Managing African Armyworm Outbreaks in Sub-Saharan Africa: Current Strategies and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. Outbreaks in Sub-Saharan Africa
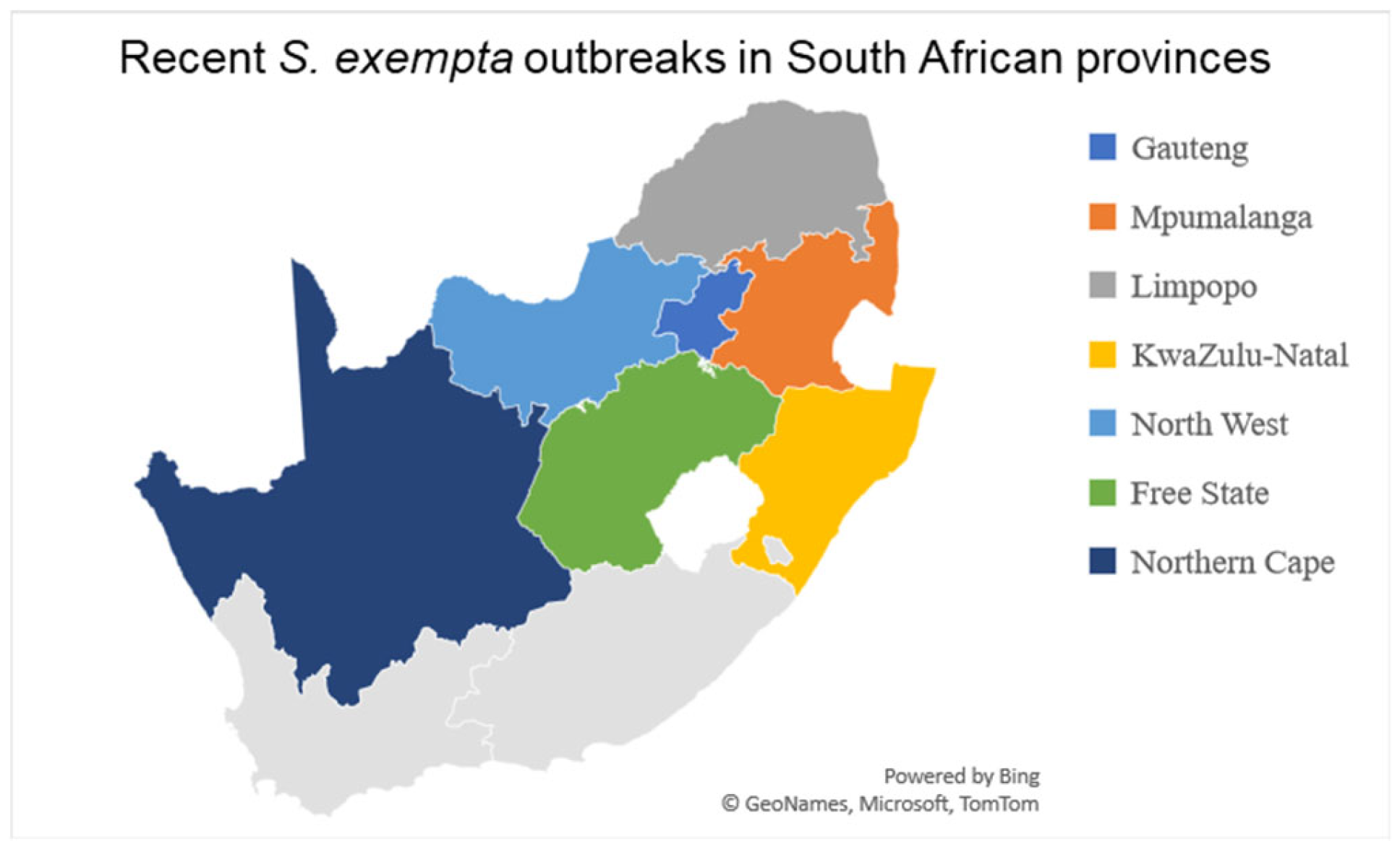
Outbreaks in South Africa
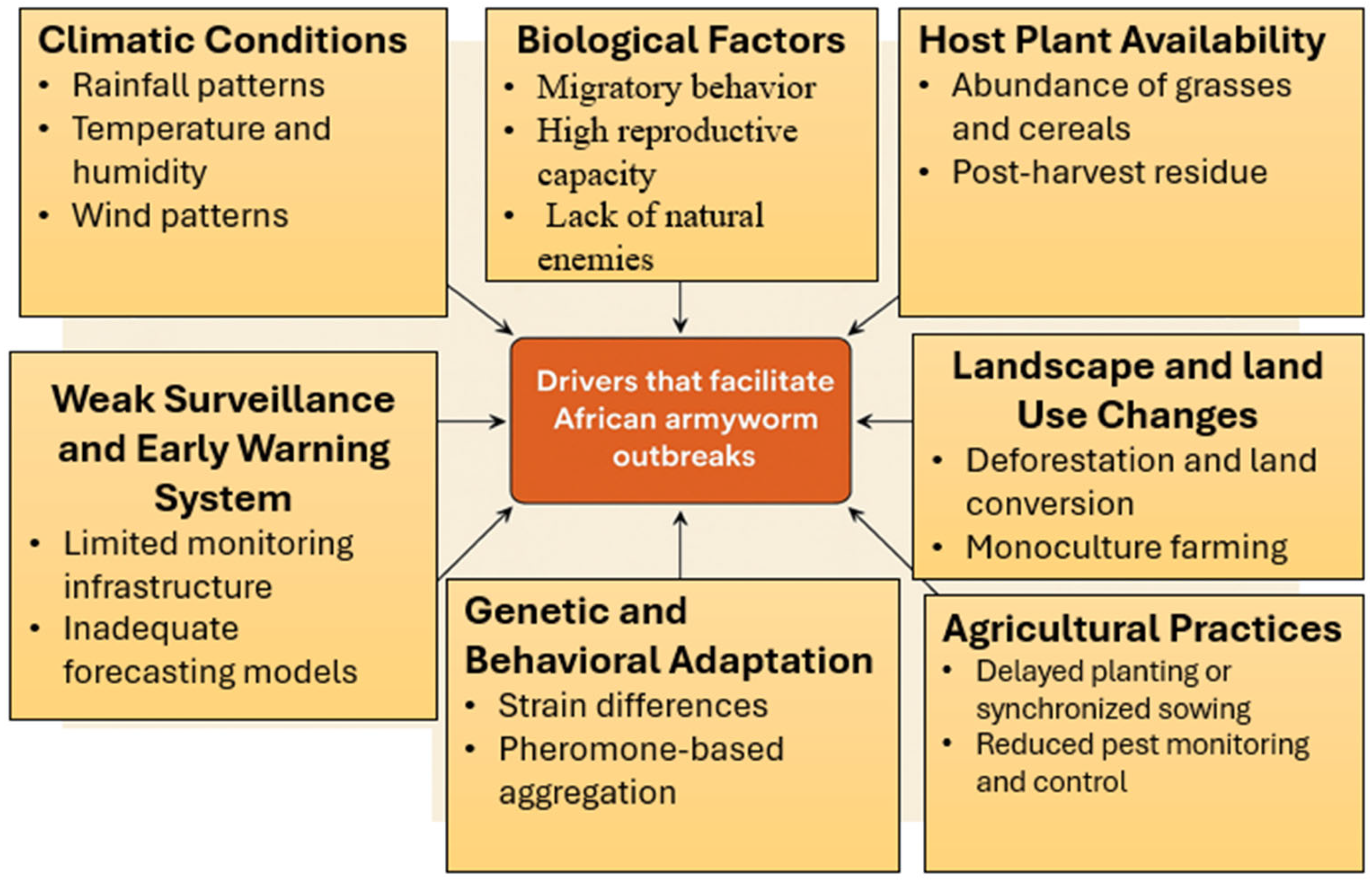
3. Drivers Facilitating African Armyworm Outbreaks
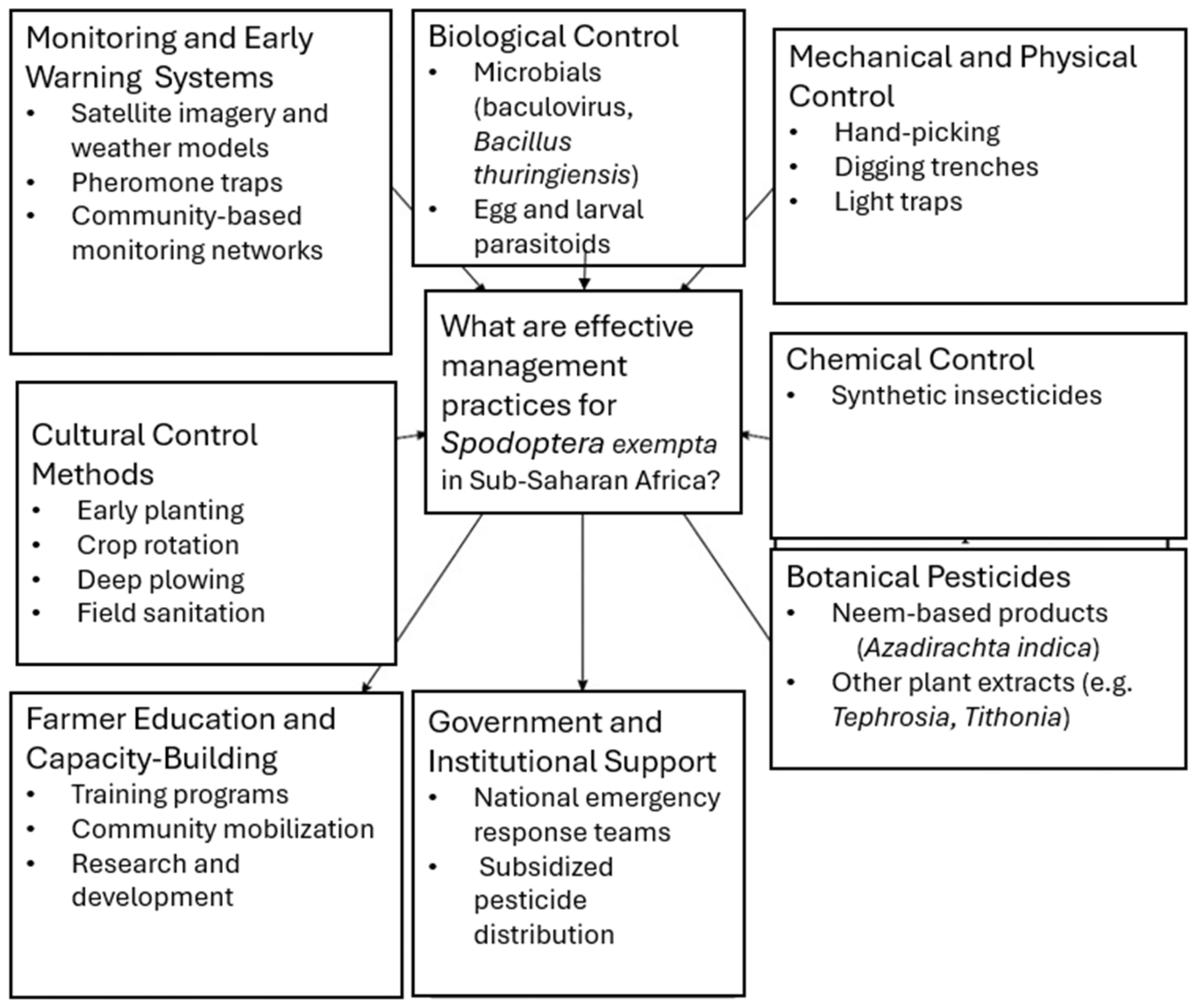
4. Management of African Armyworm
4.1. Surveillance and Predictive Tools for Early Detection of African Armyworm
4.2. Biological and Cultural Control of African Armyworm
4.3. Plant-Derived Compounds for African Armyworm Control
4.4. Integrated Pest Management of the African Armyworm
4.5. Challenges in the Current Management of the African Armyworm
4.6. Socio-Economic Consequences
4.7. Governance and Policy Frameworks
5. Advancing Technologies and African Armyworm Management
5.1. Innovative Digital Technologies
5.2. Genetically Modified Bacillus thuringiensis (Bt) Maize
5.3. Sterile Insect Technique
5.4. Molecular Technologies in the Management of African Armyworm
5.5. Nanotechnology
6. Technology-Enabled Integrated Pest Management Strategies for African Armyworm
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| Bt | Bacillus thuringiensis |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| Cry | Crystal proteins |
| DALRRD | Department of Agriculture, Land Reform, and Rural Development |
| dsRNA | Double-stranded RNA |
| FAO | Food and Agriculture Organization of the United Nations |
| GIS | Geographic information systems |
| GPS | Global positioning systems |
| IoT | Internet of Things |
| IPM | Integrated pest management |
| NPV | Nucleopolyhedrovirus |
| RNAi | RNA interference |
| SDMs | Species distribution models |
| SIT | Sterile insect technique |
| SpexNPV | Spodoptera exempta nucleopolyhedrovirus |
| SSA | Sub-Saharan Africa |
| ULV | Ultra-low volume |
| UV | Ultraviolet |
| Vips | Vegetative insecticidal proteins |
References
- Rose, J.W.; Dewhurst, C.F.; Page, W.W. The African Armyworm Handbook: The Status, Biology, Ecology, Epidemiology and Management of Spodoptera exempta (Lepidoptera: Noctuidae), 2nd ed.; Natural Resources Institute, University of Greenwich: London, UK, 2000; ISBN 0-85954-523-7. [Google Scholar]
- Centre for Agriculture and Bioscience International (CABI). Spodoptera exempta (Black armyworm). CABI Compendium, 29809 Datasheet, 6 April 2020. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.29809 (accessed on 29 April 2025).
- Fox, K.J. Migrant Lepidoptera in New Zealand 1972–1973. N. Z. Entomol. 1973, 5, 268–271. [Google Scholar] [CrossRef]
- Baker, G.L. An outbreak of Spodoptera exempta (Walker) (Lepidoptera: Noctuidae) in the highlands of Papua New Guinea. PNG Agric. J. 1978, 29, 11–25. [Google Scholar]
- Haggis, M.J. Distribution, Frequency of Attack and Seasonal Incidence of the African Armyworm Spodoptera exempta (Walk.) (Lep.: Noctuidae), with Particular Reference to Africa and Southwestern Arabia; Tropical Development and Research Institute: London, UK, 1984. [Google Scholar]
- Li, M.; Jin, Z.; Qi, Y.; Zhao, H.; Yang, N.; Guo, J.; Chen, B.; Xian, X.; Liu, W. Risk assessment of Spodoptera exempta against food security: Estimating the potential global overlapping areas of wheat, maize, and rice under climate change. Insects 2024, 15, 348. [Google Scholar] [CrossRef]
- Gómez-Undiano, I.; Musavi, F.; Mushobozi, W.L.; David, G.M.; Day, R.; Early, R.; Wilson, K. Predicting potential global and future distributions of the African armyworm (Spodoptera exempta) using species distribution models. Sci. Rep. 2022, 12, 16234. [Google Scholar]
- Centre for Coordination of Agricultural Research and Development for Southern Africa (CCARDESA). African Armyworm Outbreak Threatens Food Security in Southern Africa. CCARDESA. 6 February 2025. Available online: https://www.ccardesa.org/african-armyworm-outbreak-threatens-food-security-southern-africa (accessed on 29 April 2025).
- Cheke, R.A. Potential rates of increase of solitarious and gregarious phases of the African armyworm Spodoptera exempta (Lepidoptera: Noctuidae). Ecol. Entomol. 1995, 20, 319–325. [Google Scholar] [CrossRef]
- Kriel, G. SA Experiences Worst African Armyworm Infestation on Record. Farmer’s Weekly. 27 February 2025. Available online: https://www.farmersweekly.co.za/agri-news/south-africa/sa-experiences-worst-african-armyworm-infestation-on-record/02/ (accessed on 29 April 2025).
- Sokame, B.M.; Kipkorir, B.; Agboka, K.M.; Niassy, S.; Belayneh, Y.; Elkahky, M.; Tonnang, H.E. A system dynamics model for predicting African armyworm occurrence and population dynamics. Agric. Ecosyst. Environ. 2025, 380, 109378. [Google Scholar] [CrossRef]
- Rose, D.J.W.; Rose, D.J.W.; Rainey, R.C. The significance of low-density populations of the African armyworm Spodoptera exempta (Walk.). Phil. Trans. R. Soc. Lond. B 1979, 287, 393–402. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Curbing the March of African Armyworm. Reliefweb News and Press Release 15 June 2023. Available online: https://reliefweb.int/report/kenya/curbing-march-african-armyworm (accessed on 29 April 2025).
- Mncwango, S. African Armyworm Detected on Farms in Three Provinces. Farmer’s Weekly. 20 February 2025. Available online: https://www.farmersweekly.co.za/agri-news/south-africa/african-armyworm-detected-on-farms-in-three-provinces/02/ (accessed on 29 April 2025).
- Matsimela, M. Update on the Armyworm Outbreak in the Free State. African Farming. 28 March 2025. Available online: https://www.africanfarming.com/2025/03/28/update-on-the-armyworm-outbreak-in-the-free-state/ (accessed on 29 April 2025).
- van Burick, N. Armyworms Still Marching. African Farming. 5 April 2025. Available online: https://www.africanfarming.com/2025/04/05/armyworms-still-marching/ (accessed on 29 April 2025).
- Hoo, S.K. Agriculture Department Confirms African Armyworm Infestations in Northern Cape. Diamond Fields Advertiser. March 2025. Available online: https://dfa.co.za/news/2025-03-30-agriculture-department-confirms-african-armyworm-infestations-in-northern-cape/ (accessed on 29 April 2025).
- Tucker, M.R.; Pedgley, D.E. Rainfall and outbreaks of the African armyworm, Spodoptera exempta (Walker) (Lepidoptera: Noctuidae). Bull. Ent. Res. 1983, 73, 195–199. [Google Scholar] [CrossRef]
- Brown, E.S.; Betts, E.; Rainey, R.C. Seasonal changes in distribution of the African armyworm, Spodoptera exempta (wlk.) (Lep., Noctuidae), with special reference to Eastern Africa. Bull. Entomol. Res. 1969, 58, 661–728. [Google Scholar] [CrossRef]
- Wilson, K.; Gatehouse, A.G. Seasonal and geographical variation in the migratory potential of outbreak populations of the African armyworm moth, Spodoptera exempta. J. Anim. Ecol. 1993, 62, 169–181. [Google Scholar] [CrossRef]
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.; Borgemeister, C. Future risks of pest species under changing climatic conditions. PLoS ONE 2016, 11, e0153237. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.; Kunert, K.J.; Maling’a, J.; Foyer, C.H. Defining biotechnological solutions for insect control in sub-Saharan Africa. Food Energy Secur. 2020, 9, e191. [Google Scholar] [CrossRef]
- Subedi, B.; Poudel, A.; Aryal, S. The impact of climate change on insect pest biology and ecology: Implications for pest management strategies, crop production, and food security. J. Agric. Food Res. 2023, 14, 100733. [Google Scholar] [CrossRef]
- Karuppaiah, V.; Sujayanad, G.K. Impact of climate change on population dynamics of insect pests. World J. Agric. Sci. 2012, 8, 240–246. [Google Scholar]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. R-reproductive strategy. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer: Cham, Switzerland, 2019; Volume 10, pp. 978–983. [Google Scholar]
- Lee, K.P.; Simpson, S.J.; Raubenheimer, D. A comparison of nutrient regulation between solitarious and gregarious phases of the specialist caterpillar, Spodoptera exempta (Walker). J. Insect Physiol. 2004, 50, 1171–1180. [Google Scholar] [CrossRef]
- Cheke, R.A.; Tratalos, J.A. Migration, patchiness, and population processes illustrated by two migrant pests. BioScience 2007, 57, 145–154. [Google Scholar] [CrossRef]
- Dewhurst, C.F.; Page, W.W.; Rose, D.J.W. The relationship between outbreaks, rainfall and low density populations of the African armyworm, Spodoptera exempta, in Kenya. Entomol. Exp. Appl. 2001, 98, 285–294. [Google Scholar] [CrossRef]
- Mazzi, D.; Dorn, S. Movement of insect pests in agricultural landscapes. Ann. Appl. Biol. 2012, 160, 97–113. [Google Scholar] [CrossRef]
- Brown, M.E.; Mugo, S.; Petersen, S.; Klauser, D. Designing a pest and disease outbreak warning system for farmers, agronomists and agricultural input distributors in East Africa. Insects 2022, 13, 232. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Carvajal-Yepes, M.; Kumar, P.L.; Kawarazuka, N.; Liu, Y.; Mulema, A.A.; McCutcheon, S.; Ibabao, X. Sustainable management of transboundary pests requires holistic and inclusive solutions. Food Secur. 2022, 14, 1449–1457. [Google Scholar] [CrossRef]
- Mushobozi, W.L.; Grzywacz, D.; Musebe, R.; Kimani, M.; Wilson, K. New approaches to improve the livelihoods of poor farmers and pastoralists in Tanzania through monitoring and control of African armyworm, Spodoptera exempta. Asp. Appl. Biol. 2005, 75, 37–45. [Google Scholar]
- Rose, D.J.W.; Page, W.W.; Dewhurst, C.F.; Riley, J.R.; Reynolds, D.R.; Pedgley, D.E.; Tucker, M.R. Downwind migration of the African armyworm moth, Spodoptera exempta, studied by mark-and-capture and by radar. Ecol. Entomol. 1985, 10, 299–313. [Google Scholar] [CrossRef]
- Van Huis, A.; Cressman, K.; Magor, J.I. Preventing desert locust plagues: Optimizing management interventions. Entomol. Exp. Appl. 2007, 122, 191–214. [Google Scholar] [CrossRef]
- Pandey, M.; Suwal, B.; Kayastha, P.; Suwal, G.; Khanal, D. Desert locust invasion in Nepal and possible management strategies: A review. J. Agric. Food Res. 2021, 5, 100166. [Google Scholar] [CrossRef]
- Mathulwe, L.L. Spodoptera exempta, African Armyworm, in KwaZulu-Natal Grazing Pastures. Research & Technology Bulletin. 2024. Available online: https://www.kzndard.gov.za/images/Documents/researchandtechnologydevelopment/publications/Research_and_Technology_Bulletins/African-Armyworm-in-KwaZulu-Natal-Grazing-Pastures.pdf (accessed on 16 June 2025).
- Faithpraise, F.; Idung, J.; Chatwin, C.; Young, R.; Birch, P. Modelling the control of African armyworm (Spodoptera exempta) infestations in cereal crops by deploying naturally beneficial insects. Biosyst. Eng. 2015, 129, 268–276. [Google Scholar] [CrossRef]
- Noar, R.D.; Jahant-Miller, C.J.; Emerine, S.; Hallberg, R. Early warning systems as a component of integrated pest management to prevent the introduction of exotic pests. J. Integr. Pest Manag. 2021, 12, 16. [Google Scholar] [CrossRef]
- Persson, B. Population fluctuations of the African armyworm, Spodoptera exempta (Walker) (Lepidoptera: Noctuidae), in outdoor cages in Kenya. Bull. Ent. Res. 1981, 71, 289–297. [Google Scholar] [CrossRef]
- Grzywacz, D.; Mushobozi, W.L.; Parnell, M.; Jolliffe, F.; Wilson, K. Evaluation of Spodoptera exempta nucleopolyhedrovirus (SpexNPV) for the field control of African armyworm (Spodoptera exempta) in Tanzania. Crop Prot. 2008, 27, 17–24. [Google Scholar] [CrossRef]
- Abbaszadeh, G.; Dhillon, M.K.; Srivastava, C.; Gautam, R.D. Effect of climatic factors on bioefficacy of biopesticides in insect pest management. Biopestic. Int. 2011, 7, 1–14. [Google Scholar]
- Wilson, K.; Grzywacz, D.; Curcic, I.; Scoates, F.; Harper, K.; Rice, A.; Paul, N.; Dillon, A. A novel formulation technology for baculoviruses protects biopesticide from degradation by ultraviolet radiation. Sci. Rep. 2020, 10, 13301. [Google Scholar] [CrossRef] [PubMed]
- Broza, M.; Brownbridge, M.; Hamal, M.; Sneh, B. Control of the African army worm Spodoptera exempta Walker (Lepidoptera: Noctuidae) in Kenyan fields with highly effective strains of Bacillus thuringiensis Berliner. Biocontrol Sci. Technol. 1991, 1, 127–135. [Google Scholar] [CrossRef]
- Hamal, M.; Brownbridge, M.; Broza, M.; Sneh, B. Screening for highly effective isolates of Bacillus thuringiensis against Spodoptera exempta and Spodoptera littoralis. Phytoparasitica 1991, 19, 9–21. [Google Scholar] [CrossRef]
- Bai, C.; Degheele, D.; Jansens, S.; Lambert, B. Activity of insecticidal crystal proteins and strains of Bacillus thuringiensis against Spodoptera exempta (Walker). J. Invertebr. Pathol. 1993, 62, 211–215. [Google Scholar] [CrossRef]
- Akeme, C.N.; Ngosong, C.; Sumbele, S.A.; Aslan, A.; Tening, A.S.; Krah, C.Y.; Kamanga, B.M.; Denih, A.; Nambangia, O.J. Different controlling methods of fall armyworm (Spodoptera frugiperda) in maize farms of small-scale producers in Cameroon. IOP Conf. Ser. Earth Environ. Sci. 2021, 911, 012053. [Google Scholar] [CrossRef]
- Ahissou, B.R.; Sawadogo, W.M.; Bokonon-Ganta, A.H.; Somda, I.; Verheggen, F. Integrated pest management options for the fall armyworm Spodoptera frugiperda in West Africa: Challenges and opportunities. A review. Biotechnol. Agron. Soc. Environ. 2021, 25, 192–207. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Tanzubil, P.B.; McCaffery, A.R. Effects of azadirachtin and aqueous neem seed extracts on survival, growth and development of the African armyworm, Spodoptera exempta. Crop Prot. 1990, 9, 383–386. [Google Scholar] [CrossRef]
- Tanzubil, P.B.; McCaffery, A.R. Effects of azadirachtin on reproduction in the African armyworm (Spodoptera exempta). Entomol. Exp. Appl. 1990, 57, 115–121. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crops and Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.F.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Secur. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Cairns, J.; Hamadziripi, E.; Wilson, K.; Hughes, D.; Chelal, J.; McCloskey, P.; Kehs, A.; Clinton, N.; Cressman, K.; et al. Multi-scale remote sensing for fall armyworm monitoring and early warning systems. In Proceedings of the IGARSS 2020–2020 IEEE International Geoscience and Remote Sensing Symposium, Waikoloa, HI, USA, 26 September–2 October 2020; pp. 4886–4889. [Google Scholar]
- Tepa-Yotto, G.; Winsou, J.K.; Dahoueto, B.; Tamò, M. Assessing new scouting approaches for field sampling of Spodoptera frugiperda and its parasitoids. In Proceedings of the 1st International Electronic Conference on Entomology, Online, 1–15 July 2021; pp. 1–8. [Google Scholar]
- Togola, A.; Beyene, Y.; Bocco, R.; Tepa-Yotto, G.; Gowda, M.; Too, A.; Boddupalli, P. Fall armyworm (Spodoptera frugiperda) in Africa: Insights into biology, ecology and impact on staple crops, food systems and management approaches. Front. Agron. 2025, 7, 1538198. [Google Scholar] [CrossRef]
- Rao, M.S.; Prasad, T.V.; Gayatri, D.L.A.; Rao, C.R.; Srinivas, K.; Pratibha, G.; Prabhakar, M.; Singh, V.K. Impact of intercropping and low-external input integrated pest management on Spodoptera frugiperda (J.E. Smith) in maize. Field Crops Res. 2025, 326, 109868. [Google Scholar]
- Talha, M.A.; Rahman, M.M.; Hossain, M.S.; Ali, M.R.; Reza, M.; Khan, M.S.I.; Hossain, M.E. Development of an integrated pest management package for effective control of Spodoptera litura (Fab.) in tropical sugar beet production in Bangladesh. Asian J. Adv. Agric. Res. 2025, 25, 130–136. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.T.; Chinwada, P.; Rwomushana, I.; Goergen, G.; Subramanian, S. Integrated management of Spodoptera frugiperda 6 years post detection in Africa: A review. Curr. Opin. Insect Sci. 2022, 52, 100928. [Google Scholar] [CrossRef]
- Zanzana, K.; Dannon, E.A.; Sinzogan, A.A.; Toffa, J.M. Fall armyworm management in a changing climate: An overview of climate-responsive integrated pest management (IPM) strategies for long-term control. Egypt. J. Biol. Pest Control 2024, 34, 54. [Google Scholar] [CrossRef]
- Karakkottil, P.; Pulamte, L.; Kumar, V. Strategic analysis of collaborative networks in Spodoptera frugiperda (Lepidoptera: Noctuidae) research for improved pest management strategies. Neotrop. Entomol. 2024, 53, 937–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Qi, Y.; Li, M.; Yang, N.; Guo, J.; Xian, X.; Liu, W. Global potential geographical distribution of the Southern armyworm (Spodoptera eridania) under climate change. Biology 2023, 12, 1040. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (heteroptera) to climate change. Glob. Change Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Stange, E.E.; Ayres, M.P. Climate change impacts: Insects. In Encyclopedia of Life Sciences (eLS); John Wiley & Sons, Ltd: Chichester, UK, 2010. [Google Scholar]
- Sun, Y.C.; Guo, H.J.; Ge, F. Progress in research on the responses of insects to global climate change. Chin. J. Appl. Entomol. 2017, 54, 539–552. [Google Scholar]
- Ward, N.L.; Masters, G.J. Linking climate change and species invasion: An illustration using insect herbivores. Glob. Change Biol. 2007, 13, 1605–1615. [Google Scholar] [CrossRef]
- Ge, F.; Chen, F.J.; Wu, G.; Sun, Y.C. Research advances on the response of insects to elevated CO2 in China. Chin. Bull. Entomol. 2010, 47, 229–235. [Google Scholar]
- Yu, S.J.; Nguyen, S.N.; Abo-Elghar, G.E. Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith). Pestic. Biochem. Physiol. 2003, 77, 1–11. [Google Scholar] [CrossRef]
- Che, W.; Shi, T.; Wu, Y.; Yang, Y. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 2013, 106, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Moraiet, M.A.; Ahmad, S. Insecticides: Impact on the environment and human health. In Environmental Deterioration and Human Health; Malik, A., Grohmann, E., Akhtar, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 99–123. [Google Scholar]
- Reeson, A.F.; Wilson, K.; Gunn, A.; Hails, R.S.; Goulson, D. Baculovirus resistance in the noctuid Spodoptera exempta is phenotypically plastic and responds to population density. Proc. R. Soc. Lond. B 1998, 265, 1787–1791. [Google Scholar] [CrossRef]
- Wilson, K.; Reeson, A.F. Density-dependent prophylaxis: Evidence from Lepidoptera–baculovirus interactions? Ecol. Entomol. 1998, 23, 100–101. [Google Scholar] [CrossRef]
- Grzywacz, D.; Stevenson, P.C.; Mushobozi, W.L.; Belmain, S.; Wilson, K. The use of indigenous ecological resources for pest control in Africa. Food Secur. 2014, 6, 71–86. [Google Scholar] [CrossRef]
- Abro, Z.; Kimathi, E.; Groote, H.D.; Tefera, T.; Sevgan, S.; Niassy, S.; Kassie, M. Socioeconomic and health impacts of fall armyworm in Ethiopia. PLoS ONE 2021, 16, e0257736. [Google Scholar] [CrossRef]
- Cheke, R.A.; Tucker, M.R. An evaluation of potential economic returns from the strategic control approach to the management of African armyworm Spodoptera exempta (Lepidoptera: Noctuidae) populations in Eastern Africa. Crop Prot. 1995, 14, 91–103. [Google Scholar] [CrossRef]
- Makgoba, M.C.; Tshikhudo, P.P.; Nnzeru, L.R.; Makhado, R.A. Impact of fall armyworm (Spodoptera frugiperda) (J.E. Smith) on small-scale maize farmers and its control strategies in the Limpopo province, South Africa. Jàmbá J. Disaster Risk Stud. 2021, 13, 1016. [Google Scholar]
- Srinivasan, R.; Tamò, M.; Subramanian, S. The case for integrated pest management in Africa: Transition from a pesticide-based approach. Curr. Opin. Insect Sci. 2022, 54, 100970. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Sutherst, R.W. Pest species distribution modelling: Origins and lessons from history. Biol. Invasions 2014, 16, 239–256. [Google Scholar] [CrossRef]
- Méndez-Vázquez, L.J.; Lira-Noriega, A.; Lasa-Covarrubias, R.; Cerdeira-Estrada, S. Delineation of site-specific management zones for pest control purposes: Exploring precision agriculture and species distribution modeling approaches. Comput. Electron. Agric. 2019, 167, 105101. [Google Scholar] [CrossRef]
- Shaurub, E.-S.H. Remote sensing and geographic information system applications as early-warning tools in monitoring fall armyworm, Spodoptera frugiperda: A review. Int. J. Trop. Insect Sci. 2024, 44, 2241–2258. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Yuan, L.; Yang, G.; Chen, L.; Zhao, C. Using satellite multispectral imagery for damage mapping of armyworm (Spodoptera frugiperda) in maize at a regional scale: Using satellite images to map regional armyworm damage in maize. Pest. Manag. Sci. 2016, 72, 335–348. [Google Scholar] [CrossRef]
- Guimapi, R.A.; Niassy, S.; Mudereri, B.T.; Abdel-Rahman, E.M.; Tepa-Yotto, G.T.; Subramanian, S.; Mohamed, S.A.; Thunes, K.H.; Kimathi, E.; Agboka, K.M.; et al. Harnessing data science to improve integrated management of invasive pest species across Africa: An application to fall armyworm (Spodoptera frugiperda) (J.E. Smith) (Lepidoptera: Noctuidae). Glob. Ecol. Conserv. 2022, 35, e02056. [Google Scholar] [CrossRef]
- Lello, F.; Dida, M.; Mkiramweni, M.; Matiko, J.; Akol, R.; Nsabagwa, M.; Katumba, A. Fruit fly automatic detection and monitoring techniques: A review. Smart Agric. Technol. 2023, 5, 100294. [Google Scholar] [CrossRef]
- Kariyanna, B.; Sowjanya, M. Unravelling the use of artificial intelligence in management of insect pests. Smart Agric. Technol. 2024, 8, 100517. [Google Scholar] [CrossRef]
- Oyege, I.; Sibitenda, H.; Bhaskar, M.S.B. Deep learning applications for real-time and early detection of fall armyworm, African armyworm, and maize stem borer. Mach. Learn. Appl. 2024, 18, 100596. [Google Scholar] [CrossRef]
- Vidya Madhuri, E.; Rupali, J.S.; Sharan, S.P.; Sai Pooja, N.; Sujatha, G.S.; Singh, D.P.; Ahmad, K.; Kumar, A.; Prabha, R. Transforming pest management with artificial intelligence technologies: The future of crop protection. J. Crop Health 2025, 77, 48. [Google Scholar] [CrossRef]
- Shuang, L.I.; Feng, S.-Q.; Ullah, H.; Tu, X.-B.; Zhang, Z.-H. IPM-biological and integrated management of desert locust. J. Integr. Agric. 2022, 21, 3467–3487. [Google Scholar]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, C.; Taratiel, D.; Casanova, J.L. Desert locust detection using earth observation satellite data in Mauritania. J. Arid Environ. 2019, 164, 29–37. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Zhang, K.; Zhao, Y.; Ren, Y.; Chen, Y.; Zhuang, H.; Chen, S. Autonomous detection of Spodoptera frugiperda by feeding symptoms directly from UAV RGB imagery. Appl. Sci. 2022, 12, 2592. [Google Scholar] [CrossRef]
- Botha, A.S.; Erasmus, A.; du Plessis, H.; Van den Berg, J. Efficacy of Bt maize for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in South Africa. J. Econ. Entomol. 2019, 112, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Venter, J.G. The Response of Lepidopteran Pests to Commercialised Bt Maize in South Africa. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2015. [Google Scholar]
- Tabashnik, B.E.; Carrière, Y. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China, and India. J. Econ. Entomol. 2019, 112, 2513–2523. [Google Scholar] [CrossRef]
- Van den Berg, J.; Prasanna, B.M.; Midega, C.A.; Ronald, P.C.; Carrière, Y.; Tabashnik, B.E. Managing fall armyworm in Africa: Can Bt maize sustainably improve control? J. Econ. Entomol. 2021, 114, 1934–1949. [Google Scholar] [CrossRef]
- Moscardini, V.F.; Marques, L.H.; Santos, A.C.; Rossetto, J.; Silva, O.A.; Rampazzo, P.E.; Castro, B.A. Efficacy of Bacillus thuringiensis (Bt) maize expressing Cry1f, Cry1a. 105, Cry2ab2 and Vip3aa20 proteins to manage the fall armyworm (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2020, 137, 105269. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, H.; Kaur, S. Vegetative insecticidal protein (Vip): A potential contender from Bacillus thuringiensis for efficient management of various detrimental agricultural pests. Front. Microbiol. 2021, 12, 659736. [Google Scholar] [CrossRef] [PubMed]
- Tharun Kumar, C.J.; Subhash, A.; Rishika, K.S.; Gupta, M.; Singh, S.; Kalia, V.; Kaur, S. Toxicological impacts of neoteric Vip3a toxins from Bacillus thuringiensis on survival and development of Spodoptera frugiperda and S. litura. Biocontrol Sci. Technol. 2025, 35, 636–652. [Google Scholar] [CrossRef]
- Zhu, C.; Ruan, L.; Peng, D.; Yu, Z.; Sun, M. Vegetative insecticidal protein enhancing the toxicity of Bacillus thuringiensis subsp. Kurstaki against Spodoptera exigua. Lett. Appl. Microbiol. 2006, 42, 109–114. [Google Scholar] [CrossRef]
- Soares Figueiredo, C.; Nunes Lemes, A.R.; Sebastião, I.; Desidério, J.A. Synergism of the Bacillus thuringiensis Cry1, Cry2, and Vip3 proteins in Spodoptera frugiperda control. Appl. Biochem. Biotechnol. 2019, 188, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Hendrichs, J.; Robinson, A. Sterile insect technique. In Encyclopedia of Insects; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 953–957. [Google Scholar]
- Sayed, W.A.A.; El-Helaly, A.; Jamal, Z.A.; El-Bendary, H. Effect of a low cost diet on the cotton leaf worm, Spodoptera littoralis nucleopolyhedrosis virus pathogenicity and sterile insect technique. Egypt. J. Biol. Pest Control 2021, 31, 117. [Google Scholar] [CrossRef]
- Sengupta, M.; Vimal, N.; Angmo, N.; Seth, R.K. Effect of irradiation on reproduction of female Spodoptera litura (Fabr.) (Lepidoptera: Noctuidae) in relation to the inherited sterility technique. Insects 2022, 13, 898. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, X.-T.; Ge, S.-S.; Yang, X.-M.; Wu, K.-M. Mating competitiveness of male Spodoptera frugiperda (Smith) irradiated by X-rays. Insects 2023, 14, 137. [Google Scholar] [CrossRef]
- Sun, X.; He, W.; Jiang, S.; Ge, S.; Chu, B.; Liang, G.; Yang, X.; Wu, K. The evaluation on control potential using X-ray to irradiate adult Spodoptera frugiperda (Lepidoptera: Noctuidae). Pest Manag. Sci. 2025, 81, 1432–1443. [Google Scholar] [CrossRef]
- Hassan, R.S.; Sileem, T.M.; Sayed, W. Radiation dose optimization for improving the sterile insect technique of the cotton leaf worm, Spodoptera littoralis (Lepidoptera: Noctuidae). Menoufia J. Plant Prot. 2024, 9, 159–171. [Google Scholar] [CrossRef]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Kumari, P.; Jasrotia, P.; Kumar, D.; Kashyap, P.L.; Kumar, S.; Mishra, C.N.; Kumar, S.; Singh, G.P. Biotechnological approaches for host plant resistance to insect pests. Front. Genet. 2022, 13, 914029. [Google Scholar] [CrossRef] [PubMed]
- Ramarasu, A.; Asokan, R.; Pavithra, B.S.; Sridhar, V. Innovative molecular approaches for pest management. In Genetic Methods and Tools for Managing Crop Pests; Chakravarthy, A.K., Ed.; Springer Nature: Singapore, 2022; pp. 27–43. [Google Scholar]
- Kumar, R.; Yadav, P.; Alamgir, V.D.B.; Singh, P. Molecular methods for the control of insect pests. In Recent Trends in Plant Protection; Mishra, M.K., Kumar, A., Kumar, S., Eds.; New India Publishing Agency: New Delhi, India, 2024; pp. 37–46. [Google Scholar]
- Ali, M.W.; Zheng, W.; Sohail, S.; Li, Q.; Zheng, W.; Zhang, H. A genetically enhanced sterile insect technique against the fruit fly, Bactrocera dorsalis (Hendel) by feeding adult double-stranded RNA. Sci. Rep. 2017, 7, 4063. [Google Scholar] [CrossRef]
- Marec, F.; Vreysen, M.J.B. Advances and challenges of using the sterile insect technique for the management of pest lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Knipple, D.C. Recent advances in RNA interference research in insects: Implications for future insect pest management strategies. Crop Prot. 2013, 45, 36–40. [Google Scholar] [CrossRef]
- Di Lelio, I.; Varricchio, P.; Di Prisco, G.; Marinelli, A.; Lasco, V.; Caccia, S.; Casartelli, M.; Giordana, B.; Rao, R.; Gigliotti, S.; et al. Functional analysis of an immune gene of Spodoptera littoralis by RNAi. J. Insect Physiol. 2014, 64, 90–97. [Google Scholar] [CrossRef]
- Christiaens, O.; Tardajos, M.G.; Martinez Reyna, Z.L.; Dash, M.; Dubruel, P.; Smagghe, G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Wan, X.-S.; Shi, M.-R.; Xu, J.; Liu, J.-H.; Ye, H. Interference efficiency and effects of bacterium-mediated RNAi in the fall armyworm (Lepidoptera: Noctuidae). J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef]
- Dhandapani, R.K.; Gurusamy, D.; Palli, S.R. Protamine–lipid–dsRNA nanoparticles improve RNAi efficiency in the fall armyworm, Spodoptera frugiperda. J. Agric. Food Chem. 2022, 70, 6634–6643. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. BioTechniques 2020, 68, 283–290. [Google Scholar] [CrossRef]
- Bi, H.-L.; Xu, J.; Tan, A.-J.; Huang, Y.-P. CRISPR/Cas9-mediated targeted gene mutagenesis in Spodoptera litura: CRISPR/Cas9 targeted gene in S. litura. Insect Sci. 2016, 23, 469–477. [Google Scholar] [CrossRef]
- Suresh, J.; Naganna, R.; Nikhil, B.S.K.; Reddy, S.S.; Reddy, C.N. The CRISPR/Cas mediated genome editing: A novel insect pest management strategy. Asian J. Microbiol. Biotechnol. Environ. Sci. 2021, 23, 205–212. [Google Scholar]
- Akbar, R.; Mohi-ud-din, S.; Rafiq, S.; Nisa, R.T.; Sheikh, M.A.; Akhter, A.; Iqbal, I.; Jan, U. Review on: CRISPR-Cas 9: A novel genome editing tool for insect pest management. Biol. Forum Int. J. 2023, 15, 550–553. [Google Scholar]
- Wu, K.; Shirk, P.D.; Taylor, C.E.; Furlong, R.B.; Shirk, B.D.; Pinheiro, D.H.; Siegfried, B.D. CRISPR/Cas9-mediated knockout of the abdominal-A homeotic gene in fall armyworm moth (Spodoptera frugiperda). PLoS ONE 2018, 13, e0208647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-H.; Xu, J.; Cui, Z.; Dong, X.-T.; Ye, Z.-F.; Niu, D.-J.; Huang, Y.-P.; Dong, S.-L. Functional characterization of SlitPBP3 in Spodoptera litura by CRISPR/Cas9 mediated genome editing. Insect Biochem. Mol. Biol. 2016, 75, 1–9. [Google Scholar] [CrossRef]
- Koutroumpa, F.A.; Monsempes, C.; François, M.-C.; de Cian, A.; Royer, C.; Concordet, J.-P.; Jacquin-Joly, E. Heritable genome editing with CRISPR/Cas9 induces anosmia in a crop pest moth. Sci. Rep. 2016, 6, 29620. [Google Scholar] [CrossRef]
- Singh, S.; Rahangdale, S.; Pandita, S.; Saxena, G.; Upadhyay, S.K.; Mishra, G.; Verma, P.C. CRISPR/Cas9 for insect pests management: A comprehensive review of advances and applications. Agriculture 2022, 12, 1896. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Jiang, J.; Wang, A.; Wang, C.; Shen, Y.; Huang, B.; An, C.; Cui, B.; Zhao, X.; et al. Advances in controlled-release pesticide formulations with improved efficacy and targetability. J. Agric. Food Chem. 2021, 69, 12579–12597. [Google Scholar] [CrossRef]
- Tao, R.; You, C.; Qu, Q.; Zhang, X.; Deng, Y.; Ma, W.; Huang, C. Recent advances in the design of controlled- and sustained-release micro/nanocarriers of pesticide. Environ. Sci. Nano 2023, 10, 351–371. [Google Scholar] [CrossRef]
- Nimisha, T.; Deepthy, K.B. Nanotechnology applications in insect pest management—A review. J. Eco-Friendly Agric. 2020, 15, 1–6. [Google Scholar]
- Nonci, N.; Noveriza, R.; Meilin, A.; Melati, M.; Manzila, I.; Pustika, A.B.; Rahma, H.; Najamuddin, E.; Muis, A. Toxicity test of compound essential oil nanoemulsion on fall armyworm (Spodoptera frugiperda J.E. Smith) eggs. Cogent Food Agric. 2025, 11, 2435584. [Google Scholar] [CrossRef]
- Bharani, R.S.A.; Namasivayam, S.K.R. Biogenic silver nanoparticles mediated stress on developmental period and gut physiology of major lepidopteran pest Spodoptera litura (Fab.) (Lepidoptera: Noctuidae)—An eco-friendly approach of insect pest control. J. Environ. Chem. Eng. 2017, 5, 453–467. [Google Scholar] [CrossRef]
- Jameel, M.; Shoeb, M.; Khan, M.T.; Ullah, R.; Mobin, M.; Farooqi, M.K.; Adnan, S.M. Enhanced insecticidal activity of thiamethoxam by zinc oxide nanoparticles: A novel nanotechnology approach for pest control. ACS Omega 2020, 5, 1607–1615. [Google Scholar] [CrossRef]
- Alimohamadian, M.; Aramideh, S.; Mirfakhraie, S.; Frozan, M. Silica nanoparticle: A potential of non-invasive and as a natural insecticide application for beet armyworm, Spodoptera exigua Hubner (Lep.: Noctuidae) control. Agric. Eng. Int. CIGR J. 2022, 24, 248–257. [Google Scholar]
- Asghar, M.S.; Sarwar, Z.M.; Almadiy, A.A.; Shami, A.; El Hadi Mohamed, R.A.; Ahmed, N.; Waghulade, M.S.; Alam, P.; Abd Al Galil, F.M. Toxicological effects of silver and zinc oxide nanoparticles on the biological and life table parameters of Helicoverpa armigera (Noctuidae: Lepidoptera). Agriculture 2022, 12, 1744. [Google Scholar] [CrossRef]
- Muthusamy, R.; Ramkumar, G.; Kumarasamy, S.; Chi, N.T.L.; Al Obaid, S.; Alfarraj, S.; Karuppusamy, I. Synergism and toxicity of iron nanoparticles derived from Trigonella foenum-graecum against pyrethroid treatment in S. litura and H. armigera (Lepidoptera: Noctuidae). Environ. Res. 2023, 231, 116079. [Google Scholar] [CrossRef]
- Wang, J.; Tao, M.; Xu, L.; Fan, N.; Zhao, C.; Xiao, Z.; Wang, Z. Chitosan nanocarriers loaded with salicylic acid for controlling fall armyworm (Spodoptera frugiperda) and alleviating oxidative stress in maize plants. Environ. Sci. Nano 2023, 10, 3295–3306. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, M.Y.; Aguirre-Uribe, L.A.; Ramírez-Barrón, S.N.; Pérez-Luna, Y.D.C.; Castro-del Ángel, E.; Juárez, A.H. Insecticidal efficacy of zinc oxide and silicon dioxide nanoparticles against larvae of Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae): Efficacy of nanoparticles on Spodoptera frugiperda. J. Exp. Nanosci. 2025, 20, 2466532. [Google Scholar]
- Pittarate, S.; Rajula, J.; Rahman, A.; Vivekanandhan, P.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Insecticidal effect of zinc oxide nanoparticles against Spodoptera frugiperda under laboratory conditions. Insects 2021, 12, 1017. [Google Scholar] [CrossRef]
- Shabir, A.; Sarwar, Z.M.; Ali, H. Eco-friendly approaches of zinc oxide and silver nitrate nanoparticles along with plant extracts against Spodoptera litura (Fabricius) under laboratory conditions. Sci. Prog. 2023, 106, 00368504231219171. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Usha Rani, P.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Pittarate, S.; Perumal, V.; Kannan, S.; Mekchay, S.; Thungrabeab, M.; Suttiprapan, P.; Sengottayan, S.-N.; Krutmuang, P. Insecticidal efficacy of nanoparticles against Spodoptera frugiperda (J.E. Smith) larvae and their impact in the soil. Heliyon 2023, 9, e16133. [Google Scholar]
- Yousef, H.A.; Fahmy, H.M.; Arafa, F.N.; Abd Allah, M.Y.; Tawfik, Y.M.; El Halwany, K.K.; El-Ashmanty, B.A.; Al-Anany, F.S.; Mohamed, M.A.; Bassily, M.E. Nanotechnology in pest management: Advantages, applications, and challenges. Int. J. Trop. Insect Sci. 2023, 43, 1387–1399. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Tadesse, M.G.; Eid, E.M.; Abou Fayssal, S.; Adelodun, B.; Choi, K.S.; Širić, I.; Kumar, P.; et al. Essential oil composition of aerial part of Pluchea ovalis (Pers.) DC., silver nanoparticles synthesis, and larvicidal activities against fall armyworm. Sustainability 2022, 14, 15785. [Google Scholar] [CrossRef]
- Abbas, U.; Majeed, M.Z.; Alkherb, W.A.H.; Alshehri, M.A.; Alkeridis, L.A.; Sayed, S.; Majeed, M.I.; Riaz, M.A. Evaluation of silver nanoformulated plant extracts against larvae of the fall armyworm, Spodoptera frugiperda (J. E. Smith), under laboratory and field conditions. Entomol. Res. 2024, 54, e70003. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, J.; Dong, M.; Shen, J.; Yan, S. Nanocarrier-based eco-friendly RNA pesticides for sustainable management of plant pathogens and pests. Nanomaterials 2024, 14, 1874. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.R.; Noman, M.; Zhang, Z.; Wang, J.; Lu, Z.; Cai, Y.; Ahmed, T.; Li, B.; Wang, Y.; et al. Integrating RNA interference and nanotechnology: A transformative approach in plant protection. Plants 2025, 14, 977. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Barbole, R.S.; Banerjee, S.S.; Chate, G.P.; Biradar, A.V.; Khandare, J.J.; Giri, A.P. Budding trends in integrated pest management using advanced micro- and nano-materials: Challenges and perspectives. J. Environ. Manag. 2016, 184, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Jafir, M.; Irfan, M.; Zia-ur-Rehman, M.; Hafeez, F.; Ahmad, J.N.; Sabir, M.A.; Zulfiqar, U.; Iqbal, R.; Zulfiqar, F.; Moosa, A. The global trend of nanomaterial usage to control the important agricultural arthropod pests: A comprehensive review. Plant Stress 2023, 10, 100208. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Speciat. Bioavailab. 2018, 30, 123–134. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Zhang, Z.; Fu, H.; White, J.C.; Lynch, I. Nanomaterial transformation in the soil–plant system: Implications for food safety and application in agriculture. Small 2020, 16, 2000705. [Google Scholar] [CrossRef] [PubMed]
- Punniyakotti, P.; Vinayagam, S.; Rajamohan, R.; Priya, S.; Moovendhan, M.; Sundaram, T. Environmental fate and ecotoxicological behaviour of pesticides and insecticides in non-target environments: Nanotechnology-based mitigation strategies. J. Environ. Chem. Eng. 2024, 12, 113349. [Google Scholar] [CrossRef]
- Hafeez, A.; Husain, M.A.; Singh, S.P.; Chauhan, A.; Khan, M.T.; Kumar, N.; Chauhan, A.; Soni, S.K. Implementation of drone technology for farm monitoring & pesticide spraying: A review. Inf. Process. Agric. 2023, 10, 192–203. [Google Scholar]
- Matthews, G.A. New technology for desert locust control. Agronomy 2021, 11, 1052. [Google Scholar] [CrossRef]
- Dara, S.K. The new integrated pest management paradigm for the modern age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef]
- Seelan, S.K.; Laguette, S.; Casady, G.M.; Seielstad, G.A. Remote sensing applications for precision agriculture: A learning community approach. Remote Sens. Environ. 2003, 88, 157–169. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinyanjui, G.; Mawcha, K.T.; Malinga, L.N.; Soobramoney, K.; Ṋethononda, P.; Assefa, Y.; Okonkwo, C.O.; Ndolo, D. Managing African Armyworm Outbreaks in Sub-Saharan Africa: Current Strategies and Future Directions. Insects 2025, 16, 645. https://doi.org/10.3390/insects16060645
Kinyanjui G, Mawcha KT, Malinga LN, Soobramoney K, Ṋethononda P, Assefa Y, Okonkwo CO, Ndolo D. Managing African Armyworm Outbreaks in Sub-Saharan Africa: Current Strategies and Future Directions. Insects. 2025; 16(6):645. https://doi.org/10.3390/insects16060645
Chicago/Turabian StyleKinyanjui, Grace, Kahsay Tadesse Mawcha, Lawrence Nkosikhona Malinga, Kaitlyn Soobramoney, Phophi Ṋethononda, Yoseph Assefa, Chibuzor Onyinye Okonkwo, and Dennis Ndolo. 2025. "Managing African Armyworm Outbreaks in Sub-Saharan Africa: Current Strategies and Future Directions" Insects 16, no. 6: 645. https://doi.org/10.3390/insects16060645
APA StyleKinyanjui, G., Mawcha, K. T., Malinga, L. N., Soobramoney, K., Ṋethononda, P., Assefa, Y., Okonkwo, C. O., & Ndolo, D. (2025). Managing African Armyworm Outbreaks in Sub-Saharan Africa: Current Strategies and Future Directions. Insects, 16(6), 645. https://doi.org/10.3390/insects16060645







