dynamics of a Parasitoid in the Field. Ecol. Entomol. 2006, 31, 470–480. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.; Pinto, D.M. Nectar-Providing Plants Enhance the Energetic State of Herbivores as Well as Their Parasitoids under Field Conditions. Ecol. Entomol. 2009, 34, 221–227. [Google Scholar] [CrossRef]
- Balmer, O.; Géneau, C.E.; Belz, E.; Weishaupt, B.; Förderer, G.; Moos, S.; Ditner, N.; Juric, I.; Luka, H. Wildflower Companion Plants Increase Pest Parasitation and Yield in Cabbage Fields: Experimental Demonstration and Call for Caution. Biol. Control 2014, 76, 19–27. [Google Scholar] [CrossRef]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-Country Evidence That Crop Diversification Promotes Ecological Intensification of Agriculture. Nat. Plants 2016, 2, 16014. [Google Scholar] [CrossRef]
- Berndt, L.A.; Wratten, S.D.; Hassan, P.G. Effects of Buckwheat Flowers on Leafroller (Lepidoptera: Tortricidae) Parasitoids in a New Zealand Vineyard. Agric. Entomol. 2002, 4, 39–45. [Google Scholar] [CrossRef]
- Miall, J.H.; Abram, P.K.; Cappuccino, N.; Bennett, A.M.R.; Fernández-Triana, J.L.; Gibson, G.A.P.; Mason, P.G. Addition of Nectar Sources Affects a Parasitoid Community without Improving Pest Suppression. J. Pest Sci. 2021, 94, 335–347. [Google Scholar] [CrossRef]
- Heimpel, G.E. Linking Parasitoid Nectar Feeding and Dispersal in Conservation Biological Control. Biol. Control 2019, 132, 36–41. [Google Scholar] [CrossRef]
- Lavandero, I.B.; Wratten, S.D.; Didham, R.K.; Gurr, G. Increasing Floral Diversity for Selective Enhancement of Biological Control Agents: A Double-Edged Sward? Basic Appl. Ecol. 2006, 7, 236–243. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Romeis, J.; Van Rijn, P. Nectar and Pollen Feeding by Insect Herbivores and Implications for Multitrophic Interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef]
- Carrié, R.J.G.; George, D.R.; Wäckers, F.L. Selection of Floral Resources to Optimise Conservation of Agriculturally-Functional Insect Groups. J. Insect Conserv. 2012, 16, 635–640. [Google Scholar] [CrossRef]
- Matray, S.; Herz, A. Do Floral Resources Affect Fitness of Adult Cydia Pomonella (Linnaeus 1758) (Lepidoptera: Tortricidae)? Bull. Entomol. Res. 2021, 111, 726–732. [Google Scholar] [CrossRef]
- Santos, L.A.O.; Botelho Costa, M.; Lavigne, C.; Fernandes, O.A.; Bischoff, A.; Franck, P. Influence of the Margin Vegetation on the Conservation of Aphid Biological Control in Apple Orchards. J. Insect Conserv. 2018, 22, 465–474. [Google Scholar] [CrossRef]
- Lee, J.C. Flourishing with Sugars-Following the Fate of Parasitoids in the Field. Curr. Opin. Insect Sci. 2024, 61, 101158. [Google Scholar] [CrossRef]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging Success of Parasitoid Wasps on Flowers: Interplay of Insect Morphology, Floral Architecture and Searching Behavior. Entomol. Exp. Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Vattala, H.D.; Wratten, S.D.; Phillips, C.B.; Wäckers, F.L. The Influence of Flower Morphology and Nectar Quality on the Longevity of a Parasitoid Biological Control Agent. Biol. Control 2006, 39, 179–185. [Google Scholar] [CrossRef]
- van Rijn, P.C.J.; Wäckers, F.L. Nectar Accessibility Determines Fitness, Flower Choice and Abundance of Hoverflies That Provide Natural Pest Control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Colazza, S.; Peri, E.; Cusumano, A. Chemical Ecology of Floral Resources in Conservation Biological Control. Annu. Rev. Entomol. 2023, 68, 13–29. [Google Scholar] [CrossRef]
- Géneau, C.E.; Wäckers, F.L.; Luka, H.; Daniel, C.; Balmer, O. Selective Flowers to Enhance Biological Control of Cabbage Pests by Parasitoids. Basic Appl. Ecol. 2012, 13, 85–93. [Google Scholar] [CrossRef]
- Arnó, J.; Oveja, M.F.; Gabarra, R. Selection of Flowering Plants to Enhance the Biological Control of Tuta Absoluta Using Parasitoids. Biol. Control 2018, 122, 41–50. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Wildflower Plantings Enhance the Abundance of Natural Enemies and Their Services in Adjacent Blueberry Fields. Biol. Control 2015, 91, 94–103. [Google Scholar] [CrossRef]
- Pandey, S.; Rahman, A.; Gurr, G.M. Australian Native Flowering Plants Enhance the Longevity of Three Parasitoids of Brassica Pests. Entomol. Exp. Appl. 2018, 166, 265–276. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Alvarez-Baca, J.K.; Alfaro-Tapia, A.; Gontijo, L.; Lavandero, B. Manipulation of Agricultural Habitats to Improve Conservation Biological Control in South America. Neotrop. Entomol. 2019, 48, 875–898. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Artigas, J.N. Entomología Económica. Insectos de Interés Agrícola, Forestal, Médico y Veterinario (Nativos, Introducidos y Susceptibles de Ser Introducidos); Universidad de Concepción: Concepción, Chile, 1994; ISBN 956-227-101-3. [Google Scholar]
- Kumar, S.; Neven, L.G.; Zhu, H.; Zhang, R. Assessing the Global Risk of Establishment of Cydia Pomonella (Lepidoptera: Tortricidae) Using CLIMEX and MaxEnt Niche Models. J. Econ. Entomol. 2015, 108, 1708–1719. [Google Scholar] [CrossRef]
- CABI. Cydia Pomonella (Codling Moth). Available online: http://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.11396 (accessed on 3 April 2025).
- Mills, N. Selecting Effective Parasitoids for Biological Control Introductions: Codling Moth as a Case Study. Biol. Control 2005, 34, 274–282. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Mills, N.J. Clutch Size Decisions of a Gregarious Parasitoid under Laboratory and Field Conditions. Anim. Behav. 2003, 66, 1119–1128. [Google Scholar] [CrossRef]
- Devotto, L.; Valle, C.; Ceballos, R.; Gerding, M. Biology of Mastrus Ridibundus (Gravenhorst), a Potential Biological Control Agent for Area-Wide Management of Cydia Pomonella (Linneaus) (Lepidoptera: Tortricidae). J. Appl. Entomol. 2010, 134, 243–250. [Google Scholar] [CrossRef]
- Charles, J.G.; Dugdale, J.S. Non-Target Species Selection for Host-Range Testing of Mastrus Ridens. N. Z. Entomol. 2011, 34, 45–51. [Google Scholar] [CrossRef]
- Retamal, R.; Zaviezo, T.; Malausa, T.; Fauvergue, X.; Le Goff, I.; Toleubayev, K. Genetic Analyses and Occurrence of Diploid Males in Field and Laboratory Populations of Mastrus Ridens (Hymenoptera: Ichneumonidae), a Parasitoid of the Codling Moth. Biol. Control 2016, 101, 69–77. [Google Scholar] [CrossRef]
- Zaviezo, T.; Retamal, R.; Urvois, T.; Fauvergue, X.; Blin, A.; Malausa, T. Effects of Inbreeding on a Gregarious Parasitoid Wasp with Complementary Sex Determination. Evol. Appl. 2018, 11, 243–253. [Google Scholar] [CrossRef]
- Sandanayaka, W.R.M.; Jenkins, H.K.; Davis, V.A.; Page-Weir, N.E.M.; Chhagan, A.; Blin, A.; Zaviezo, T. Assessment of Fitness Traits of Pure and Mixed Crosses of Mastrus Ridens Populations to Improve Classical Biological Control of Codling Moth. Biol. Control 2022, 169, 104899. [Google Scholar] [CrossRef]
- Reiche, K. Estudios críticos sobre la flora de Chile. An. De La Univ. De Chile 1896, 1, 215–265. [Google Scholar]
- Reiche, K. Estudios críticos sobre la flora de Chile. An. De La Univ. De Chile 1902, 3, 90–91, 258–380. [Google Scholar]
- Reiche, K. Estudios críticos sobre la flora de Chile. An. De La Univ. De Chile 1905, 4, 1–459. [Google Scholar]
- Reiche, K. Estudios críticos sobre la flora de Chile. An. De La Univ. De Chile 1910, 5, 243–245, 272–306, 312–317. [Google Scholar]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de las plantas vasculares de Chile. Gayana Botánica 2018, 75, 1–430. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; 2025. Available online: https://powo.science.kew.org/ (accessed on 5 February 2025).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Morrant, D.S.; Schumann, R.; Petit, S. Field Methods for Sampling and Storing Nectar from Flowers with Low Nectar Volumes. Ann. Bot. 2009, 103, 533–542. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar/ (accessed on 22 June 2025).
- Tew, N.E.; Memmott, J.; Vaughan, I.P.; Bird, S.; Stone, G.N.; Potts, S.G.; Baldock, K.C.R. Quantifying Nectar Production by Flowering Plants in Urban and Rural Landscapes. J. Ecol. 2021, 109, 1747–1757. [Google Scholar] [CrossRef]
- Power, E.F.; Stabler, D.; Borland, A.M.; Barnes, J.; Wright, G.A. Analysis of Nectar from Low-Volume Flowers: A Comparison of Collection Methods for Free Amino Acids. Methods Ecol. Evol. 2018, 9, 734–743. [Google Scholar] [CrossRef]
- Petanidou, T.; Van Laere, A.J.; Smets, E. Change in Floral Nectar Components from Fresh to Senescent Flowers of Capparis Spinosa (Capparidaceae), a Nocturnally Flowering Mediterranean Shrub. Plant Syst. Evol. 1996, 199, 79–92. [Google Scholar] [CrossRef]
- Wist, T.J.; Davis, A.R. Floral Nectar Production and Nectary Anatomy and Ultrastructure of Echinacea Purpurea (Asteraceae). Ann. Bot. 2006, 97, 177–193. [Google Scholar] [CrossRef]
- Díaz-Forestier, J.; Gómez, M.; Montenegro, G. Nectar Volume and Floral Entomofauna as a Tool for the Implementation of Sustainable Apicultural Management Plans in Quillaja Saponaria Mol. Agrofor. Syst. 2009, 76, 149–162. [Google Scholar] [CrossRef]
- Lloyd, S.; Ayre, D.J.; Whelan, R.J. A Rapid and Accurate Visual Assessment of Nectar Production Can Reveal Patterns of Temporal Variation in Banksia Ericifolia (Proteaceae). Aust. J. Bot. 2002, 50, 595–600. [Google Scholar] [CrossRef]
- Alignier, A.; Lenestour, N.; Jeavons, E.; van Baaren, J.; Aviron, S.; Uroy, L.; Ricono, C.; Le Lann, C. Floral Resource Maps: A Tool to Explain Flower-Visiting Insect Abundance at Multiple Spatial Scales. Landsc. Ecol. 2023, 38, 1511–1525. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A Global Database of Plant Traits. Glob. Change Biol. 2011, 17, 2905. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Matteson, K.C.; Minor, E.S. Evaluating the Dependence of Urban Pollinators on Ornamental, Non-Native, and ‘Weedy’ Floral Resources. Urban Ecosyst. 2019, 22, 293–302. [Google Scholar] [CrossRef]
- Ammann, L.; Bosem-Baillod, A.; Eckerter, P.W.; Entling, M.H.; Albrecht, M.; Herzog, F. Comparing Floral Resource Maps and Land Cover Maps to Predict Predators and Aphid Suppression on Field Bean. Landsc. Ecol. 2022, 37, 431–441. [Google Scholar] [CrossRef]
- Wäckers, F.L. Assessing the Suitability of Flowering Herbs as Parasitoid Food Sources: Flower Attractiveness and Nectar Accessibility. Biol. Control 2004, 29, 307–314. [Google Scholar] [CrossRef]
- Koptur, S. Nectar as Fuel for Plant Protectors. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, UK, 2005; pp. 75–108. ISBN 9780511542220. [Google Scholar]
- Irvin, N.A.; Hoddle, M.S.; Castle, S.J. The Effect of Resource Provisioning and Sugar Composition of Foods on Longevity of Three Gonatocerus Spp., Egg Parasitoids of Homalodisca Vitripennis. Biol. Control 2007, 40, 69–79. [Google Scholar] [CrossRef]
- Wade, M.R.; Wratten, S.D. Excised or Intact Inflorescences? Methodological Effects on Parasitoid Wasp Longevity. Biol. Control 2007, 40, 347–354. [Google Scholar] [CrossRef]
- Nafziger, T.D.; Fadamiro, H.Y. Suitability of Some Farmscaping Plants as Nectar Sources for the Parasitoid Wasp, Microplitis Croceipes (Hymenoptera: Braconidae): Effects on Longevity and Body Nutrients. Biol. Control 2011, 56, 225–229. [Google Scholar] [CrossRef]
- Balzan, M.V.; Wäckers, F.L. Flowers to Selectively Enhance the Fitness of a Host-Feeding Parasitoid: Adult Feeding by Tuta Absoluta and Its Parasitoid Necremnus Artynes. Biol. Control 2013, 67, 21–31. [Google Scholar] [CrossRef]
- Ricou, C.; Schneller, C.; Amiaud, B.; Plantureux, S.; Bockstaller, C. A Vegetation-Based Indicator to Assess the Pollination Value of Field Margin Flora. Ecol. Indic. 2014, 45, 320–331. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.L.; Kaufman, L.V.; Larraz, V.; van Lenteren, J.C. Nectar Exploitation by Herbivores and Their Parasitoids Is a Function of Flower Species and Relative Humidity. Biol. Control 2009, 50, 299–306. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E. Impact of Flowering Buckwheat on Lepidopteran Cabbage Pests and Their Parasitoids at Two Spatial Scales. Biol. Control 2005, 34, 290–301. [Google Scholar] [CrossRef]
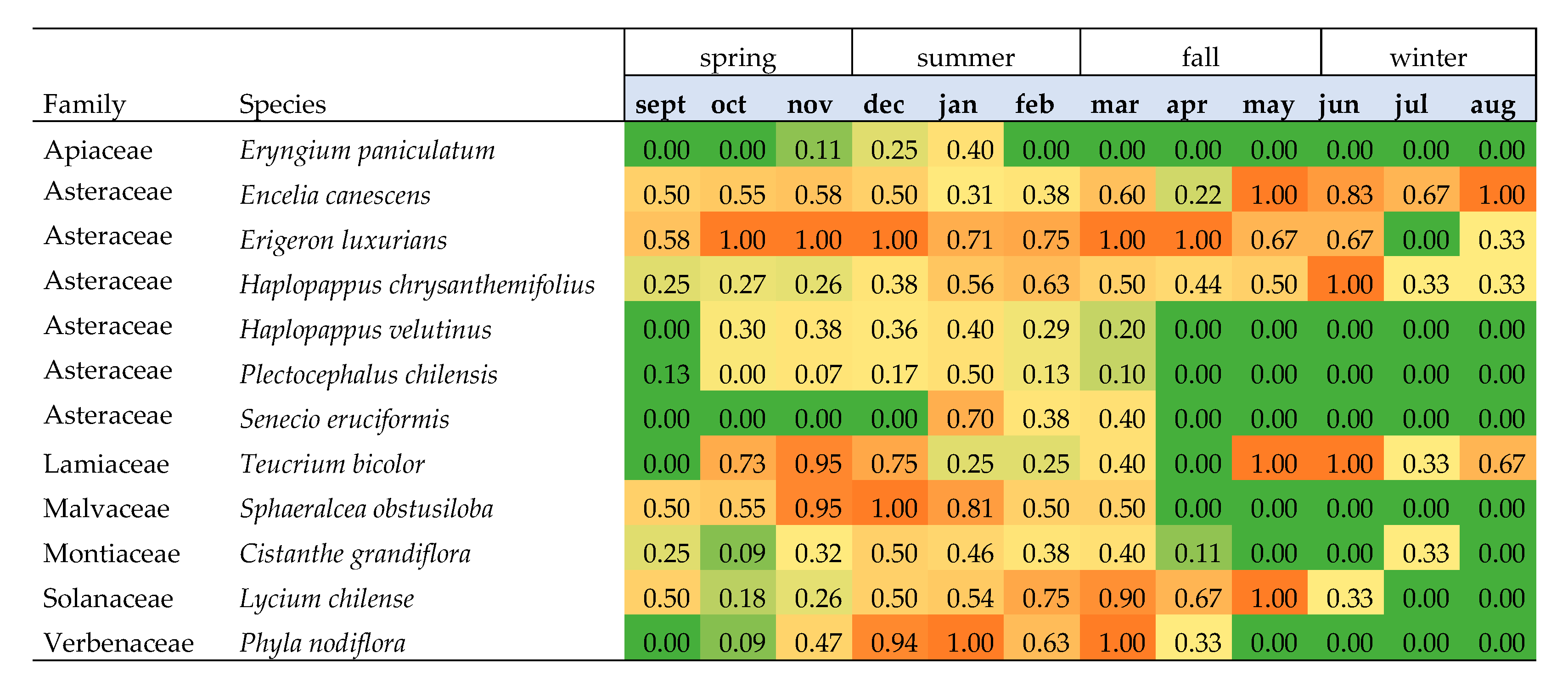
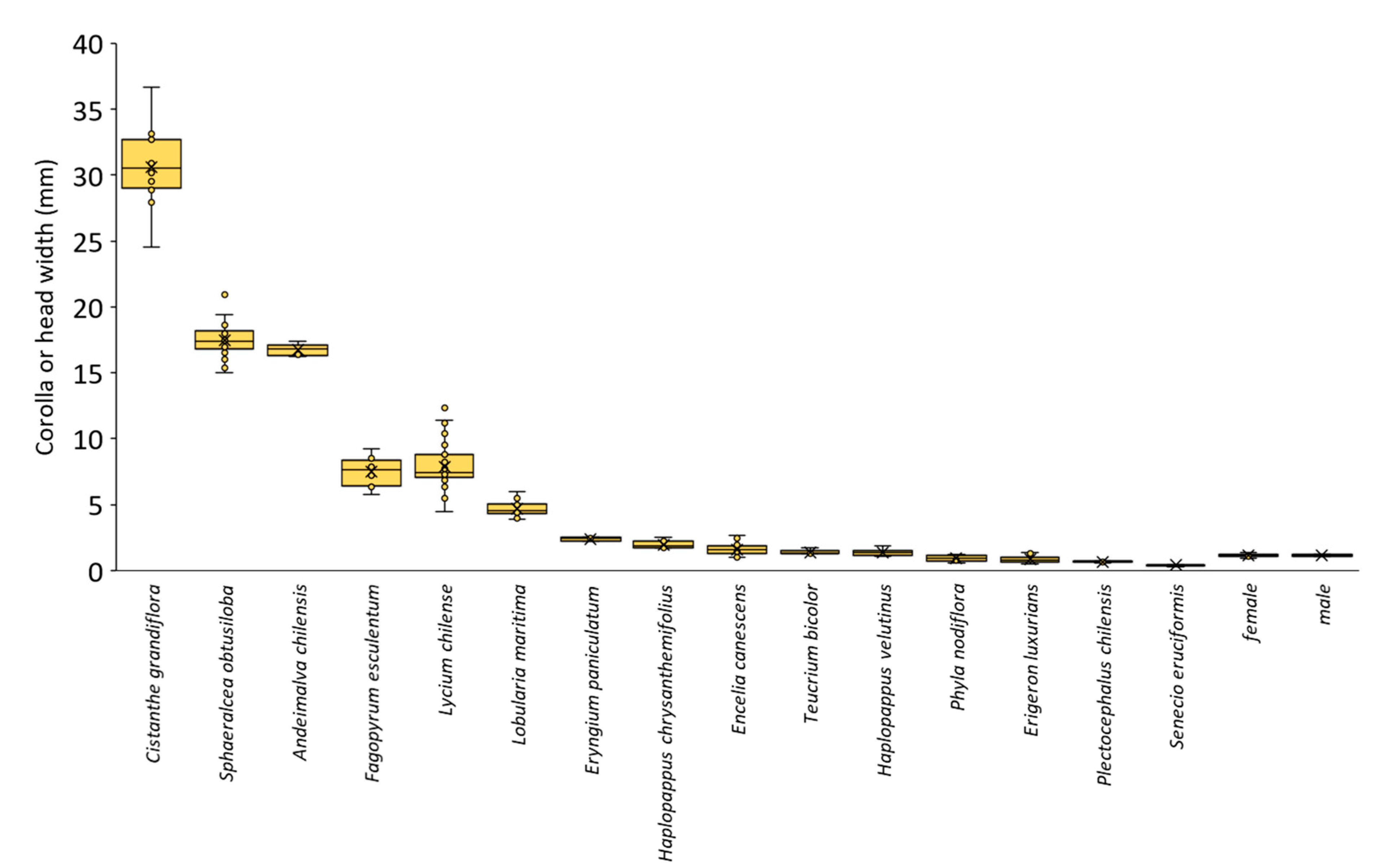


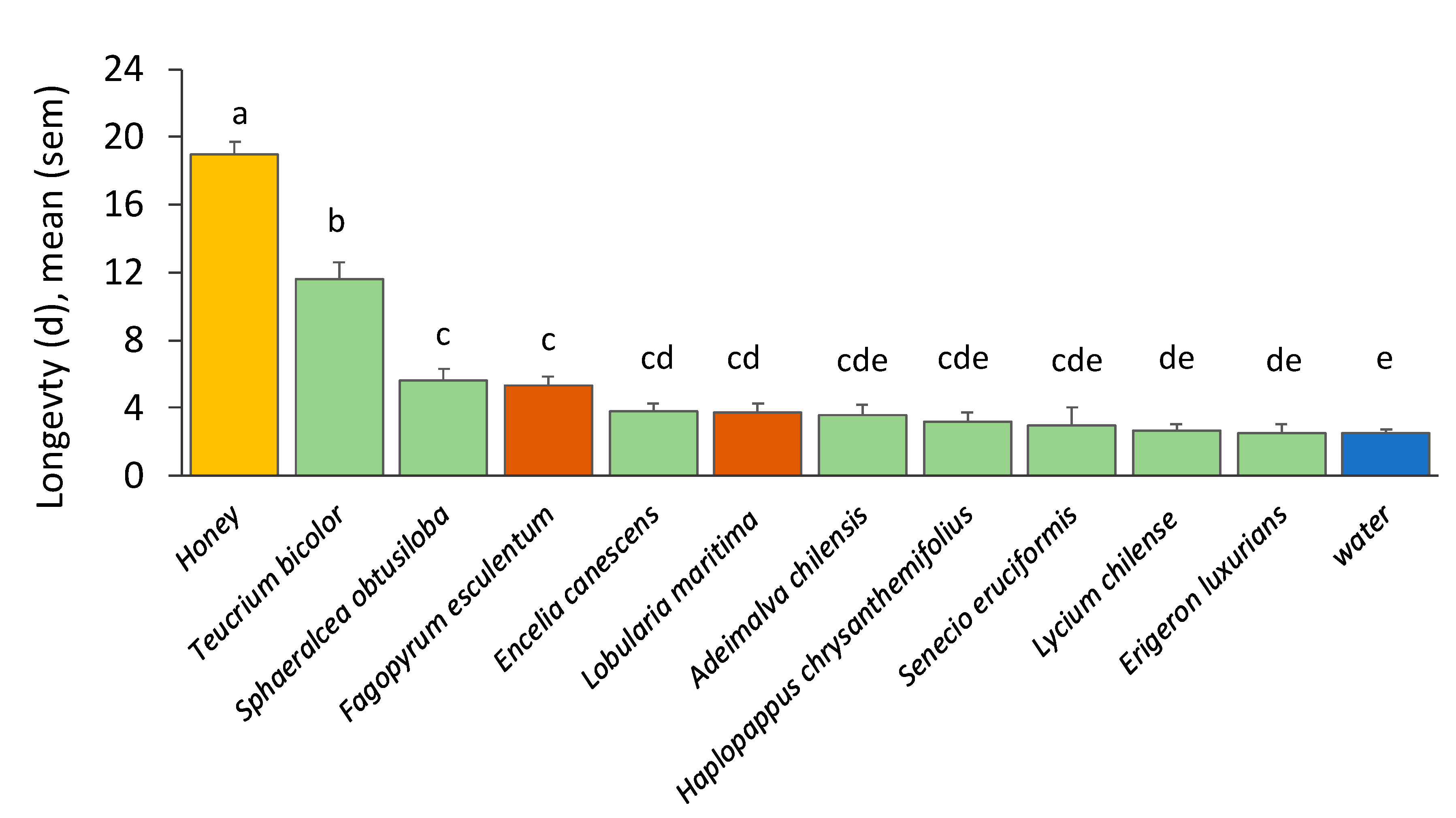
| Family/Scientific Name | Life Form | Flower Arrangement | Flower Type | Biogeographic Origin |
|---|---|---|---|---|
| Apiaceae | ||||
| Eryngium paniculatum Cav. & Dombey ex F. Delaroche | Herb | Dense inflorescence | Tubular | Native (Chile, Argentina) |
| Asteraceae | ||||
| Encelia canescens Lam. | Subshrub | Dense inflorescence | Ligulate and tubular | Native (Bolivia, Chile, Galápagos, Peru) |
| Erigeron luxurians (Skottsb.) Solbrig | Subshrub | Dense inflorescence | Ligulate and tubular | Endemic (Central Chile) |
| Haplopappus velutinus J. Remy | Shrub | Dense inflorescence | Ligulate and tubular | Native (Chile, Argentina) |
| Haplopappus chrysanthemifolius (Less.) DC | Shrub | Dense inflorescence | Ligulate and tubular | Endemic (Central Chile) |
| Plectocephalus chilensis (Bertero ex Hook. & Arn.) G. Don | Subshrub | Dense inflorescence | Ligulate and tubular | Endemic (Northern and Central Chile) |
| Senecio eruciformis J. Remy | Subshrub | Dense inflorescence | Ligulate and tubular | Native (Chile, Argentina) |
| Lamiaceae | ||||
| Teucrium bicolor Sm. | Subshrub | Loose inflorescence | Bilabiate | Endemic (Central Chile) |
| Malvaceae | ||||
| Sphaeralcea obtusiloba (Hook.) G. Don | Subshrub | Loose inflorescence | Campanulate | Endemic (Central Chile) |
| Andeimalva chilensis (Gay) J.A. Tate | Shrub | Loose inflorescence | Campanulate | Endemic (Northern and Central Chile) |
| Montiaceae | ||||
| Cistanthe grandiflora (Lindl.) Schltdl. | Herb | Solitary | Campanulate | Endemic (Central Chile) |
| Solanaceae | ||||
| Lycium chilense var. confertifolium (Miers) F.A. Barkley | Shrub | Solitary | Campanulate | Native (Argentina, Chile) |
| Verbenaceae | ||||
| Phyla nodiflora var. minor (Gillies & Hook.) N.O’Leary & Múlgura | Subshrub | Dense inflorescence | Ligulate and tubular | Native (Argentina, Bolivia, Brazil, Chile, Ecuador, Paraguay, Peru, Uruguay) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaviezo, T.; Muñoz, A.E.; Bueno, E. Availability, Accessibility, and Suitability of Native Flowers from Central Chile to Mastrus ridens, a Parasitoid of Codling Moth. Insects 2025, 16, 665. https://doi.org/10.3390/insects16070665
Zaviezo T, Muñoz AE, Bueno E. Availability, Accessibility, and Suitability of Native Flowers from Central Chile to Mastrus ridens, a Parasitoid of Codling Moth. Insects. 2025; 16(7):665. https://doi.org/10.3390/insects16070665
Chicago/Turabian StyleZaviezo, Tania, Alejandra E. Muñoz, and Erick Bueno. 2025. "Availability, Accessibility, and Suitability of Native Flowers from Central Chile to Mastrus ridens, a Parasitoid of Codling Moth" Insects 16, no. 7: 665. https://doi.org/10.3390/insects16070665
APA StyleZaviezo, T., Muñoz, A. E., & Bueno, E. (2025). Availability, Accessibility, and Suitability of Native Flowers from Central Chile to Mastrus ridens, a Parasitoid of Codling Moth. Insects, 16(7), 665. https://doi.org/10.3390/insects16070665






