Immune Gene Expression and Locomotor Activity in Response to Vairimorpha ceranae Infection Across Five Honey Bee Subspecies
Simple Summary
Abstract
1. Introduction
1.1. Vairimorpha (Formerly Nosema) Disease
1.2. Honey Bee Subpopulations
1.3. Honey Bee Locomotion
1.4. Honey Bee Immunity
2. Materials and Methods
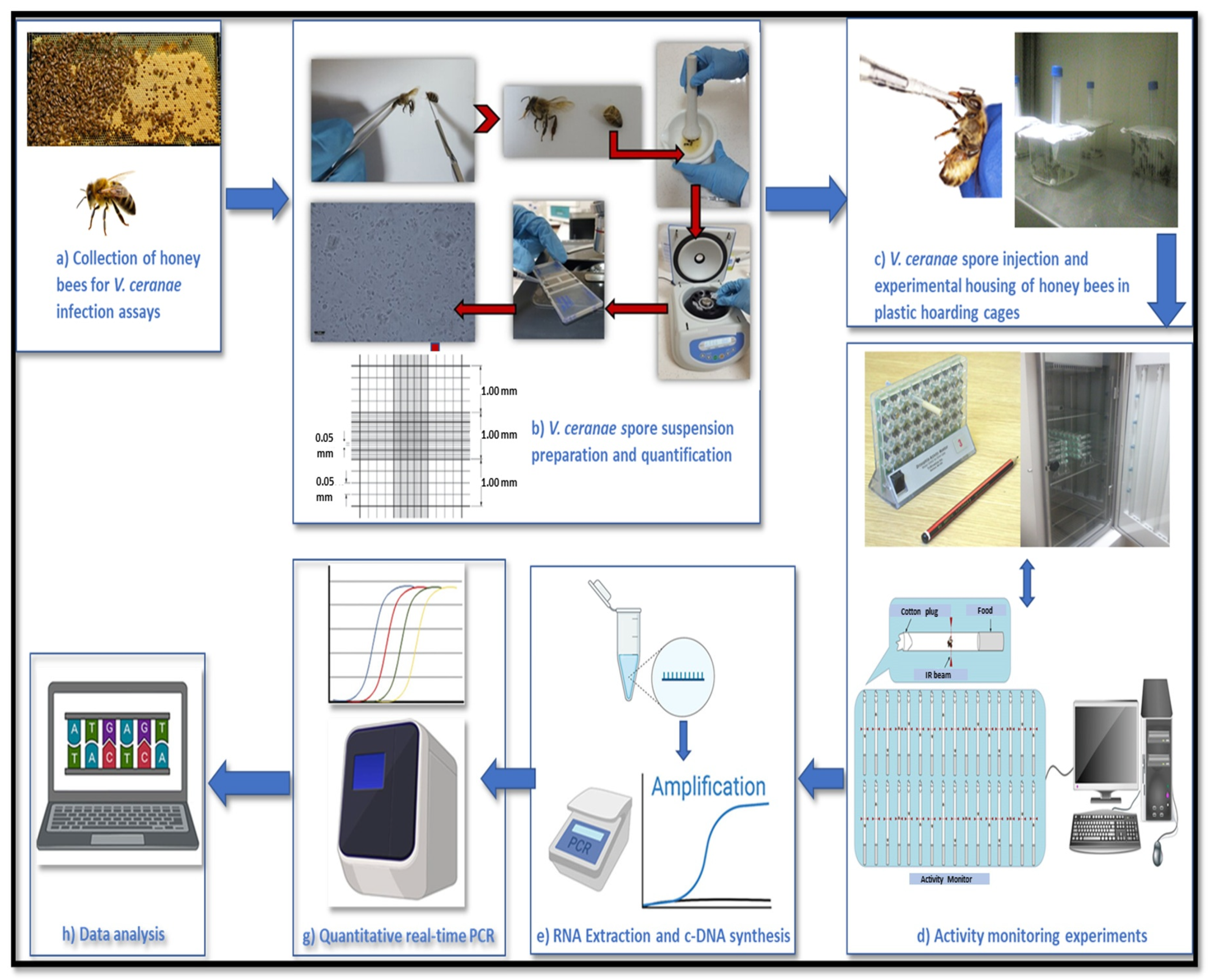
2.1. Collection of Worker Bees for V. ceranae Infection Assays
2.2. Vairimorpha ceranae Spore Suspension Preparation and Quantification
2.3. Activity Monitoring Experiments
2.4. RNA Extraction, Quantification, cDNA-Synthesis, and qPCR
2.5. Statistical Analysis
3. Results
3.1. Immune Gene Expressions
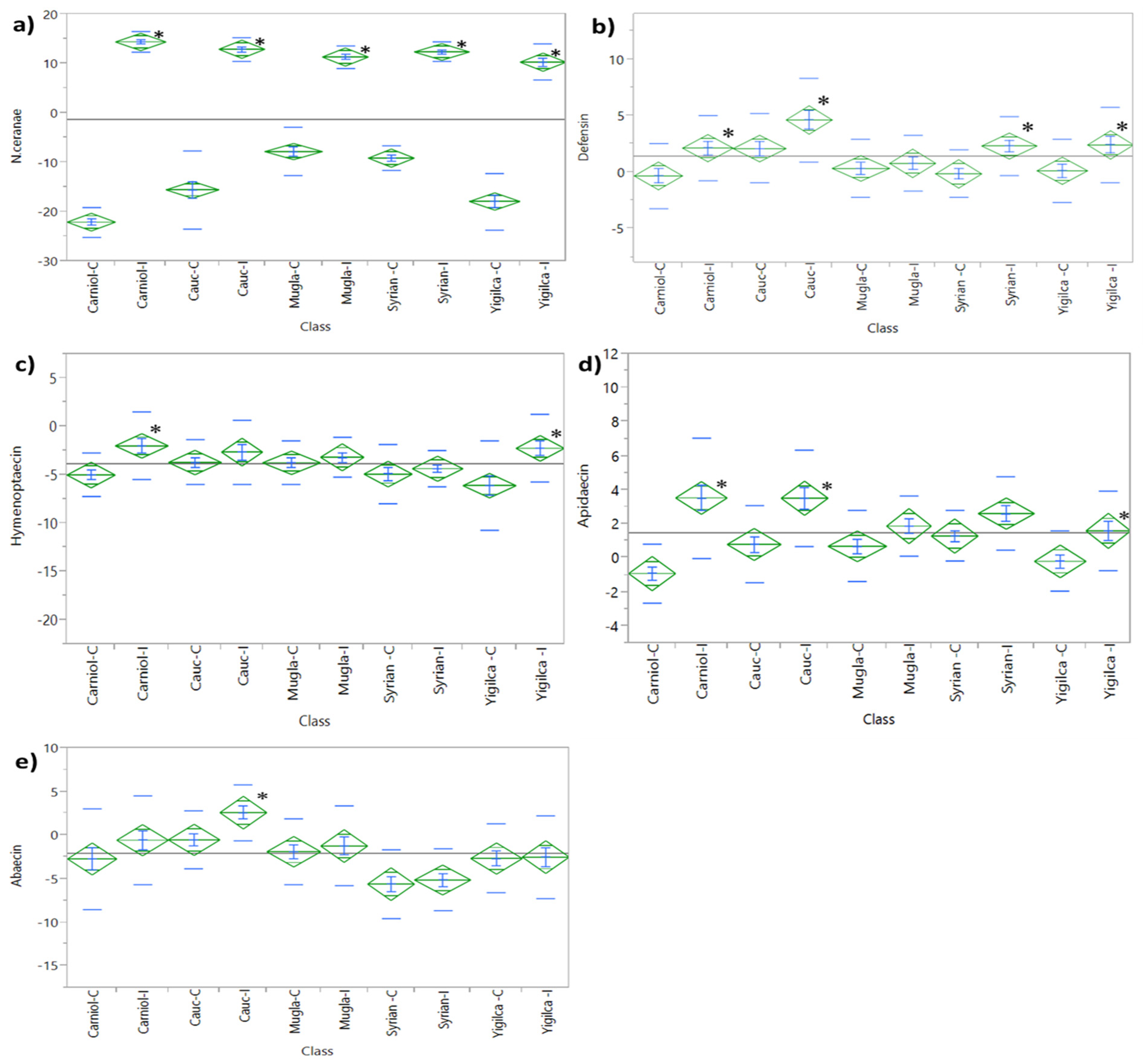
3.1.1. Defensin Expression
3.1.2. Hymenoptaecin Expression
3.1.3. Apidaecin Expression
3.1.4. Abaecin Expression
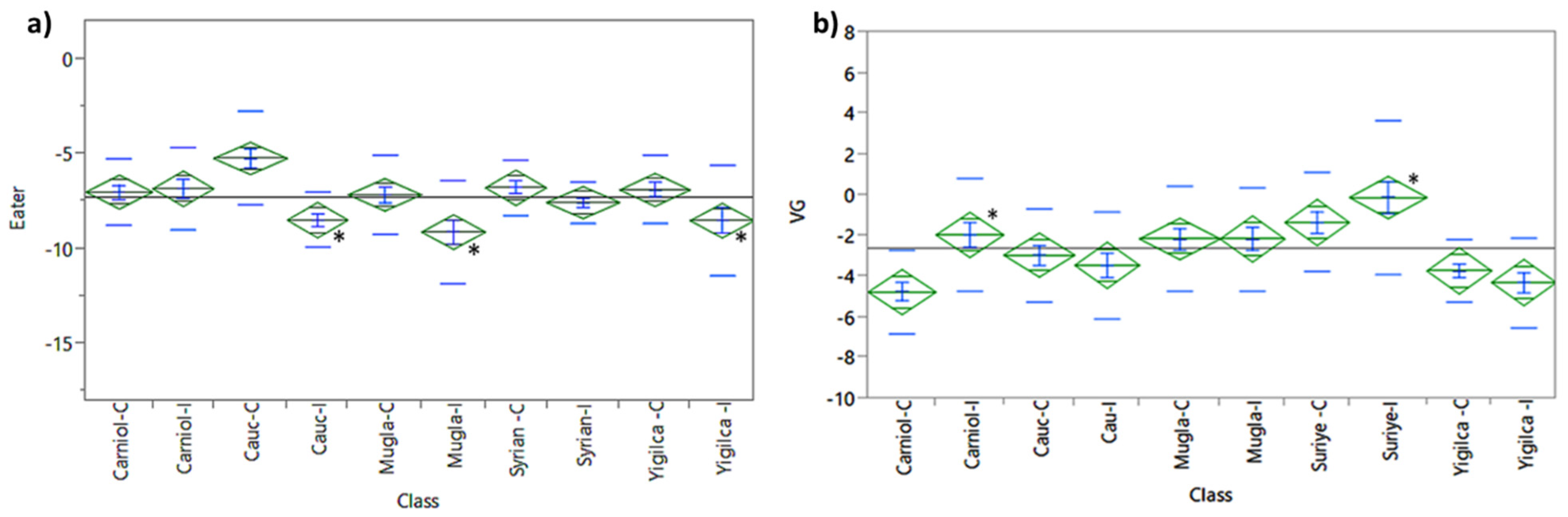
3.1.5. Eater Expression
3.1.6. Vitellogenin Expression
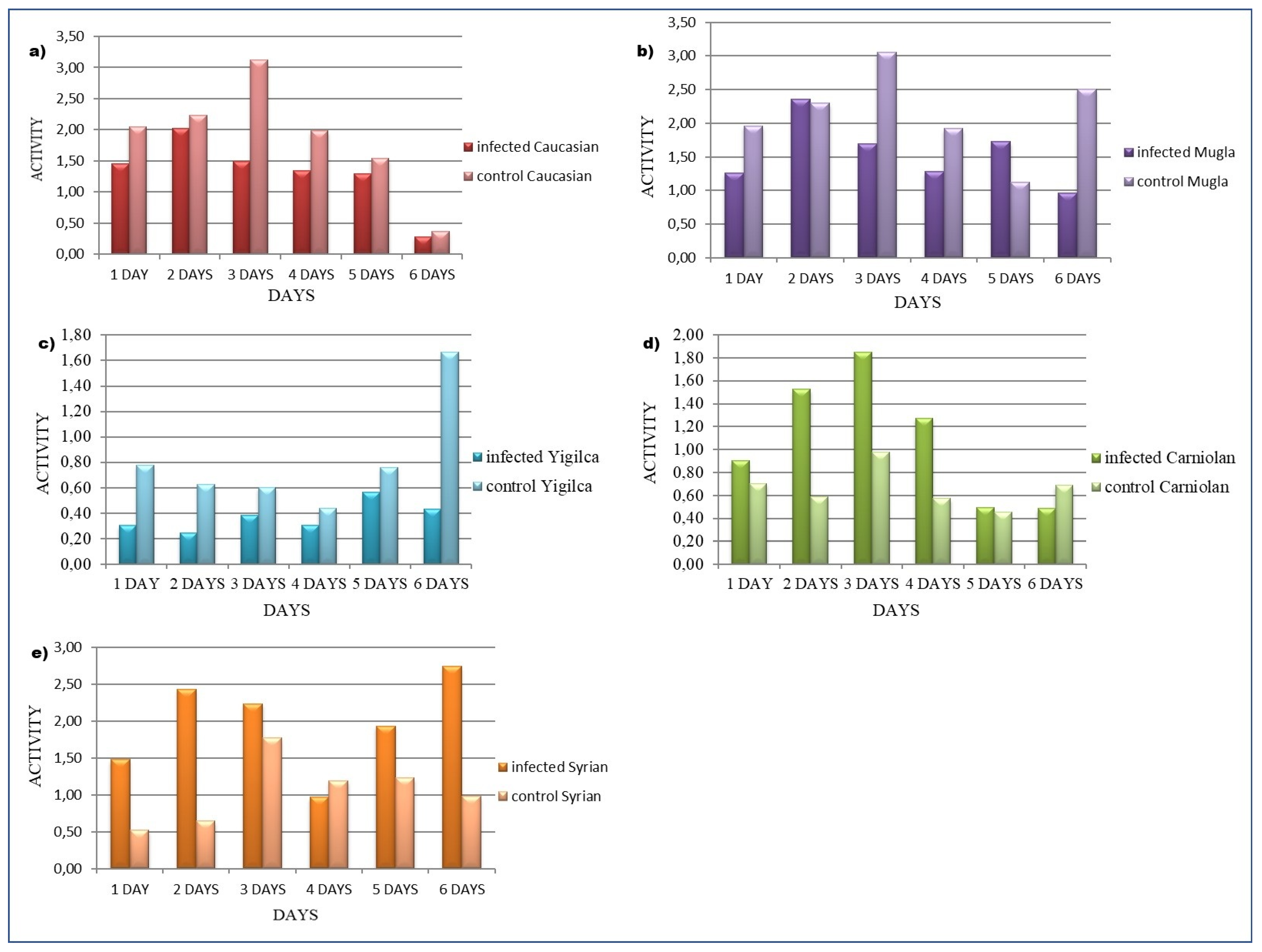
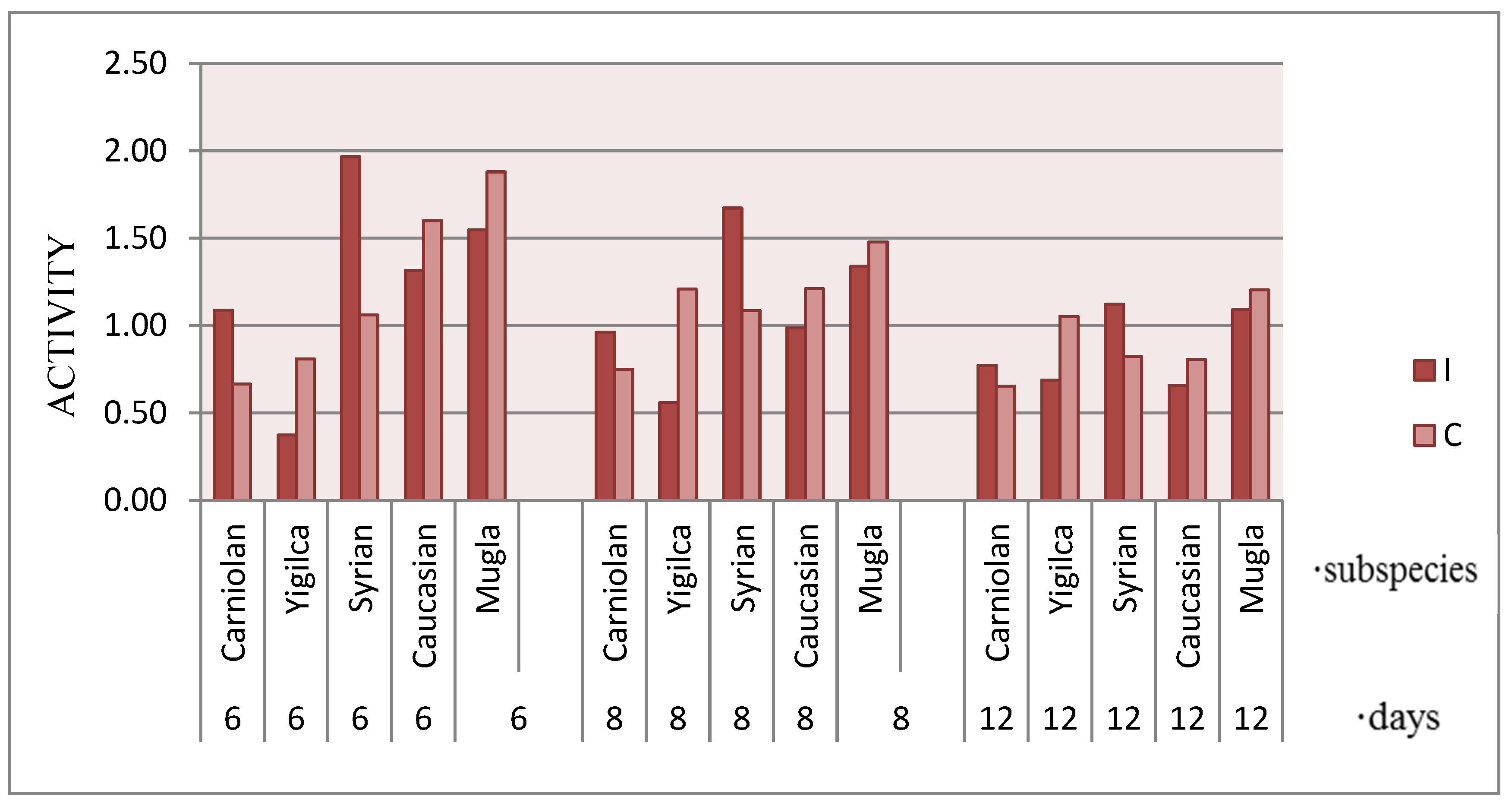
3.2. Honey Bee Locomotion
4. Discussion
4.1. Immune Gene Responses
4.2. Locomotion
4.3. Practical Implications for Beekeeping and Breeding Programs
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, L.; Ball, B. Honey Bee Pathology, 2nd ed.; Academic Press: London, UK, 1991. [Google Scholar]
- Corradi, N.; Keeling, P.J. Microsporidia: A journey through radical taxonomical revisions. Fungal Biol. Rev. 2009, 23, 1–8. [Google Scholar] [CrossRef]
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef]
- Fries, I.; Martin, R.; Meana, A.; Garcia-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Higes, M.; Martin-Hernandez, R.; Meana, A.; Cougoule, N.; Faucon, J.P. Presence of Nosema ceranae in French honey bee colonies. J. Apicult. Res. 2007, 46, 127–128. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, J.H.; Chen, Y.W.; Wang, C.H. A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 2007, 38, 30–37. [Google Scholar] [CrossRef]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef]
- Williams, G.R.; Shafer, A.B.A.; Rogers, R.E.L.; Shutler, D.; Stewart, D.T. First detection of Nosema ceranae, a microsporidian parasite of European honey bees (Apis mellifera), in Canada and central USA. J. Invertebr. Pathol. 2008, 97, 189–192. [Google Scholar] [CrossRef]
- Invernizzi, C.; Abud, C.; Tomasco, I.H.; Harriet, J.; Ramallo, G.; Campá, J.; Katz, H.; Gardiol, G.; Mendoza, Y. Presence of Nosema ceranae in honeybees (Apis mellifera) in Uruguay. J. Invertebr. Pathol. 2009, 101, 150–153. [Google Scholar] [CrossRef]
- Goodwin, M.; Houten, A.T.; Perry, J.; Blackman, R. Cost benefit analysis of using fumagillin to treat Nosema. N. Z. Beekeep. 1990, 208, 11–12. [Google Scholar]
- Malone, L.A.; Giacon, H.A.; Newton, M.R. Comparison of the responses of some New Zealand and Australian honey bees (Apis mellifera L) to Nosema apis Z. Apidologie 1995, 26, 495–502. [Google Scholar] [CrossRef]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and behavioral changes in honey bees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef]
- Naug, D.; Gibbs, A. Behavioural changes mediated by hunger in honey bees infected with Nosema ceranae. Apidologie 2009, 40, 595–599. [Google Scholar] [CrossRef]
- Higes, M.; Garcia-Palencia, P.; Martin-Hernandez, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- Buczeck, K. Honey bee colony collapse disorder (CCD). Ann. Umcs Med. Vet. 2009, 64, 1–6. [Google Scholar] [CrossRef][Green Version]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.; González-Porto, A.; Barrios, L.; Jesús del Nozal, M.; Bernal, J.; Jiménez, J.; García Palencia, P.; et al. How natural infection by Nosema ceranae causes honey bee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Kandemir, I.; Kence, M.; Kence, A. Genetic and morphometric variation in honeybee (Apis mellifera L.) populations of Turkey. Apidologie 2000, 31, 343–356. [Google Scholar] [CrossRef]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De La Rúa, P.; et al. A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apicult. Res. 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Fuchikawa, T.; Shimizu, I. Circadian rhythm of locomotor activity in the Japanese honeybee, Apis cerana japonica. Physiol. Entomol. 2007, 32, 73–80. [Google Scholar] [CrossRef]
- Bloch, G.; Toma, D.P.; Robinson, G.E. Behavioral rhythmicity, age, division of labor and period expression in the honey bee brain. J. Biol. Rhythms 2001, 16, 444–456. [Google Scholar] [CrossRef]
- Bloch, G.; Robinson, G. Chronobiology—Reversal of honeybee behavioural rhythms. Nature 2001, 410, 1048. [Google Scholar] [CrossRef]
- Moore, D. Honey bee circadian clocks: Behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 2001, 47, 843–857. [Google Scholar] [CrossRef]
- Meshi, A.; Bloch, G. Monitoring circadian rhythms of individual honey bees in a social environment reveals social influences on postembryonic ontogeny of activity rhythms. J. Biol. Rhythms 2007, 22, 343–355. [Google Scholar] [CrossRef]
- Shemesh, Y.; Cohen, M.; Bloch, G. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 2007, 21, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Toma, D.P.; Bloch, G.; Moore, D.; Robinson, G.E. Changes in period mRNA levels in the brain and division of labor in honey bee colonies. Proc. Natl. Acad. Sci. USA 2000, 97, 6914–6919. [Google Scholar] [CrossRef]
- Bloch, G. The social clock of the honeybee. J. Biol. Rhythms 2010, 25, 307–317. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Bloch, G. Circadian Rhythms and Sleep in Honey Bees. In Honeybee Neurobiology and Behavior: A Tribute to Randolf Menzel; Galizia, C.G., Eisenhardt, D., Giurfa, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 31–46. [Google Scholar] [CrossRef]
- Ruiz Díaz, J.D.; Bajwa, S.T.; Vanani, N.; Bajwa, T.A.; Cavanaugh, D.J. Slowpoke functions in circadian output cells to regulate rest rhythms. PLoS ONE 2021, 16, e0249215. [Google Scholar] [CrossRef]
- Feitoza, T.S.; Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lounibos, L.P.; Lima-Camara, T.N. Interspecific Mating Effects on Locomotor Activity Rhythms and Refractoriness of Aedes albopictus (Diptera: Culicidae) Females. Insects 2020, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Sitaraman, D.; Vecsey, C.G.; Koochagian, C. Activity Monitoring for Analysis of Sleep in Drosophila melanogaster. Cold Spring Harb. Protoc. 2024, 2024, pdb.top108095. [Google Scholar] [CrossRef]
- Pena Porter, M.; Roman, G.; Vecsey, C. Analysis of Positional Preference in Drosophila Using Multibeam Activity Monitors. Cold Spring Harb. Protoc. 2024, 2024, pdb.prot108181. [Google Scholar] [CrossRef] [PubMed]
- Giannoni-Guzmán, M.A.; Avalos, A.; Perez, J.M.; Loperena, E.J.O.; Kayım, M.; Medina, J.A.; Massey, S.E.; Kence, M.; Kence, A.; Giray, T.; et al. Measuring individual locomotor rhythms in honey bees, paper wasps and other similar-sized insects. J. Exp. Biol. 2014, 217, 1307–1315. [Google Scholar]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K.; Riessberger-Galle, U. Honey bee age-dependent resistance against American foulbrood. Apidologie 2001, 32, 91–103. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect haemocytes and their role in immunity. Insect Biochem. Mol. Bio. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003, 254, 1014–1018. [Google Scholar] [CrossRef]
- Osta, M.A.; Christophides, G.K.; Vlachou, D.; Kafatos, F.C. Innate immunity in the malaria vector Anopheles gambiae: Comparative and functional genomics. J. Exp. Biol. 2004, 207, 2551–2563. [Google Scholar] [CrossRef]
- Decker, H.; Jaenicke, E. Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev. Comp. Immunol. 2004, 28, 673–687. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Schonbaum, C.P.; Murtha, M.T.; Cavener, D.R. Developmental expression of the glucose dehydrogenase gene in Drosophila melanogaster. Genetics 1990, 124, 873–880. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 2632013271. [Google Scholar] [CrossRef]
- Ertürk-Hasdemir, D.; Silverman, N. Eater: A big bite into phagocytosis. Cell 2005, 123, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Kocks, C.; Cho, J.H.; Nehme, N.; Ulvila, J.; Pearson, A.M.; Meister, M.; Strom, C.; Conto, S.L.; Hetru, C.; Stuart, L.M.; et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 2005, 123, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D. Beepath: An ordered quantitative-PCR array for exploring honey bee immunity and disease. J. Invertebr. Pathol. 2006, 93, 135–139. [Google Scholar] [CrossRef]
- Casteels, P.; Ampe, C.; Riviere, L.; Van Damme, J.; Elicone, C.; Fleming, M.; Jacobs, F.; Tempst, P. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur. J. Biochem. 1990, 187, 381–386. [Google Scholar] [CrossRef]
- Casteels, P.; Ampe, C.; Jacobs, F.; Tempst, P. Functional and chemical of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J. Biol. Chem. 1993, 268, 7044–7054. [Google Scholar] [CrossRef] [PubMed]
- Casteels-Jonsson, K.; Zhang, W.; Capaci, T.; Casteels, P.; Tempst, P. Acute transcriptional response of the honeybee peptide--antibiotics gene repertoire posttranslational conversion of the precursor structures. J. Biol. Chem. 1994, 269, 28569–28575. [Google Scholar] [CrossRef]
- Amdam, G.V.; Omholt, W. The hive bee to forager transition in honeybee colonies: The double repressor hypothesis. J. Theor. Biol. 2003, 223, 451–464. [Google Scholar] [CrossRef]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E.; Amdam, G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007, 5, 673–677. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Reichhart, J.M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kiran, F.; Sevin, S.; Ceylan, A. Biocontrol potential of Apilactobacillus kunkeei EIR/BG-1 against infectious diseases in honey bees (Apis mellifera L.). Vet. Res. Commun. 2022, 47, 753–765. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.S.; Chen, Y.P.; Budge, G.; Cornman, R.S.; De la Rua, P.; de Miranda, J.R.; Foret, S.; Foster, L.; Gauthier, L. Standard methods for molecular research in Apis mellifera. J. Apicult. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.E.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Evans, J.D.; Lopez, D.L. Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2004, 97, 752–756. [Google Scholar] [CrossRef]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Chaimanee, V.; Pettis, J.S.; Chen, Y.; Evans, J.D.; Khongphinitbunjong, K.; Chantawannakul, P. Susceptibility of four different honey bee species to Nosema ceranae. Vet. Parasitol. 2013, 193, 260–265. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.P.; Wang, R.W.; Cheng, S.; Evans, J.D. Host-Parasite Interactions and Purifying Selection in a Microsporidian Parasite of Honey Bees. PLoS ONE 2016, 11, e0147549. [Google Scholar] [CrossRef]
- Werner, T.; Liu, G.; Kang, D.; Ekengren, S.; Steiner, H.; Hultmark, D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13772–13777. [Google Scholar] [CrossRef] [PubMed]
- Decanini, L.I.; Collins, A.M.; Evans, J.D. Variation and heritability in immune gene expression by diseased honeybees. J. Hered. 2007, 98, 195–201. [Google Scholar] [CrossRef]
- Huang, Q.; Kryger, P.; Le Conte, Y.; Moritz, R.F.A. Survival and immune response of drones of a Nosemosis tolerant honey bee strain towards N. ceranae infections. J. Invertebr. Pathol. 2012, 109, 297–302. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Evans, J.D. Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev. Comp. Immunol. 2013, 40, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, J.M.; Dolstad, H.A.; Sivalingam, M.D.; Snow, J.W. Barrier Immune Effectors Are Maintained during Transition from Nurse to Forager in the Honey Bee. PLoS ONE 2013, 8, e54097. [Google Scholar] [CrossRef]
- Sinpoo, C.; Paxton, R.J.; Disayathanoowat, T.; Krongdang, S.; Chantawannakul, P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect Physiol. 2018, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Evans, J.D.; Li, J.; Su, S.; Hamilton, M.; Chen, Y. Spore load and immune response of honey bees naturally infected by Nosema ceranae. Parasitol. Res. 2017, 116, 3265–3274. [Google Scholar] [CrossRef]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J. Insect Physiol. 2012, 58, 109020131095. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kocks, C. Recognition of pathogenic microbes by the Drosophila phagocytic pattern recognition receptor Eater. J. Biol. Chem. 2011, 286, 26524–26532. [Google Scholar] [CrossRef]
- Amdam, G.V.; Simões, Z.L.P.; Hagen, A.; Norberg, K.; Schroder, K.; Mikkelsen, O.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef]
- Alaux, C.; Folschweiller, M.; McDonnell, C.; Beslay, D.; Cousin, M.; Dussaubat, C.; Brunet, J.L.; Conte, Y.L. Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invertebr. Pathol. 2011, 106, 380–385. [Google Scholar] [CrossRef]
- BenVau, L.R.; Nieh, J.C. Larval honey bees infected with Nosema ceranae have increased vitellogenin titers as young adults. Sci. Rep. 2017, 7, 14144. [Google Scholar] [CrossRef]
- Antúnez, K.; Mendoza, Y.; Santos, E.; Invernizzi, C. Differential expression of vitellogenin in honey bees (Apis mellifera) with different degrees of Nosema ceranae infection. J. Apic. Res. 2013, 52, 227–234. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Råberg, L.; Graham, A.L.; Read, A.F. Decomposing health: Tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 37–49. [Google Scholar] [CrossRef]
- Ardia, D.R.; Gantz, J.E.; Schneider, B.C.; Strebel, S. Costs of immunity in insects: An induced immune response increases metabolic rate and decreases antimicrobial activity. Funct. Ecol. 2012, 26, 732–739. [Google Scholar] [CrossRef]
- Harpur, B.A.; Guarna, M.M.; Huxter, E.; Higo, H.; Moon, K.M.; Hoover, S.E.; Ibrahim, A.; Melathopoulos, A.P.; Desai, S.; Currie, R.W.; et al. Integrative genomics reveals the genetics and evolution of the honey bee’s social immune system. Genome Biol. Evol. 2019, 11, 937–948. [Google Scholar] [CrossRef]
- Moritz, R.; Kryger, P. Self-organization of circadian rhythms in groups of honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 1994, 34, 211–215. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee Colony Thermoregulation- Regulatory Mechanisms and Contribution of Individuals in Dependence on Age, Location and Thermal Stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Rankin, M.A. Light and temperature entrainment of a locomotor rhythm in honeybees. Physiol. Entomol. 1993, 18, 271–278. [Google Scholar] [CrossRef]
- Shimizu, I.; Kawai, Y.; Taniguchi, M.; Aoki, S. Circadian rhythm and cDNA cloning of the clock gene period in the honeybee Apis cerana japonica. Zool. Sci. 2001, 18, 779–789. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2018, 28, R879–R884. [Google Scholar]
- Evans, J.D.; Pettis, J.S. Colony-level impacts of immune responsiveness in honey bees, Apis mellifera. Evolution 2005, 59, 2270–2274. [Google Scholar]
- Mallon, E.B.; Brockmann, A.; Schmid-Hempel, P. Immune function inhibits memory formation in the honeybee Apis melifera. Proc. R. Soc. Lond. B 2003, 270, 2471–2473. [Google Scholar] [CrossRef]
- Hatjina, F.; Bienkowska, M.; Charistos, L.; Chlebo, R.; Costa, C.; Dražić, M.M.; Filipi, J.; Gregorc, A.; Ivanova, E.N.; Kezic, N.; et al. A review of methods used in some European countries for assessing the quality of honey bee queens through their physical characters and the performance of their colonies. J. Apicult. Res. 2014, 53, 337–363. [Google Scholar] [CrossRef]
- Meixner, M.D.; Francis, R.M.; Gajda, A.; Kryger, P.; Andonov, S.; Uzunov, A.; Topolska, G.; Costa, C.; Amiri, E.; Berg, S.; et al. Occurrence of parasites and pathogens in honey bee colonies used in a European genotypeenvironment Interactions experimentrs 2010/2011 and 2011/2012. J. Apicult. Res. 2014, 53, 215–229. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Guarna, M.M.; Hoover, S.E.; Huxter, E.; Higo, H.; Moon, K.M.; Domanski, D.; Bixby, M.E.; Melathopoulos, A.P.; Ibrahim, A.; Peirson, M.; et al. Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci. Rep. 2017, 7, 8381. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Nielsen, D.I. Sampling error, effective paternity, and estimating the genetic structure of honey bee colonies (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2002, 95, 513–528. [Google Scholar] [CrossRef]
- Tarpy, D.R. Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc. R. Soc. Lond. B 2003, 270, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Tarpy, D.R. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B 2007, 274, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nehme, N.T.; Quintin, J.; Cho, J.H.; Lee, J.; Lafarge, M.C.; Kocks, C.; Ferrandon, D. Relative roles of the cellular and humoral responses in the Drosophila host defense against three gram-positive bacterial infections. PLoS ONE 2011, 6, e14743. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozkar, C.Ö.; Evans, J.D. Immune Gene Expression and Locomotor Activity in Response to Vairimorpha ceranae Infection Across Five Honey Bee Subspecies. Insects 2025, 16, 593. https://doi.org/10.3390/insects16060593
Tozkar CÖ, Evans JD. Immune Gene Expression and Locomotor Activity in Response to Vairimorpha ceranae Infection Across Five Honey Bee Subspecies. Insects. 2025; 16(6):593. https://doi.org/10.3390/insects16060593
Chicago/Turabian StyleTozkar, Cansu Özge, and Jay D. Evans. 2025. "Immune Gene Expression and Locomotor Activity in Response to Vairimorpha ceranae Infection Across Five Honey Bee Subspecies" Insects 16, no. 6: 593. https://doi.org/10.3390/insects16060593
APA StyleTozkar, C. Ö., & Evans, J. D. (2025). Immune Gene Expression and Locomotor Activity in Response to Vairimorpha ceranae Infection Across Five Honey Bee Subspecies. Insects, 16(6), 593. https://doi.org/10.3390/insects16060593






