Cell Structure of the Preoral Mycangia of Xyleborus (Coleoptera: Curculiondiae) Ambrosia Beetles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing, Fungal Strains, and Culture Conditions
2.2. Microscopy
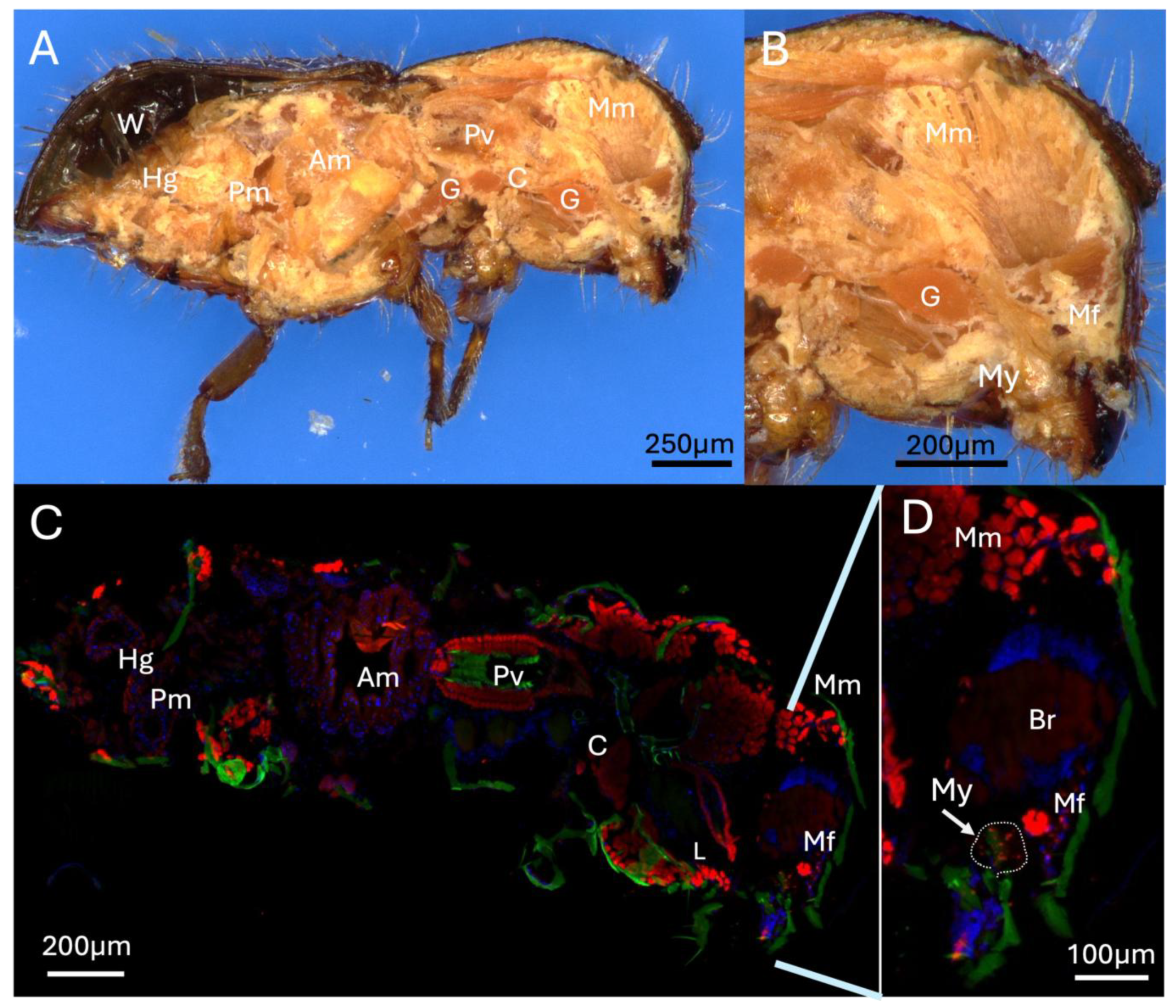
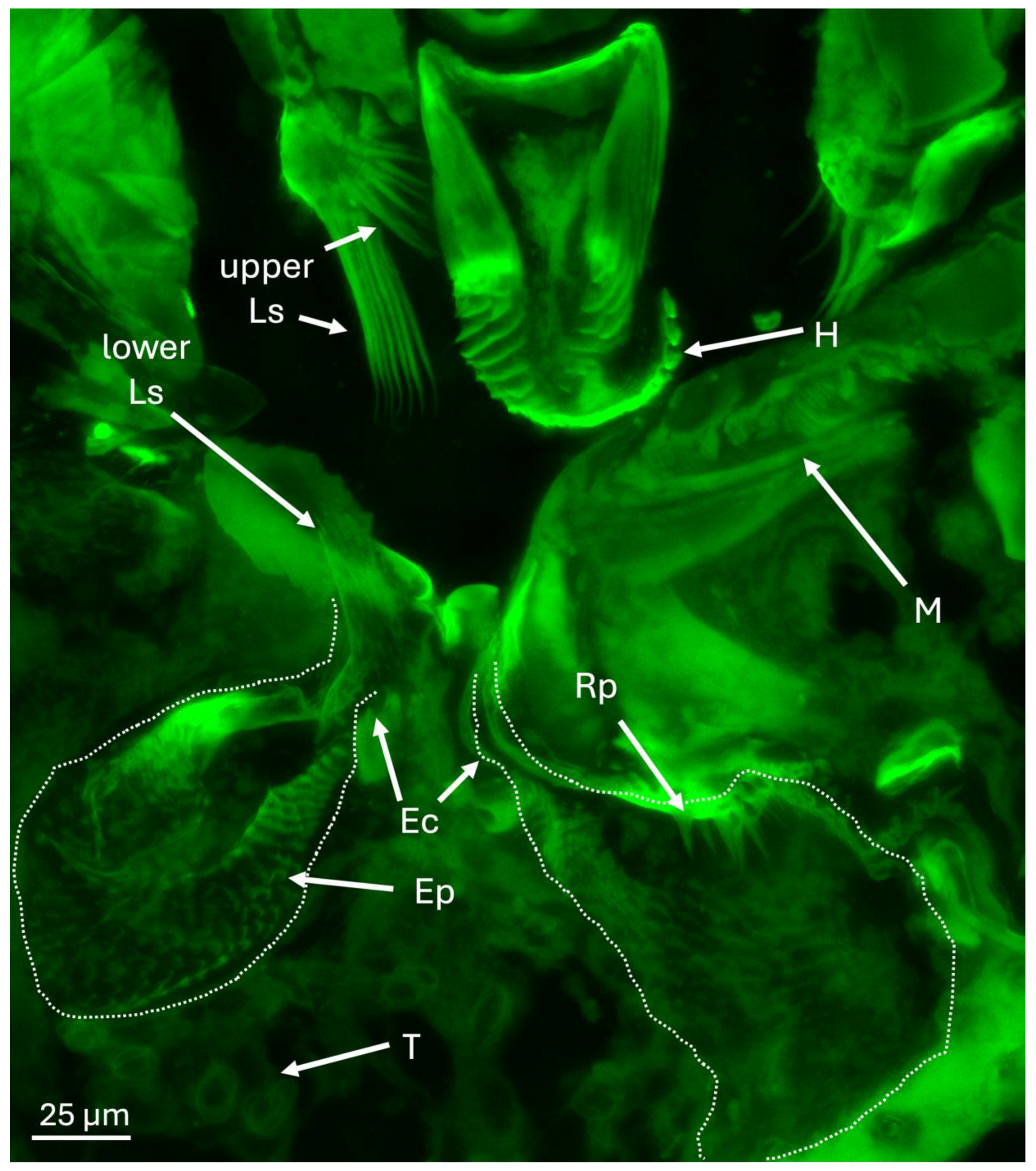
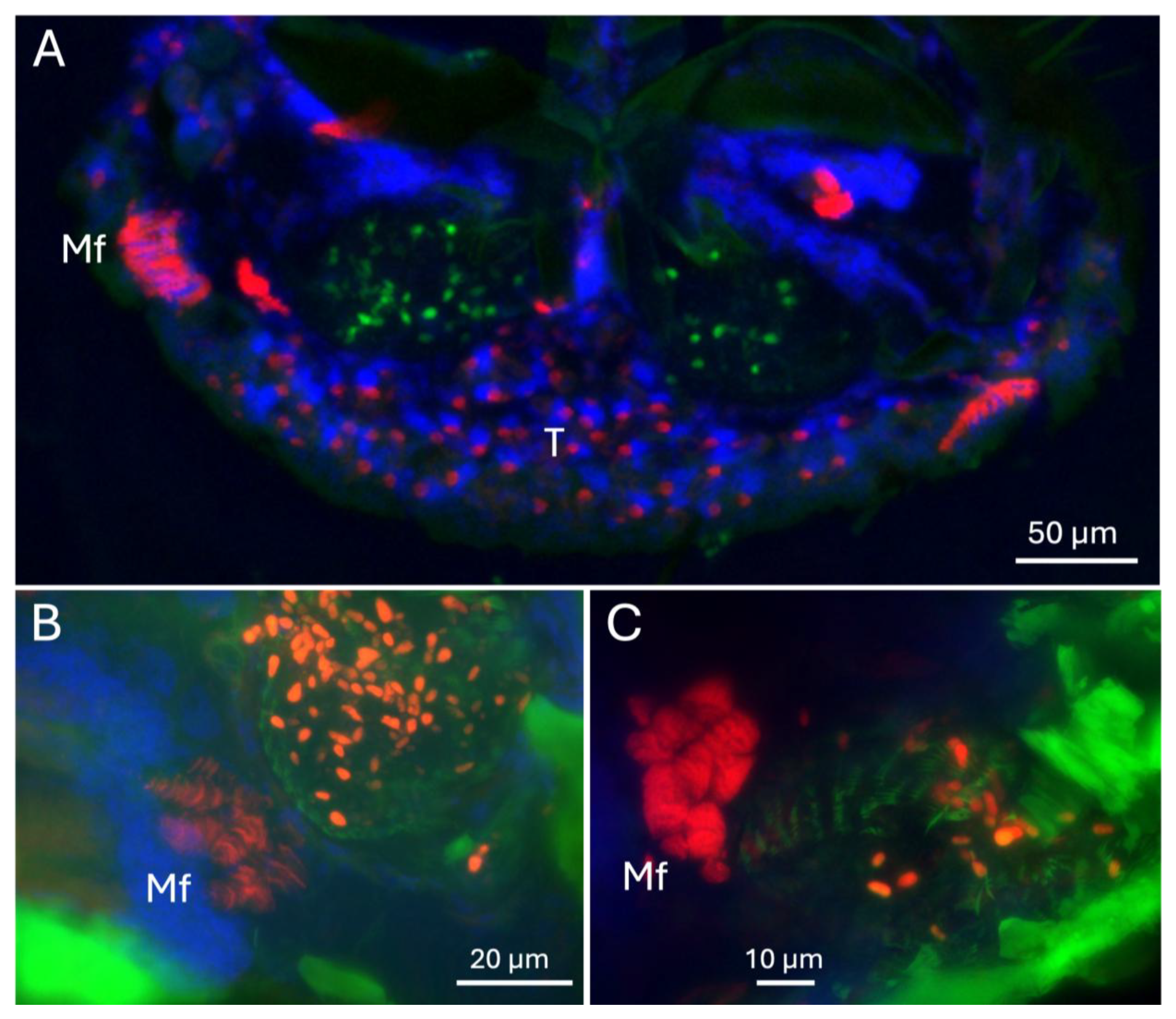
3. Results
3.1. Mandible, Muscle, and Cross-Section Analyses of the X. affinis Mycangia
3.2. Finer Details of the Surrounding External and Internal Structures of the Mycangia
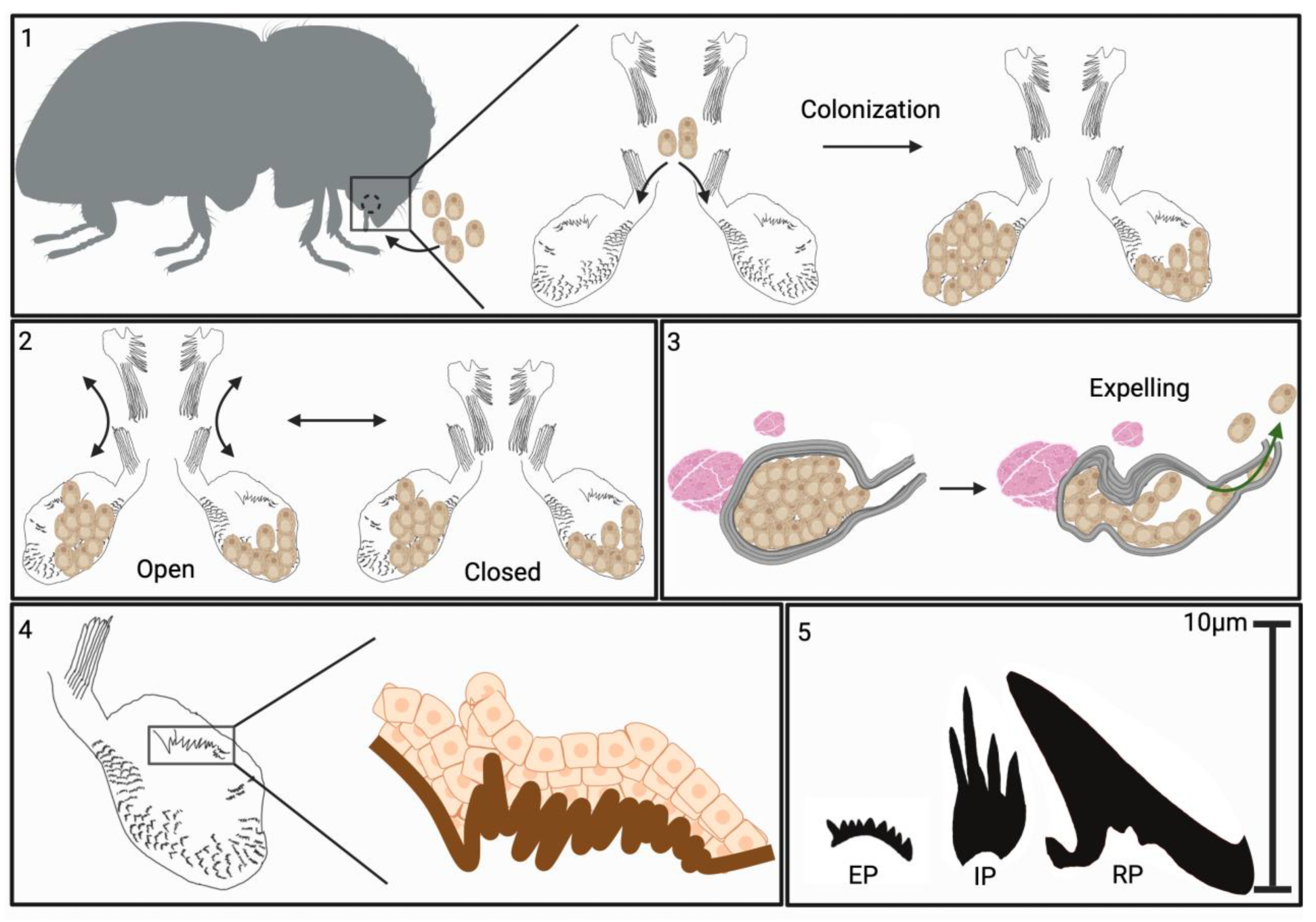
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dzurenko, M.; Hulcr, J. Ambrosia beetles. Curr. Biol. 2022, 32, R61–R62. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Keyhani, N. Fungal mutualisms and pathosystems: Life and death in the ambrosia beetle mycangia. Appl. Microbiol. Biotechnol. 2021, 105, 3393–3410. [Google Scholar] [CrossRef]
- Vanderpool, D.; Bracewell, R.R.; McCutcheon, J.P. Know your farmer: Ancient origins and multiple independent domestications of ambrosia beetle fungal cultivars. Mol. Ecol. 2018, 27, 2077–2094. [Google Scholar] [CrossRef]
- Biedermann, P.; Vega, F.; Douglas, A. Ecology and evolution of insect-fungus mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef]
- Li, Y.; Bateman, C.; Skelton, J.; Wang, B.; Black, A.; Huang, Y.; Gonzalez, A.; Jusino, M.; Nolen, Z.; Freeman, S.; et al. Preinvasion assessment of exotic bark beetle-vectored fungi to detect tree-killing pathogens. Phytopathology 2022, 112, 261–270. [Google Scholar] [CrossRef]
- Hulcr, J.; Gomez, D.; Skelton, J.; Johnson, A.; Adams, S.; Li, Y.; Jusino, M.; Smith, M. Invasion of an inconspicuous ambrosia beetle and fungus may affect wood decay in Southeastern North America. Biol. Invasions 2021, 23, 1339–1347. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, J.; Hulcr, J. Insect wood borers on commercial North American tree species growing in China: Review of Chinese peer-review and grey literature. Environ. Entomol. 2023, 52, 289–300. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, A.; Gao, L.; Wu, C.; Hulcr, J. Two new invasive Ips bark beetles (Coleoptera: Curculionidae) in mainland China and their potential distribution in Asia. Pest Manag. Sci. 2021, 77, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Johnson, A.; Hulcr, J. Potential pest bark and ambrosia beetles from Cuba not present in the continental United States. Fla. Entomol. 2020, 103, 96–102. [Google Scholar] [CrossRef]
- Prior, K.M.; Adams, D.C.; Klepzig, K.D.; Hulcr, J. When does invasive species removal lead to ecological recovery? Implications for management success. Biol. Invasions 2018, 20, 267–283. [Google Scholar] [CrossRef]
- Seibold, S.; Muller, J.; Baldrian, P.; Cadotte, M.; Stursova, M.; Biedermann, P.; Krah, F.; Bassler, C. Fungi associated with beetles dispersing from dead wood-Let’s take the beetle bus! Fungal Ecol. 2019, 39, 100–108. [Google Scholar] [CrossRef]
- Skelton, J.; Johnson, A.; Jusino, M.; Bateman, C.; Li, Y.; Hulcr, J. A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts. Proc. R. Soc. B-Biol. Sci. 2019, 286, 20182127. [Google Scholar] [CrossRef]
- Carrillo, J.; Rugman-Jones, P.; Husein, D.; Stajich, J.; Kasson, M.; Carrillo, D.; Stouthamer, R.; Eskalen, A. Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genet. Biol. 2019, 133, 103269. [Google Scholar] [CrossRef]
- Saucedo-Carabez, J.R.; Ploetz, R.C.; Konkol, J.L.; Carrillo, D.; Gazis, R. Partnerships between ambrosia beetles and fungi: Lineage-specific promiscuity among vectors of the laurel wilt pathogen, Raffaelea lauricola. Microb. Ecol. 2018, 925–940. [Google Scholar] [CrossRef]
- Kostovcik, M.; Bateman, C.; Kolarik, M.; Stelinski, L.; Jordal, B.; Hulcr, J. The ambrosia symbiosis is specific in some species and promiscuous in others: Evidence from community pyrosequencing. ISME J. 2015, 9, 126–138. [Google Scholar] [CrossRef]
- Francke-Grossmann, H. Ectosymbiosis in wood-inhabiting insects. In Symbiosis: Associations of invetebrates Birds, Ruminants and Other Biota; Henry, S.M., Ed.; Academic Press: New York, NY, USA, 1967; Volume II, pp. 142–206. [Google Scholar]
- Li, Y.; Ruan, Y.; Kasson, M.; Stanley, E.; Gillett, C.; Johnson, A.; Zhang, M.; Hulcr, J. Structure of the ambrosia beetle (Coleoptera: Curculionidae) mycangia revealed through micro-computed tomography. J. Insect Sci. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Johnson, A.J.; McKenna, D.D.; Jordal, B.H.; Cognato, A.I.; Smith, S.M.; Lemmon, A.R.; Lemmon, E.M.; Hulcr, J. Phylogenomics clarifies repeated evolutionary origins of inbreeding and fungus farming in bark beetles (Curculionidae, Scolytinae). Mol. Phylogenet Evol. 2018, 127, 229–238. [Google Scholar] [CrossRef]
- Batra, L.R. Ambrosia fungi: Extent of specificity to ambrosia beetles. Science 1966, 153, 193–195. [Google Scholar] [CrossRef]
- Mayers, C.G.; Harrington, T.C.; Biedermann, P.H.W. Mycangia define the diverse ambrosia beetle–fungus symbioses. In The Convergent Evolution of Agriculture in Humans and Insects; Schultz, T.R., Gawne, R., Peregrine, P.N., Eds.; MIT Press: Cambridge, MA, USA, 2022; pp. 105–142. [Google Scholar]
- Mayers, C.; Harrington, T.; Mcnew, D.; Roeper, R.; Biedermann, P.; Masuya, H.; Bateman, C. Four mycangium types and four genera of ambrosia fungi suggest a complex history of fungus farming in the ambrosia beetle tribe Xyloterini. Mycologia 2020, 112, 1104–1137. [Google Scholar] [CrossRef]
- Li, Y.; Simmons, D.R.; Bateman, C.C.; Short, D.P.G.; Kasson, M.T.; Rabaglia, R.J.; Hulcr, J. New fungus-insect symbiosis: Culturing, molecular, and histological methods determine saprophytic Polyporales mutualists of Ambrosiodmus ambrosia beetles. PLoS ONE 2015, 10, e0137689. [Google Scholar] [CrossRef]
- Spahr, E.; Kasson, M.; Kijimoto, T. Micro-computed tomography permits enhanced visualization of mycangia across development and between sexes inEuwallaceaambrosia beetles. PLoS ONE 2020, 15, e0236653. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Kinoshita, S.; Sasaki, O.; Cognato, A.I.; Kajimura, H. Non-destructive observation of the mycangia of Euwallacea interjectus (Blandford) (Coleoptera: Curculionidae: Scolytinae) using X-ray computed tomography. Entomol. Sci. 2019, 22, 173–181. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive tree diseases associated with ambrosia and bark beetles: Black swan events in tree pathology? Plant Dis. 2013, 97, 856–872. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Narvaez, T.; Duncan, R.E.; Saucedo, R.J.; Campbell, A.; Mantilla, J.; Carrillo, D.; Kendra, P.E. Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida, USA. J. Econ. Entomol. 2017, 110, 347–354. [Google Scholar] [CrossRef]
- Pena, J.E.; Carrillo, D.; Duncan, R.E.; Capinera, J.L.; Brar, G.; Mclean, S.; Arpaia, M.L.; Focht, E.; Smith, J.A.; Hughes, M.; et al. Susceptibility of Persea spp. and other Lauraceae to attack by redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2012, 95, 783–787. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the Southeastern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef]
- Saucedo, J.R.; Ploetz, R.C.; Konkol, J.L.; Angel, M.; Mantilla, J.; Menocal, O.; Carrillo, D. Nutritional symbionts of a putative vector, Xyloborus bispinatus, of the laurel wilt pathogen of avocado, Raffaelea lauricola. Symbiosis 2017, 75, 29–38. [Google Scholar] [CrossRef]
- Joseph, R.; Bansal, K.; Keyhani, N.O. Host switching by an ambrosia beetle fungal mutualist: Mycangial colonization of indigenous beetles by the invasive laurel wilt fungal pathogen. Environ. Microbiol. 2023, 25, 1894–1908. [Google Scholar] [CrossRef]
- Menocal, O.; Cruz, L.F.; Kendra, P.E.; Berto, M.; Carrillo, D. Flexibility in the ambrosia symbiosis of Xyleborus bispinatus. Front. Microbiol. 2023, 14, 1110474. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.F.; Menocal, O.; Mantilla, J.; Ibarra-Juarez, L.A.; Carrillo, D. Xyleborus volvulus (Coleoptera: Curculionidae): Biology and Fungal Associates. Appl. Environ. Microbiol. 2019, 85, e01190-19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, D.; Joseph, R.; Li, T.; Keyhani, N. High efficiency transformation and mutant screening of the laurel wilt pathogen, Raffaelea lauricola. Appl. Microbiol. Biotechnol. 2020, 104, 7331–7343. [Google Scholar] [CrossRef] [PubMed]
- Spahr, E.; McLaughlin, S.; Tichinel, A.; Kasson, M.; Kijimoto, T. Staining and scanning protocol for micro-computed tomography to observe the morphology of soft tissues in ambrosia beetles. Bio-Protocol 2023, 13, e4584. [Google Scholar] [CrossRef]
- Toki, W. A single case study of mycetangia-associated fungi and their abilities to assimilate wood-associated carbon sources in the ship timber beetle Elateroides flabellicornis (Coleoptera: Lymexylidae) in Japan. Symbiosis 2021, 83, 173–181. [Google Scholar] [CrossRef]
- Kubota, K.; Watanabe, K.; Zhu, X.J.; Kawakami, K.; Tanahashi, M.; Fukatsu, T. Evolutionary relationship between Platycerus stag beetles and their mycangium-associated yeast symbionts. Front. Microbiol. 2020, 11, 1436. [Google Scholar] [CrossRef]
- Takagi, R.; Kajimura, H. Ecological traits of three species of Xiphydria woodwasps from Japan: Host tree species and eggs, symbiotic fungi, and mucus in their bodies. Forests 2025, 16, 264. [Google Scholar] [CrossRef]
- Bateman, C.; Huang, Y.T.; Simmons, D.R.; Kasson, M.T.; Stanley, E.L.; Hulcr, J. Ambrosia beetle Premnobius cavipennis (Scolytinae: Ipini) carries highly divergent ascomycotan ambrosia fungus, Afroraffaelea ambrosiae gen. nov et sp nov (Ophiostomatales). Fungal Ecol. 2017, 25, 41–49. [Google Scholar] [CrossRef]
- Kasson, M.T.; Wickert, K.L.; Stauder, C.M.; Macias, A.M.; Berger, M.C.; Simmons, D.R.; Short, D.P.G.; DeVallance, D.B.; Hulcr, J. Mutualism with aggressive wood-degrading Flavodon ambrosius (Polyporales) facilitates niche expansion and communal social structure in Ambrosiophilus ambrosia beetles. Fungal Ecol. 2016, 23, 86–96. [Google Scholar] [CrossRef]
- Spahr, E.J.; Wasef, F.; Kasson, M.T.; Kijimoto, T. Developmental genetic underpinnings of a symbiosis-associated organ in the fungus-farming ambrosia beetle Euwallacea validus. Sci. Rep. 2023, 13, 14014. [Google Scholar] [CrossRef]
- Joseph, R.A.; Bansal, K.; Nguyen, J.; Bielanski, M.; Tirmizi, E.; Masoudi, A.; Keyhani, N.O. Fungi that live within animals: Application of cell cytometry to examine fungal colonization of ambrosia beetle (Xyleborus sp.) mycangia. J. Fungi 2025, 11, 184. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, R.A.; Tirmizi, E.; Masoudi, A.; Keyhani, N.O. Cell Structure of the Preoral Mycangia of Xyleborus (Coleoptera: Curculiondiae) Ambrosia Beetles. Insects 2025, 16, 644. https://doi.org/10.3390/insects16060644
Joseph RA, Tirmizi E, Masoudi A, Keyhani NO. Cell Structure of the Preoral Mycangia of Xyleborus (Coleoptera: Curculiondiae) Ambrosia Beetles. Insects. 2025; 16(6):644. https://doi.org/10.3390/insects16060644
Chicago/Turabian StyleJoseph, Ross A., Esther Tirmizi, Abolfazl Masoudi, and Nemat O. Keyhani. 2025. "Cell Structure of the Preoral Mycangia of Xyleborus (Coleoptera: Curculiondiae) Ambrosia Beetles" Insects 16, no. 6: 644. https://doi.org/10.3390/insects16060644
APA StyleJoseph, R. A., Tirmizi, E., Masoudi, A., & Keyhani, N. O. (2025). Cell Structure of the Preoral Mycangia of Xyleborus (Coleoptera: Curculiondiae) Ambrosia Beetles. Insects, 16(6), 644. https://doi.org/10.3390/insects16060644




